Chapter 2 Measurement Mass and Weight Measurement Sig















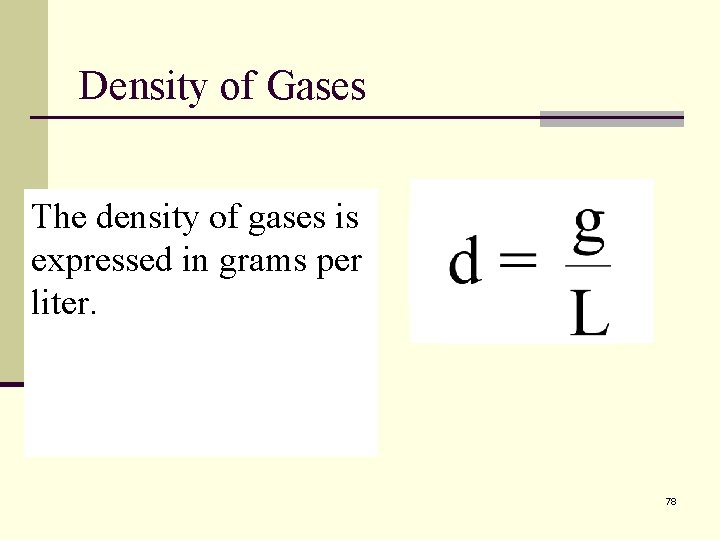


- Slides: 87

Chapter 2 - Measurement Mass and Weight Measurement Sig. Figs. And Rounding Scientific Notation Sig. Figs. in Calculations Metric system Measurement – Length, Mass, Volume, and Temperature 8. Density 9. Problem Solving 1. 2. 3. 4. 5. 6. 7. 1

Mass and Weight n Mass: The amount of matter in that body n Measured on a balance with a comparison with known masses n Weight: The measure of the earth’s gravitational attraction for that object n Measured on a scale, which measures force against a spring – Varies with location of the object n Matter: Anything that has mass and occupies space. 2

Nature of Measurement n Experiments are performed. n Numerical values or data are obtained from these measurements. 3

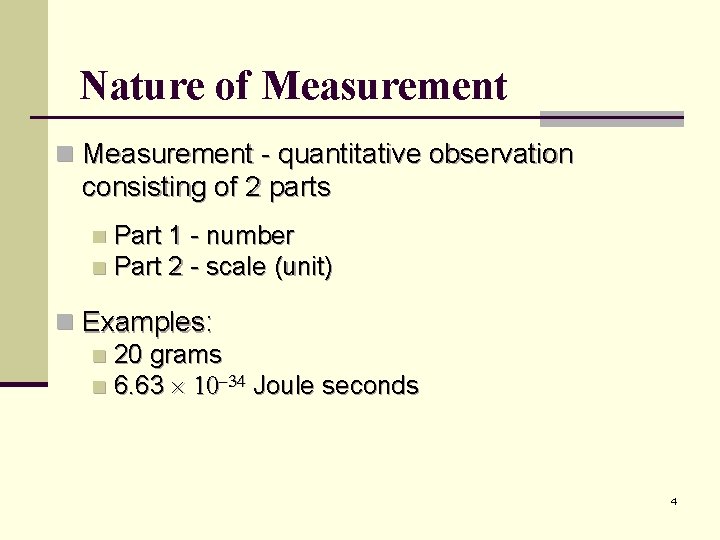
Nature of Measurement n Measurement - quantitative observation consisting of 2 parts Part 1 - number n Part 2 - scale (unit) n n Examples: n 20 grams n 6. 63 Joule seconds 4

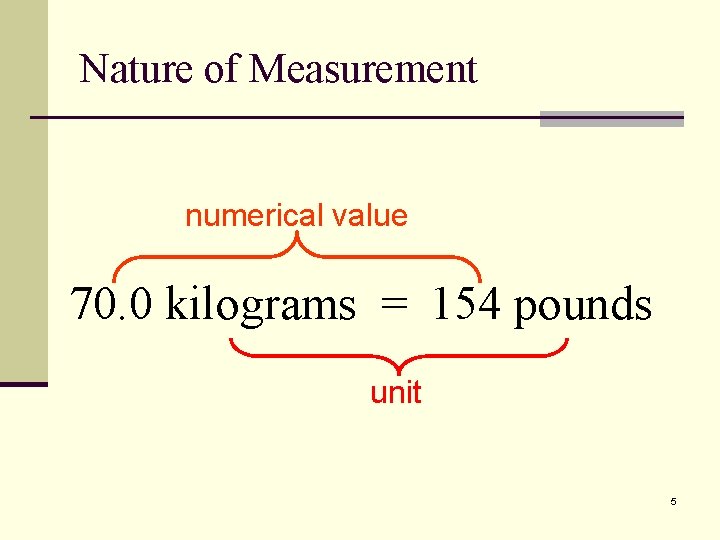
Nature of Measurement numerical value 70. 0 kilograms = 154 pounds unit 5

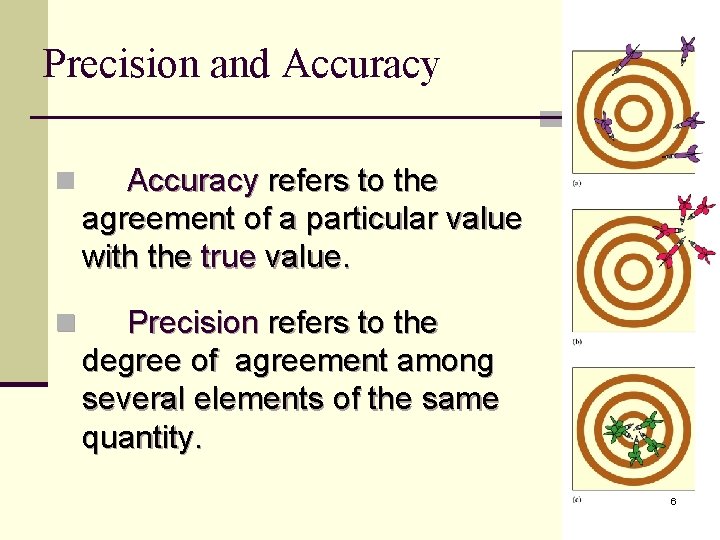
Precision and Accuracy n Accuracy refers to the agreement of a particular value with the true value. n Precision refers to the degree of agreement among several elements of the same quantity. 6

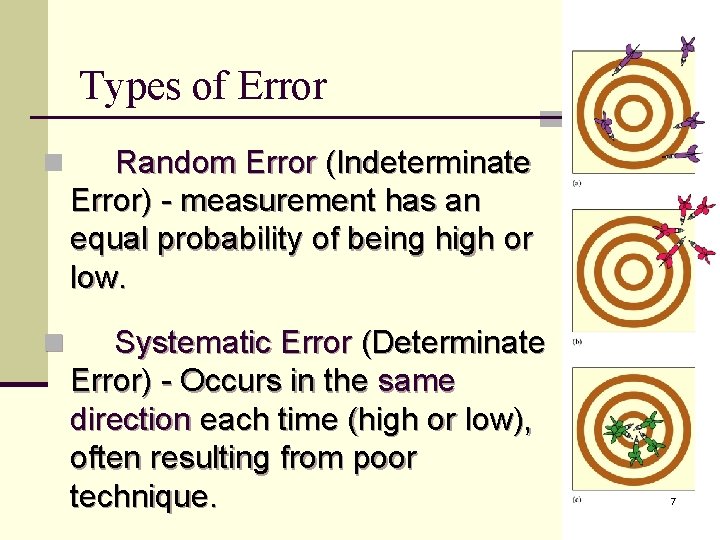
Types of Error n Random Error (Indeterminate Error) - measurement has an equal probability of being high or low. n Systematic Error (Determinate Error) - Occurs in the same direction each time (high or low), often resulting from poor technique. 7

Measurement and Significant Figures n Numbers obtained from a measurement are never exact values. n Degree of uncertainty Due to limitations of instrument n Skill of the individual n n Recorded value should indicate uncertainty n Maximum precision n n Contain all known digits Plus one digit that is estimated n These digits are know as Significant Figures 8

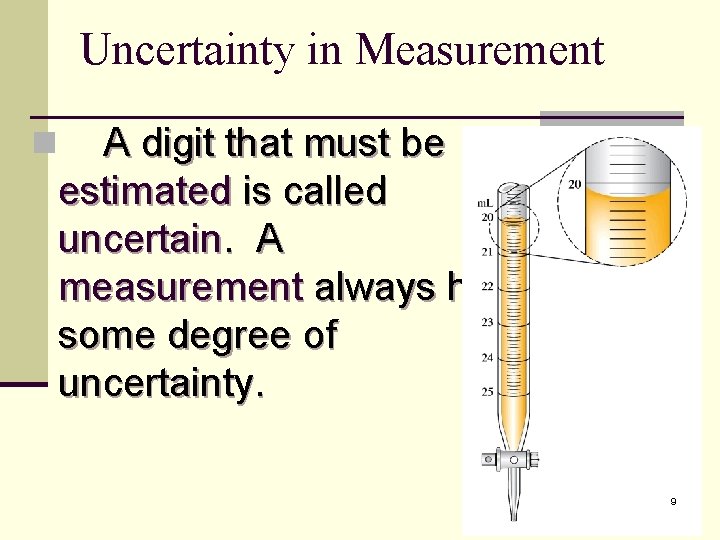
Uncertainty in Measurement A digit that must be estimated is called uncertain. A measurement always has some degree of uncertainty. n 9

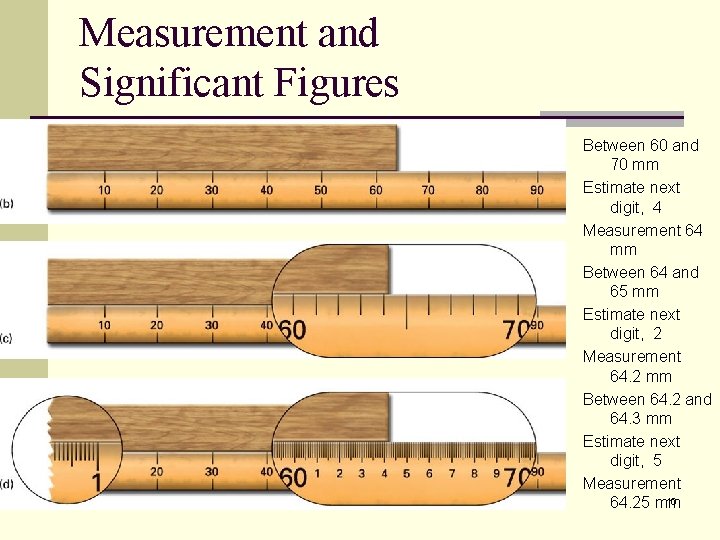
Measurement and Significant Figures Between 60 and 70 mm Estimate next digit, 4 Measurement 64 mm Between 64 and 65 mm Estimate next digit, 2 Measurement 64. 2 mm Between 64. 2 and 64. 3 mm Estimate next digit, 5 Measurement 10 64. 25 mm

Measurement and Significant Figures 11

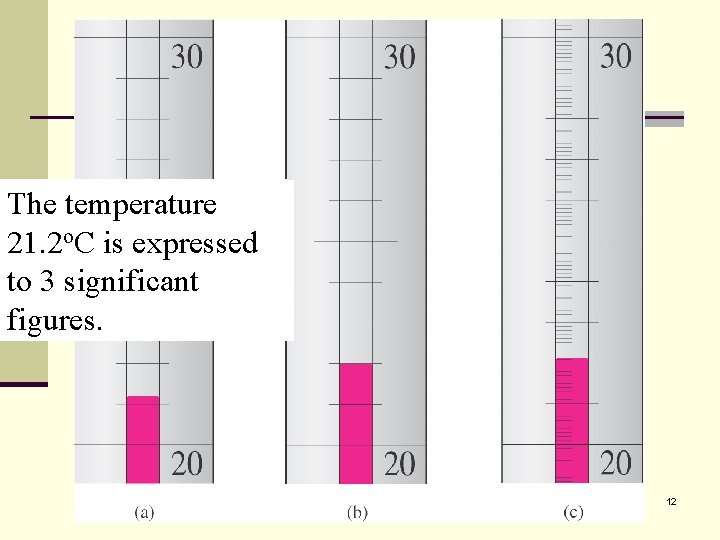
The temperature Temperature is o. C is expressed 21. 2 estimated to be o. C. The last 2 is to 3 significant 21. 2 figures. uncertain. 12

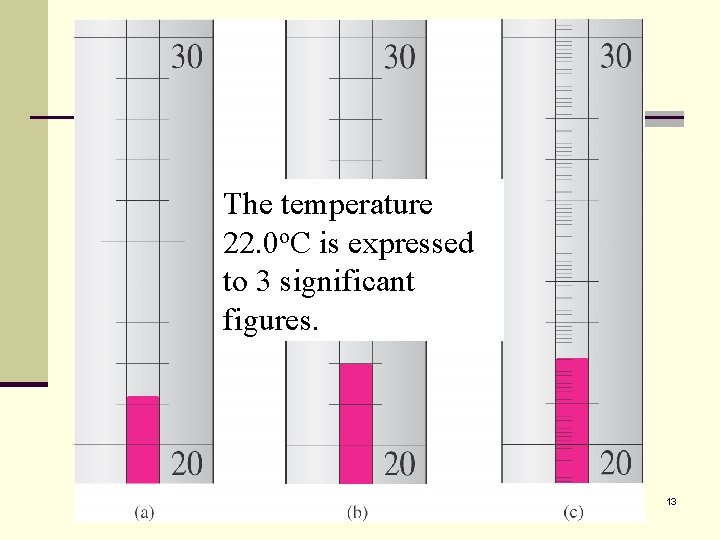
The temperature Temperature is o. C is expressed 22. 0 estimated to be to 3 osignificant 22. 0 C. The last 0 is figures. uncertain. 13

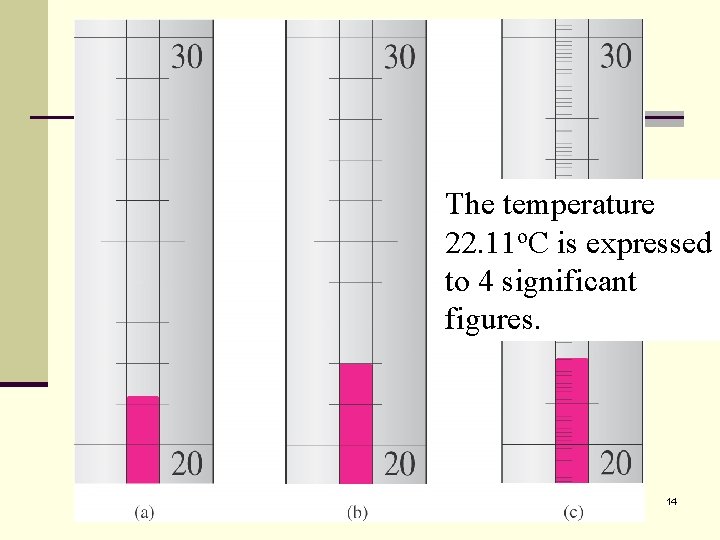
The temperature Temperature is o. C isto 22. 11 expressed estimated be o. C. The last 1 to 4 significant 22. 11 figures. is uncertain. 14

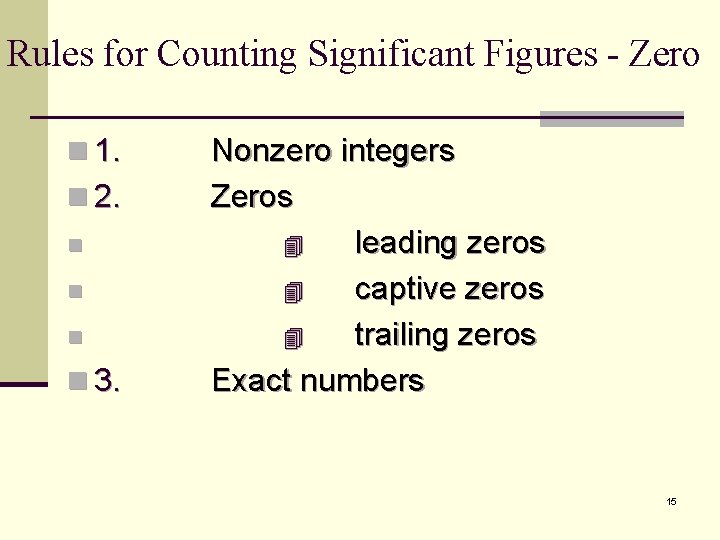
Rules for Counting Significant Figures - Zero n 1. n 2. n n 3. Nonzero integers Zeros leading zeros captive zeros trailing zeros Exact numbers 15

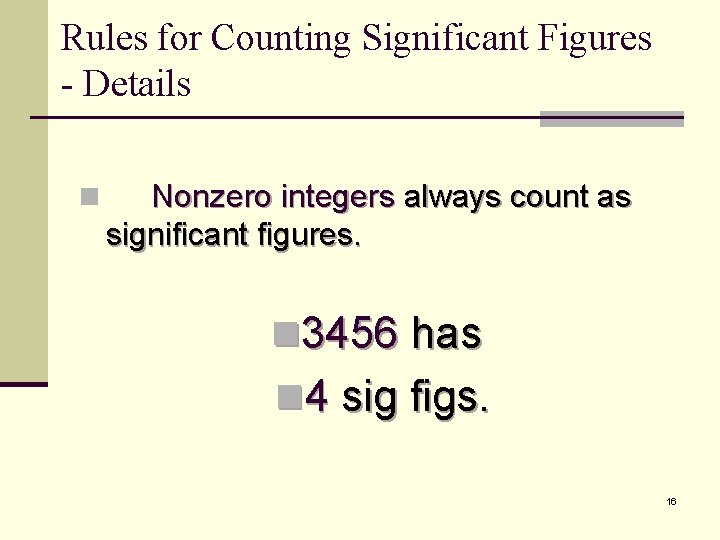
Rules for Counting Significant Figures - Details n Nonzero integers always count as significant figures. n 3456 has n 4 sig figs. 16

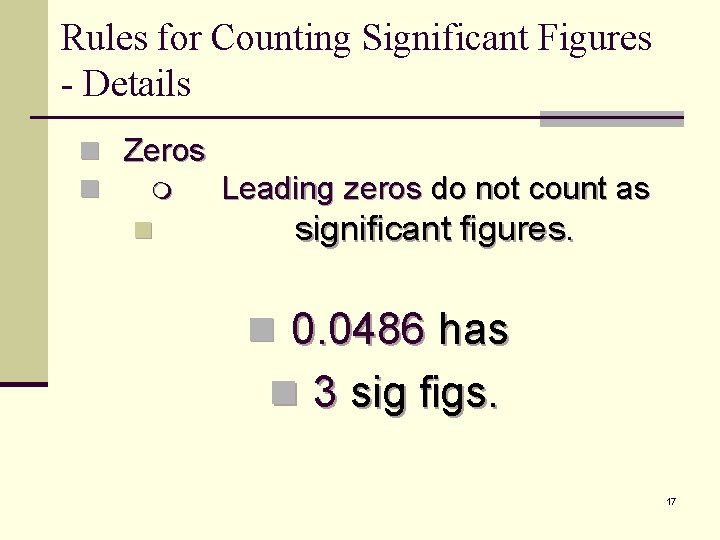
Rules for Counting Significant Figures - Details n Zeros n Leading zeros do not count as n significant figures. n 0. 0486 has n 3 sig figs. 17

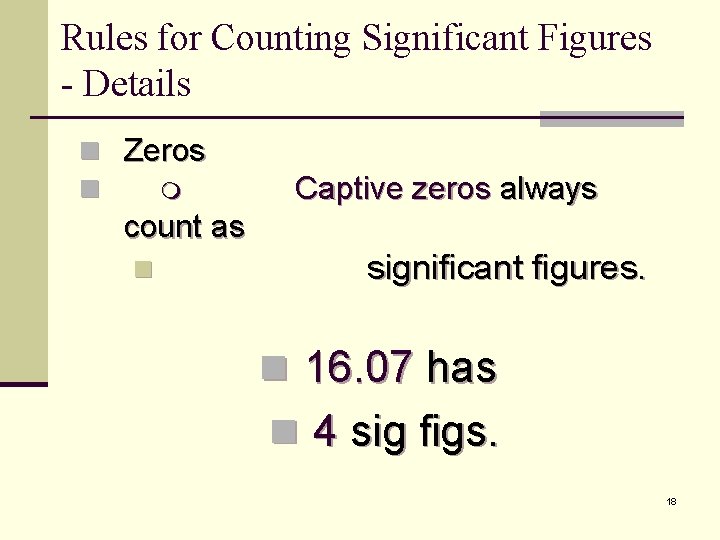
Rules for Counting Significant Figures - Details n Zeros n Captive zeros always count as n significant figures. n 16. 07 has n 4 sig figs. 18

Rules for Counting Significant Figures - Details n Zeros n Trailing zeros are significant only n if the number contains a decimal point. n 9. 300 has n 4 sig figs. 19

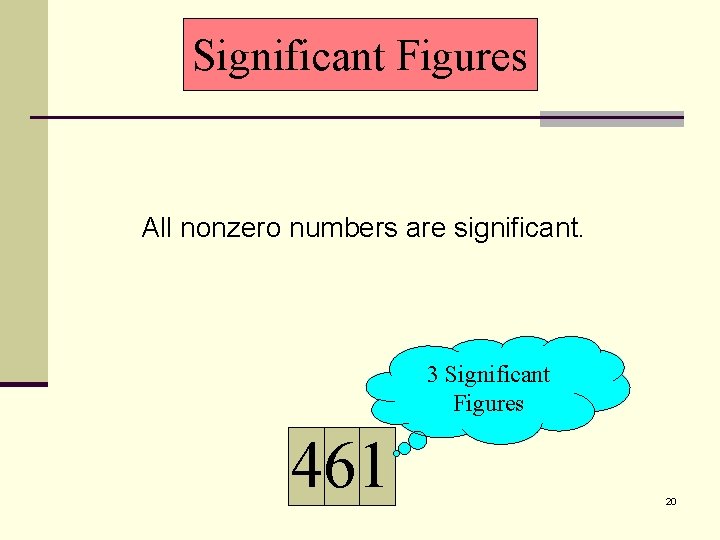
Significant Figures All nonzero numbers are significant. 3 Significant Figures 461 20

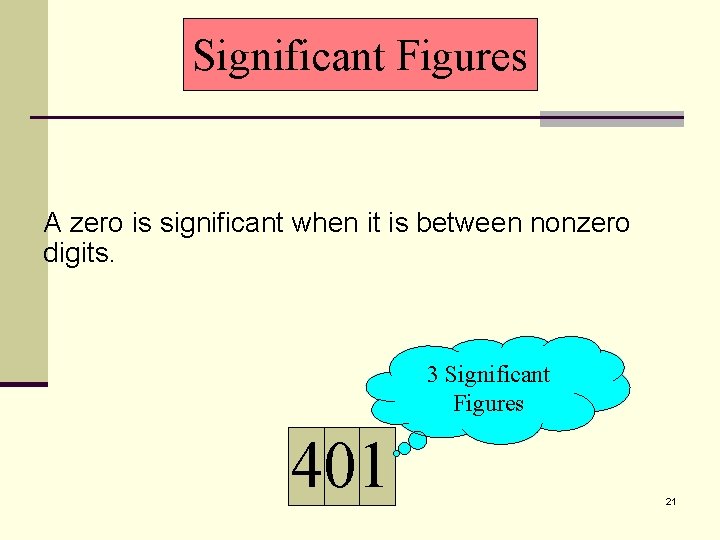
Significant Figures A zero is significant when it is between nonzero digits. 3 Significant Figures 401 21

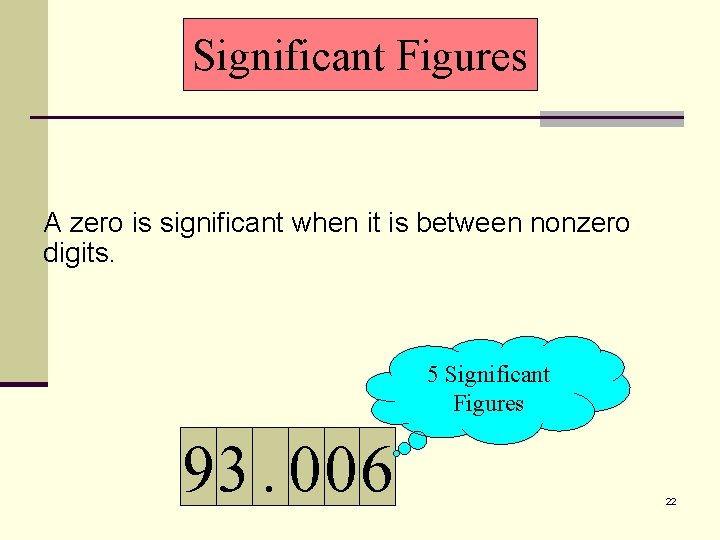
Significant Figures A zero is significant when it is between nonzero digits. 5 Significant Figures 93. 006 22

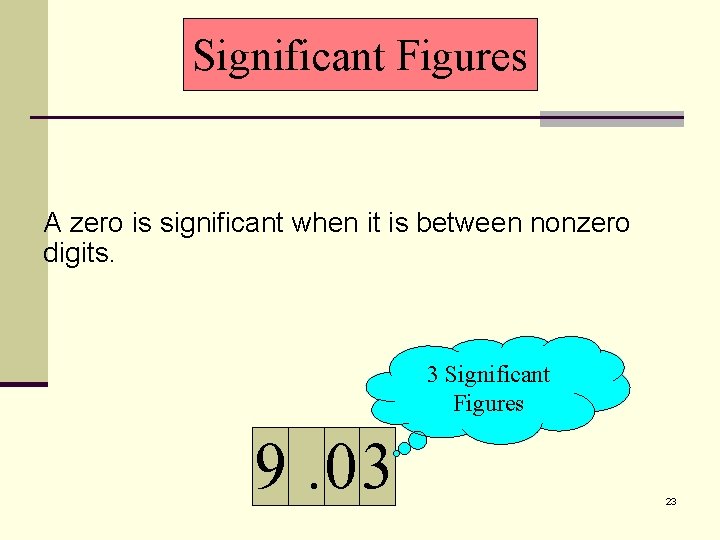
Significant Figures A zero is significant when it is between nonzero digits. 3 Significant Figures 9. 03 23

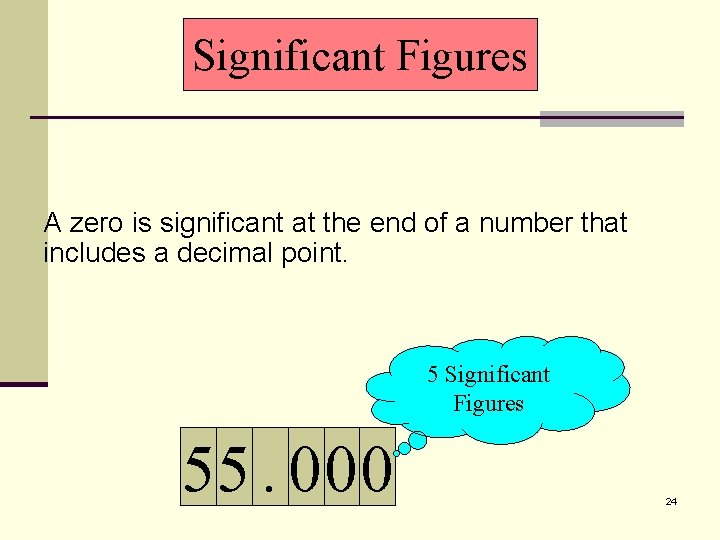
Significant Figures A zero is significant at the end of a number that includes a decimal point. 5 Significant Figures 55. 000 24

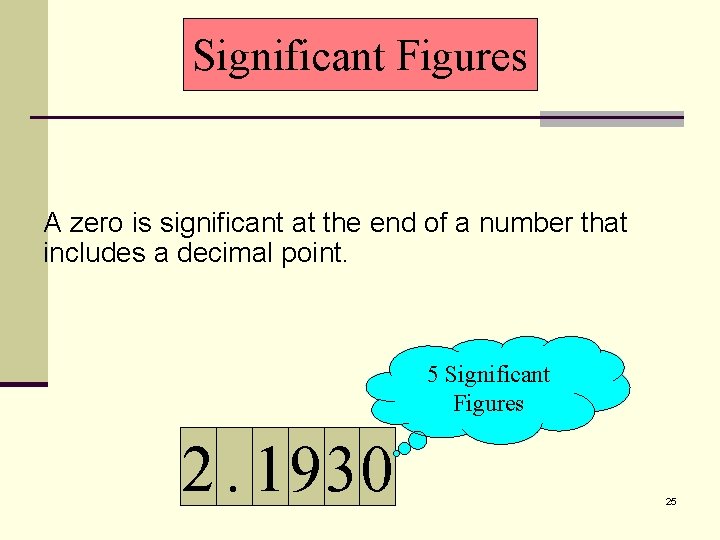
Significant Figures A zero is significant at the end of a number that includes a decimal point. 5 Significant Figures 2. 1930 25

Significant Figures A zero is not significant when it is before the first nonzero digit. 1 Significant Figure 0. 006 26

Significant Figures A zero is not significant when it is before the first nonzero digit. 3 Significant Figures 0. 709 27

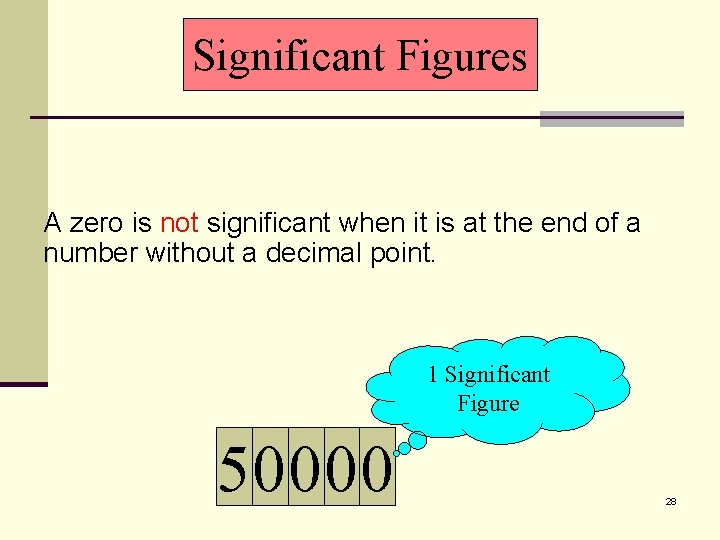
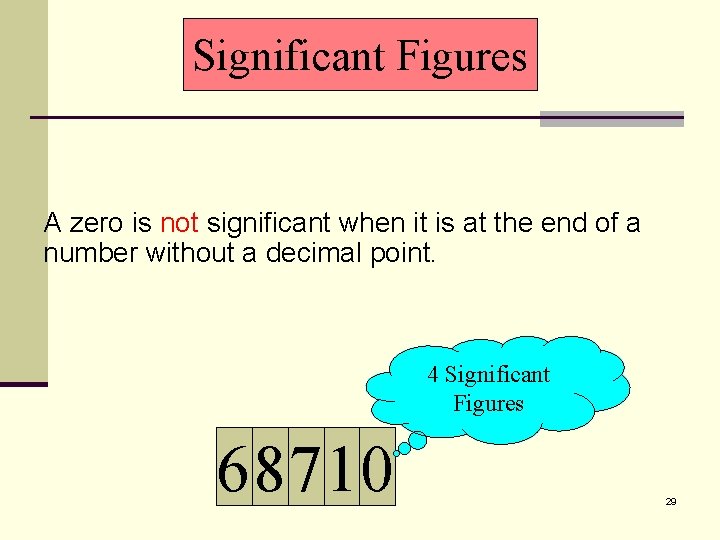
Significant Figures A zero is not significant when it is at the end of a number without a decimal point. 1 Significant Figure 50000 28

Significant Figures A zero is not significant when it is at the end of a number without a decimal point. 4 Significant Figures 68710 29

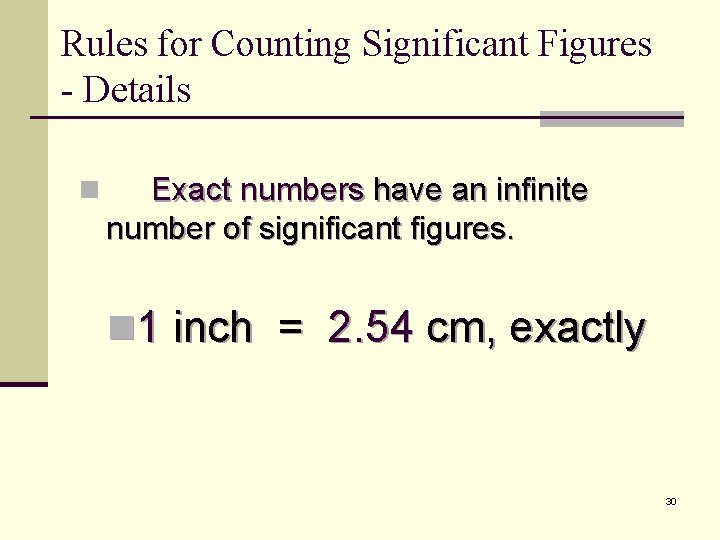
Rules for Counting Significant Figures - Details n Exact numbers have an infinite number of significant figures. n 1 inch = 2. 54 cm, exactly 30

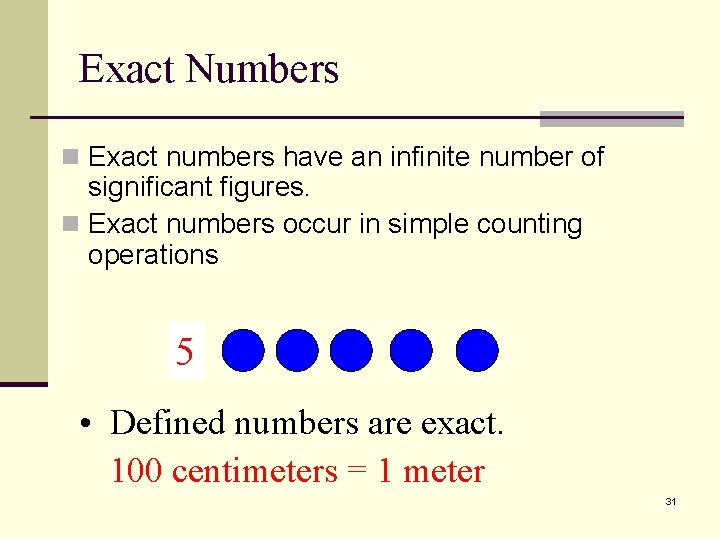
Exact Numbers n Exact numbers have an infinite number of significant figures. n Exact numbers occur in simple counting operations 12345 • Defined numbers are exact. 12 inches 100 centimeters = 1 foot = 1 meter 31

Rounding Off Numbers n Often when calculations are performed extra digits are present in the results. n It is necessary to drop these extra digits so as to express the answer to the correct number of significant figures. n When digits are dropped the value of the last digit retained is determined by a process known as rounding off numbers. 32

Rounding off Numbers n Rule 1. The first digit after those to be retained is 4 or less, all other digits are dropped. n 34. 642 = 34. 64 n Rule 2. The first digit after those to be retained is 5 or more, all other digits are dropped and the last digit is increased by one. n 34. 6426 = 34. 643 33

Rounding Off Numbers Rule 1. When the first digit after those you want to retain is 4 or less, that digit and all others to its right are dropped. The last digit retained is not changed. 4 or less 80. 873 34

Rounding Off Numbers Rule 1. When the first digit after those you want to retain is 4 or less, that digit and all others to its right are dropped. The last digit retained is not changed. 4 or less 1. 875377 35

Rounding Off Numbers Rule 2. When the first digit after those you want to retain is 5 or greater, that digit and all others to its right are dropped. The last digit retained is increased by 1. drop 5 or these greater figures increase by 1 6 5. 459672 36

Scientific Notation n Why? A convenient way of writing very small or very big numbers. n Earth age is 4, 500, 000 years n n n Estimated value to the nearest 0. 1 billion years Thus can be written as 4. 5 x 109 years. Radius of hydrogen 0. 000, 037 meters n Written 3. 7 x 10 -11 meters 37

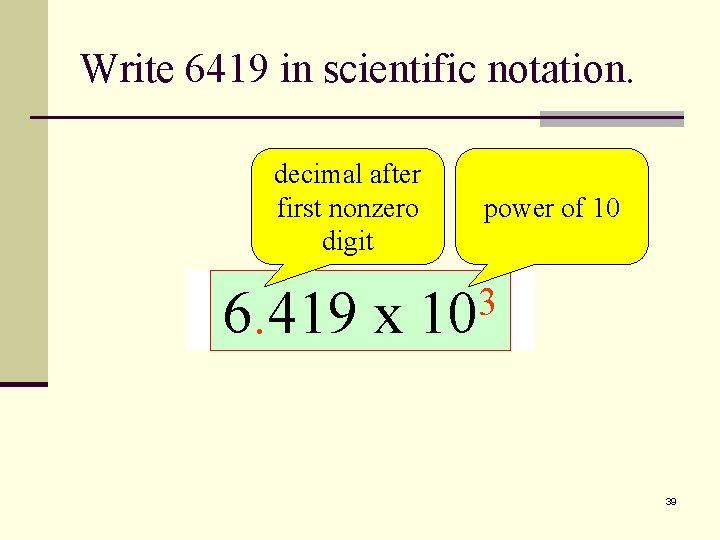
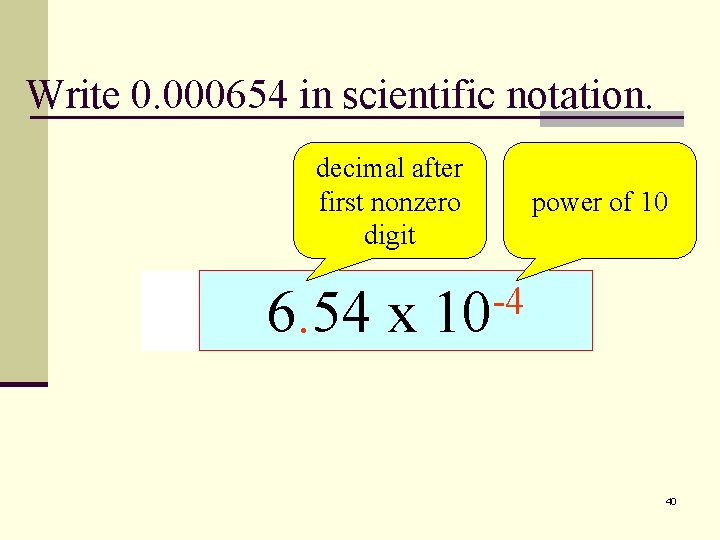
Scientific Notation n Write a number as a power of 10 n Move the decimal point in the original number so that it is located after the first nonzero digit. n Follow the new number by a multiplication sign and 10 with an exponent (power). n The exponent is equal to the number of places that the decimal point was shifted. 38

Write 6419 in scientific notation. decimal after first nonzero digit power of 10 2 1 3 641. 9 x 10 64. 19 x 10 6419. 6. 419 x 10 39

Write 0. 000654 in scientific notation. decimal after first nonzero digit 6. 54 x 0. 000654 0. 654 power of 10 -4 -2 -1 -3 10 40

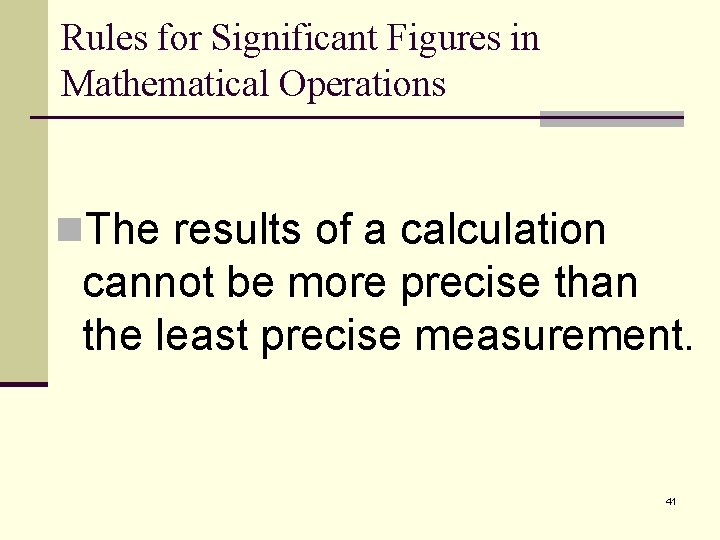
Rules for Significant Figures in Mathematical Operations n. The results of a calculation cannot be more precise than the least precise measurement. 41

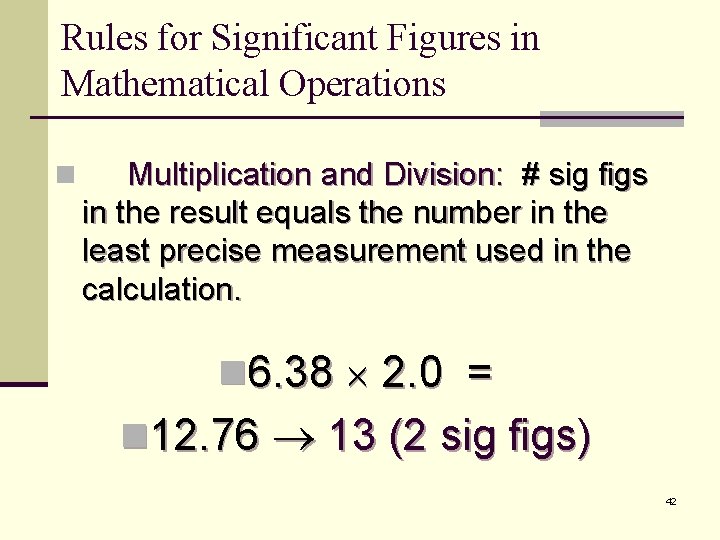
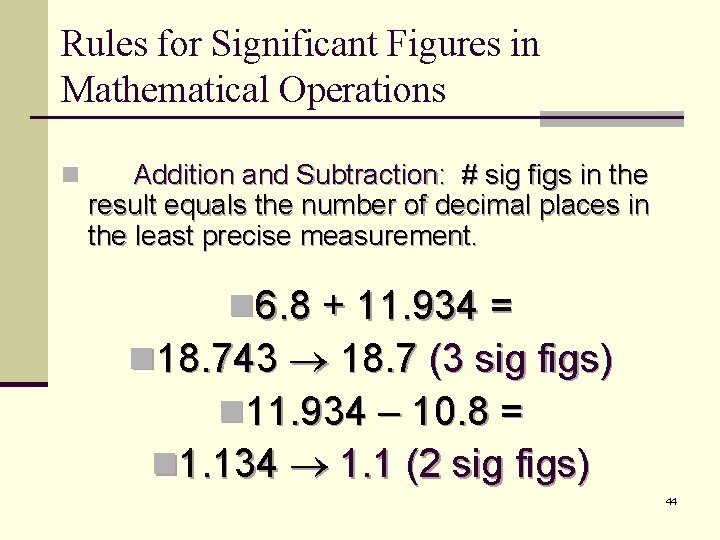
Rules for Significant Figures in Mathematical Operations n Multiplication and Division: # sig figs in the result equals the number in the least precise measurement used in the calculation. n 6. 38 2. 0 = n 12. 76 13 (2 sig figs) 42

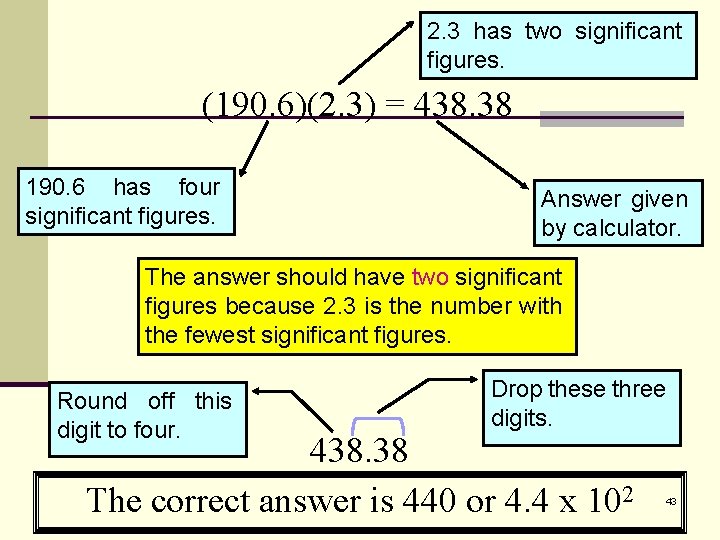
2. 3 has two significant figures. (190. 6)(2. 3) = 438. 38 190. 6 has four significant figures. Answer given by calculator. The answer should have two significant figures because 2. 3 is the number with the fewest significant figures. Round off this digit to four. Drop these three digits. 438. 38 The correct answer is 440 or 4. 4 x 102 43

Rules for Significant Figures in Mathematical Operations n Addition and Subtraction: # sig figs in the result equals the number of decimal places in the least precise measurement. n 6. 8 + 11. 934 = n 18. 743 18. 7 (3 sig figs) n 11. 934 – 10. 8 = n 1. 134 1. 1 (2 sig figs) 44

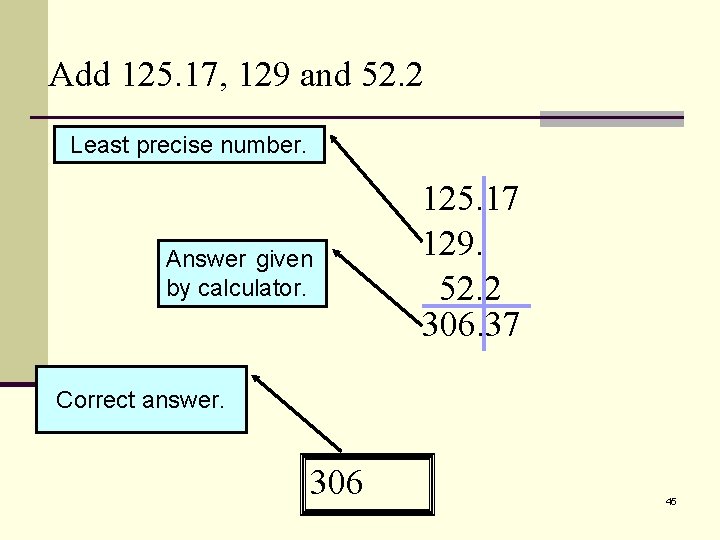
Add 125. 17, 129 and 52. 2 Least precise number. Answer given by calculator. 125. 17 129. 52. 2 306. 37 Round off to the Correct answer. nearest unit. 306. 37 45

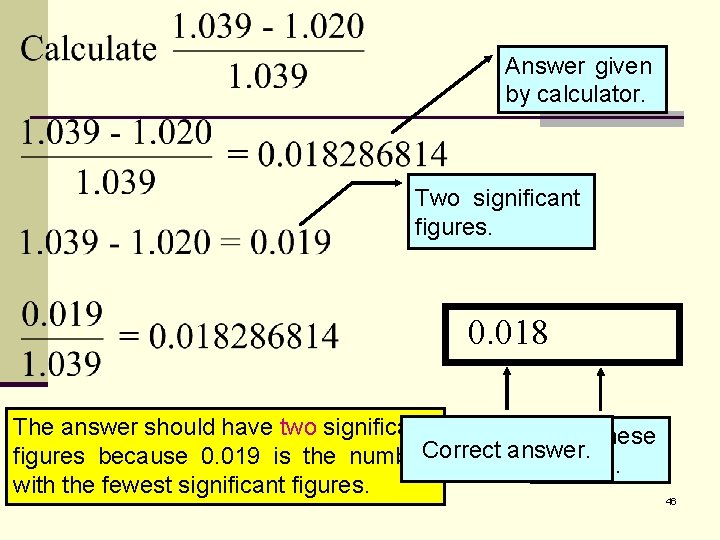
Answer given by calculator. Two significant figures. 0. 018286814 The answer should have two significant Drop these Correct answer. figures because 0. 019 is the number 6 digits. with the fewest significant figures. 46

Practice 12. 62 + 1. 5 + 0. 25 = 14. 37 = 14. 4 8 3 4 1. 08 x 10 (2. 25 x 10 ) (4. 80 x 10 ) = 195. 97 = 2. 0 x 102 0. 0007177852 = 7. 18 x 10 -4 10. 4 + 3. 75(1. 5 x 10 -4) =10. 4005625 = 10. 4 47

International System (le Système International) n. Based on metric system and units derived from metric system. 48

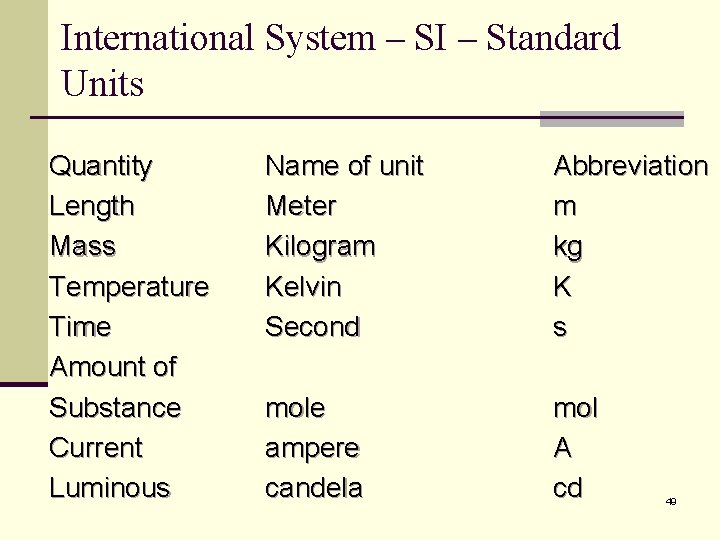
International System – SI – Standard Units Quantity Length Mass Temperature Time Amount of Substance Current Luminous Name of unit Meter Kilogram Kelvin Second Abbreviation m kg K s mole ampere candela mol A cd 49

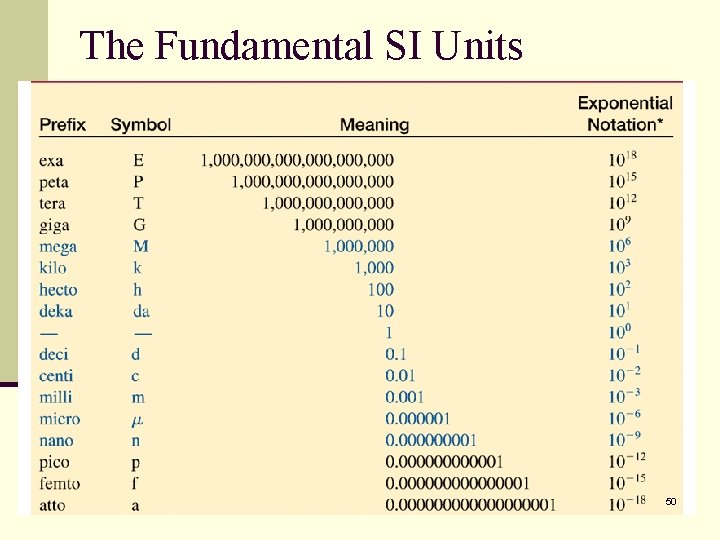
The Fundamental SI Units 50

Problem Solving n Many chemical principles can be illustrated mathematically n n Usually can be solved by many methods. Dimensional analysis offers 1. 2. 3. 4. A systematic and orderly approach Gives a clear understanding of the principles Helps you to organize and evaluate data Identifies error!!! Since unwanted units are retained if problem setup is incorrect 51

Problem Solving n Basic steps in problem solving 1. Read the problem carefully. Determine what is to solved for, and write it down. 2. Tabulate the data given in the problem with proper units. 3. Determine which principles are involved and which unit relationships are needed to solve the problem 4. Set up in a neat, organized, and logical fashion, then check to see that unwanted units cancel 5. Do the math – Check Sig Figs 6. Check the answer – is it reasonalble? 52

Conversion factors n List on Back cover of book and throughout text n Use of conversion factors n unit 1 x conversion factor = unit 2 Use conversion factors to change from one unit to other Ex. How many seconds in a day Do as many examples in the book as possible – Exercises 19 -56 53

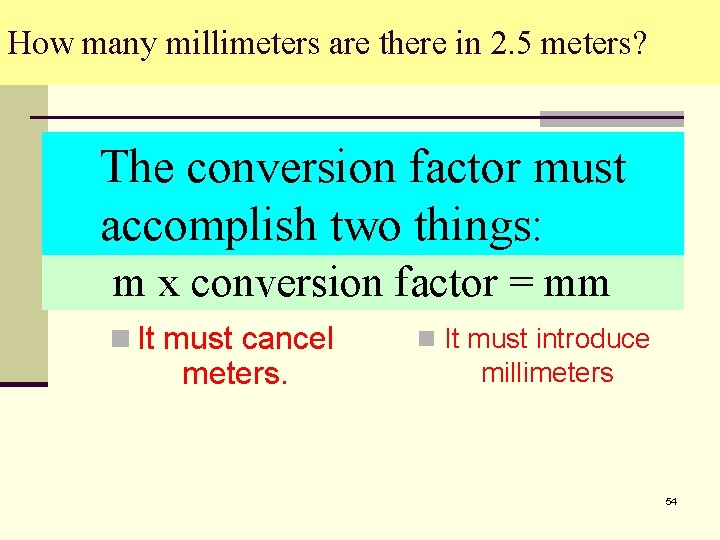
How many millimeters are there in 2. 5 meters? The conversion factor must unit 1 x conversion factor = unit 2 accomplish two things: m x conversion factor = mm n It must cancel meters. n It must introduce millimeters 54

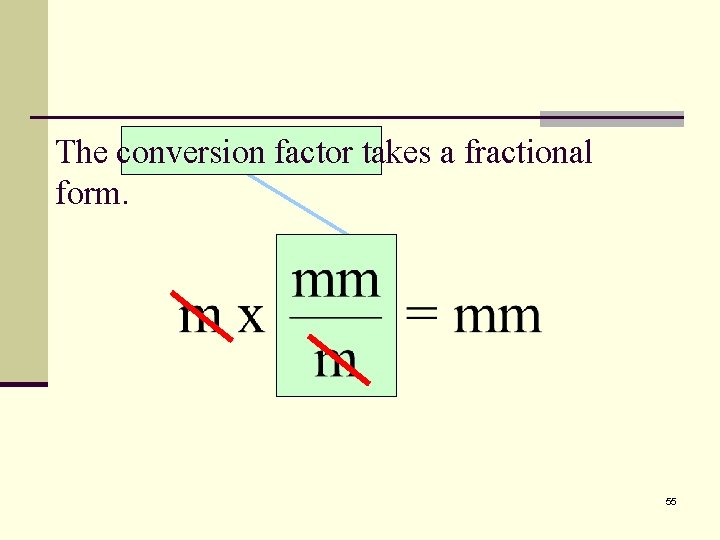
The conversion factor takes a fractional form. 55

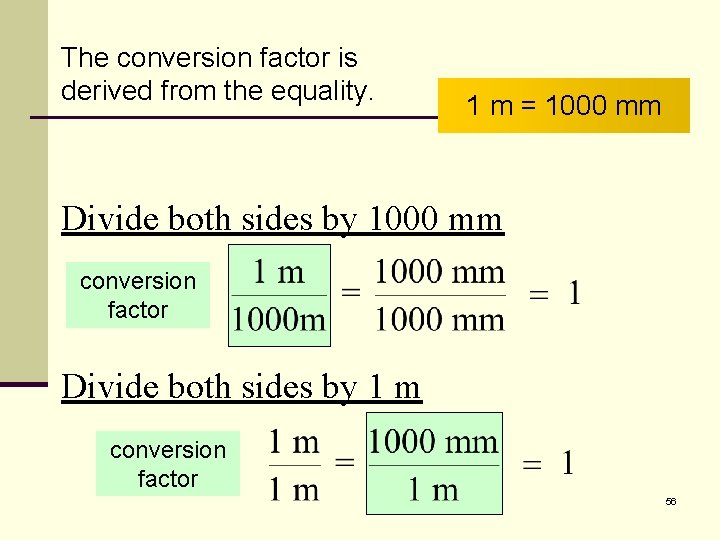
The conversion factor is derived from the equality. 1 m = 1000 mm Divide both sides by 1000 mm conversion factor Divide both sides by 1 m conversion factor 56

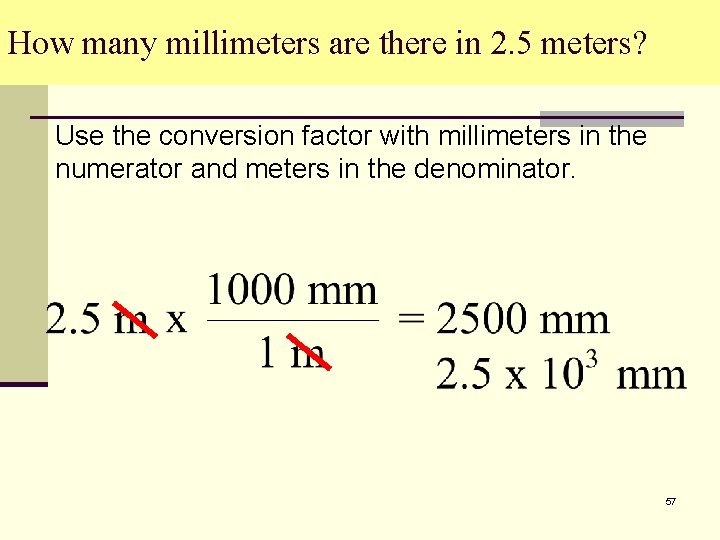
How many millimeters are there in 2. 5 meters? Use the conversion factor with millimeters in the numerator and meters in the denominator. 57

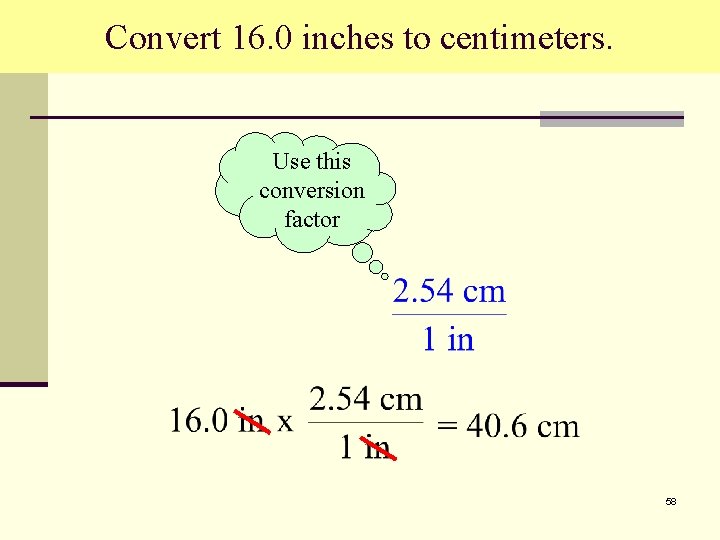
Convert 16. 0 inches to centimeters. Use this conversion factor 58

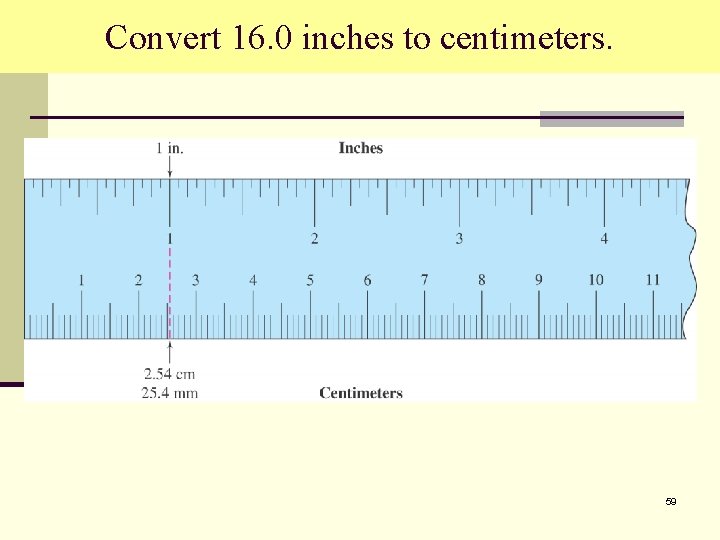
Convert 16. 0 inches to centimeters. 59

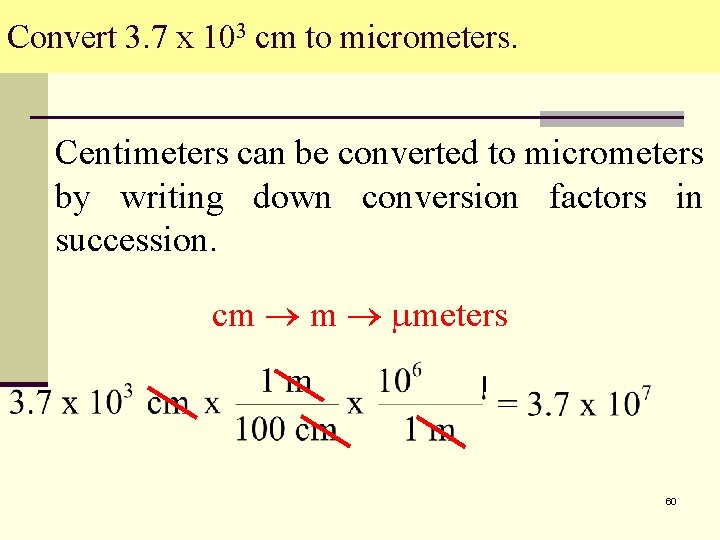
Convert 3. 7 x 103 cm to micrometers. Centimeters can be converted to micrometers by writing down conversion factors in succession. cm m meters 60

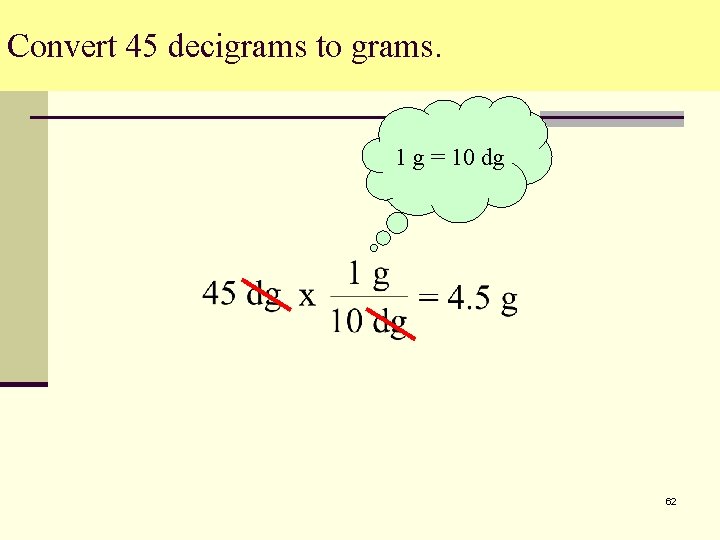
Convert 45 decigrams to grams. 1 g = 10 dg 62

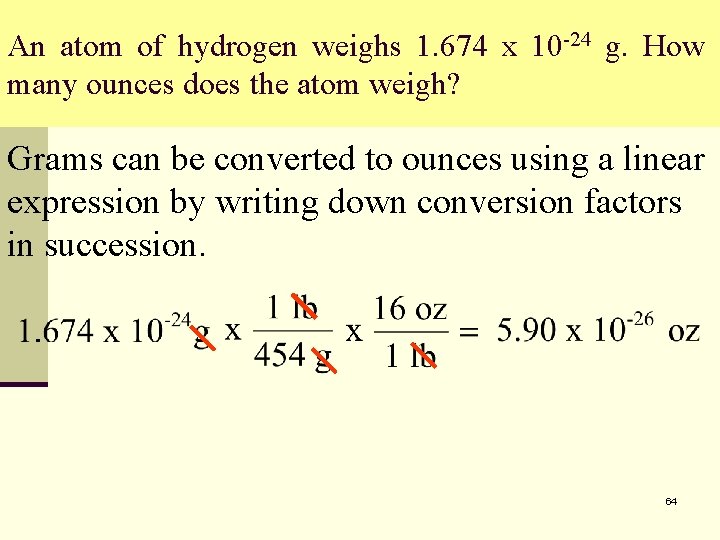
An atom of hydrogen weighs 1. 674 x 10 -24 g. How many ounces does the atom weigh? Grams can be converted to ounces using a linear expression by writing down conversion factors in succession. 64

Convert 4. 61 x 102 microliters to milliliters. Microliters can be converted to milliliters using a linear expression by writing down conversion factors in succession. L L m. L 66

Heat n A form of energy that is associated with the motion of small particles of matter. n Heat refers to the quantity of this energy associated with the system. n The system is the entity that is being heated or cooled. 67

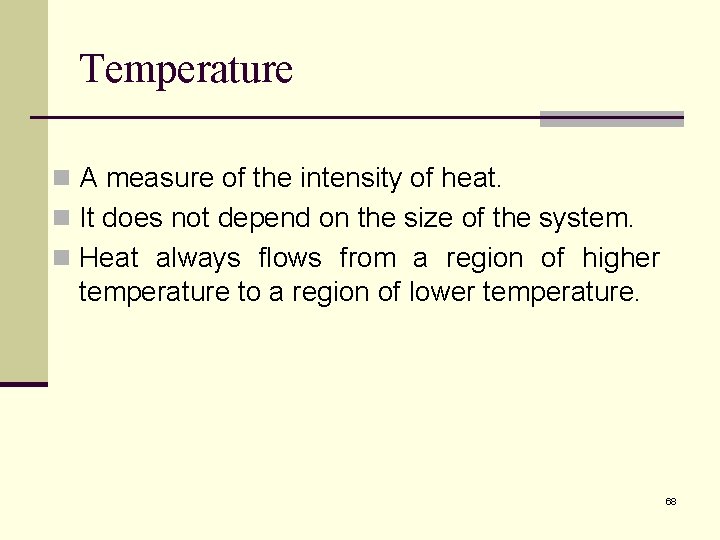
Temperature n A measure of the intensity of heat. n It does not depend on the size of the system. n Heat always flows from a region of higher temperature to a region of lower temperature. 68

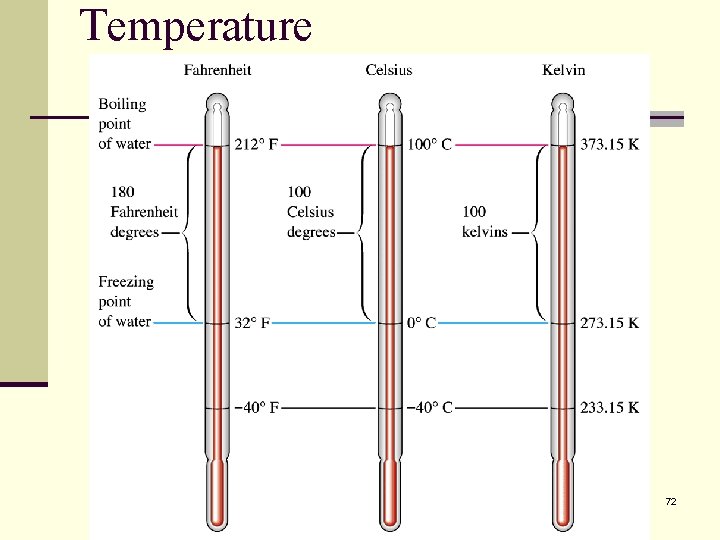
Temperature Measurement n The SI unit of temperature is the Kelvin. n There are three temperature scales: Kelvin, Celsius and Fahrenheit. n In the laboratory temperature is commonly measured with a thermometer. 69

Temperature n. Celsius scale = C n. Kelvin scale = K n. Fahrenheit scale = F 70

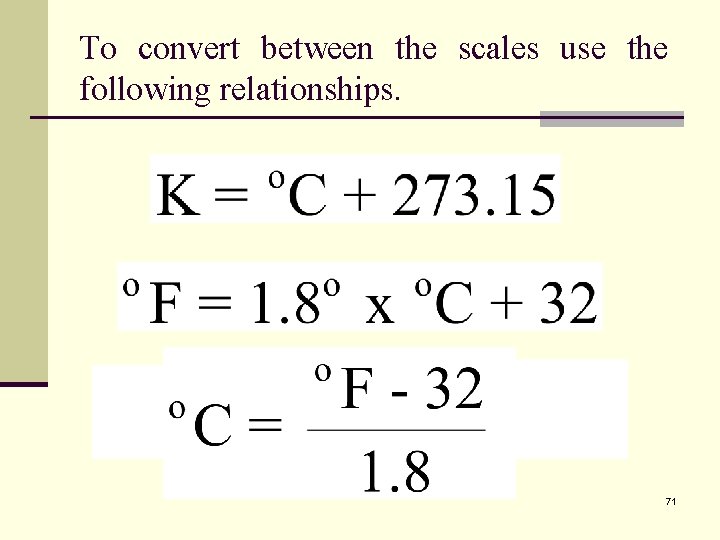
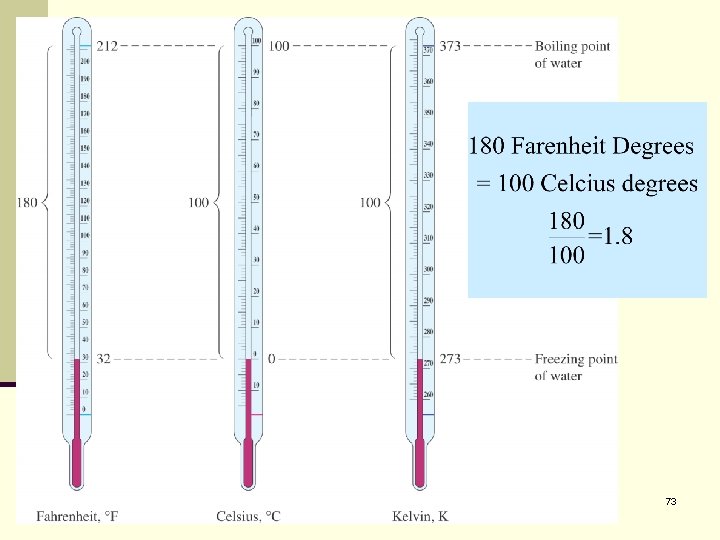
To convert between the scales use the following relationships. 71

Temperature 72

73

Temperature - Conversion 74

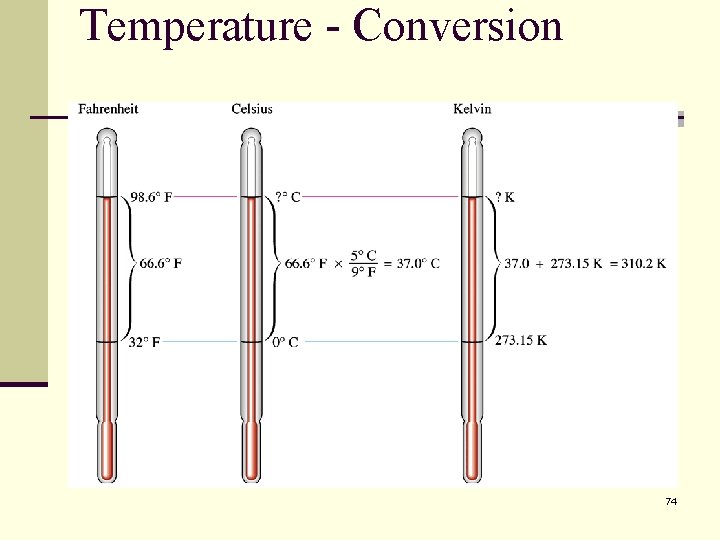
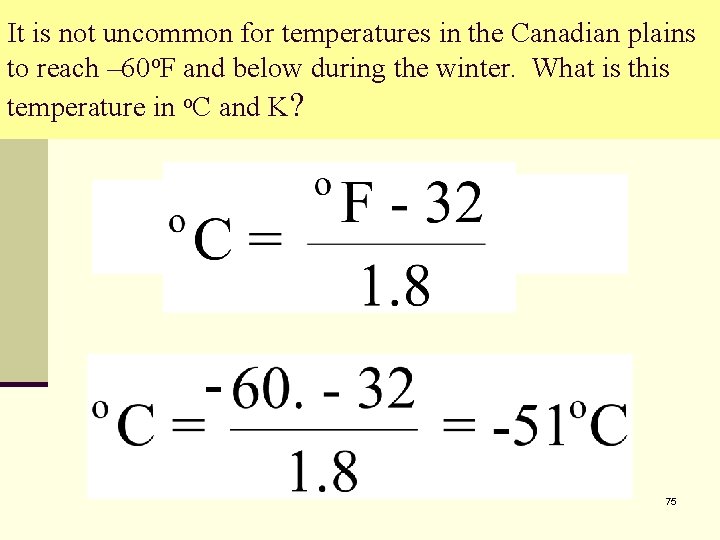
It is not uncommon for temperatures in the Canadian plains to reach – 60 o. F and below during the winter. What is this temperature in o. C and K? 75

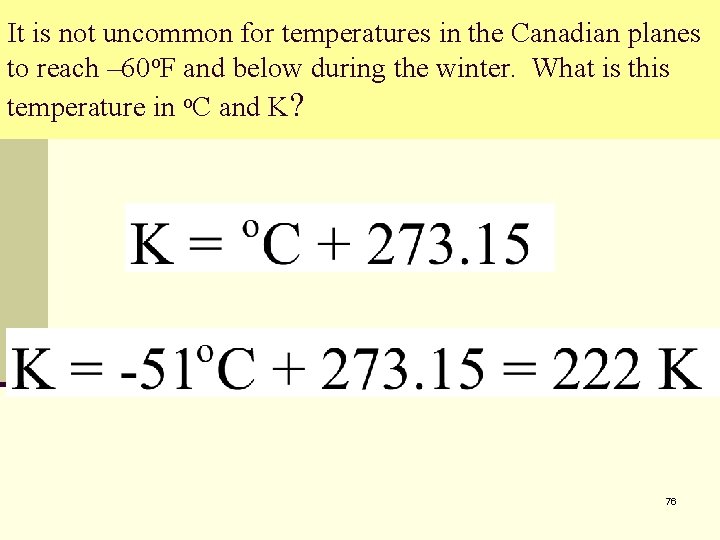
It is not uncommon for temperatures in the Canadian planes to reach – 60 o. F and below during the winter. What is this temperature in o. C and K? 76

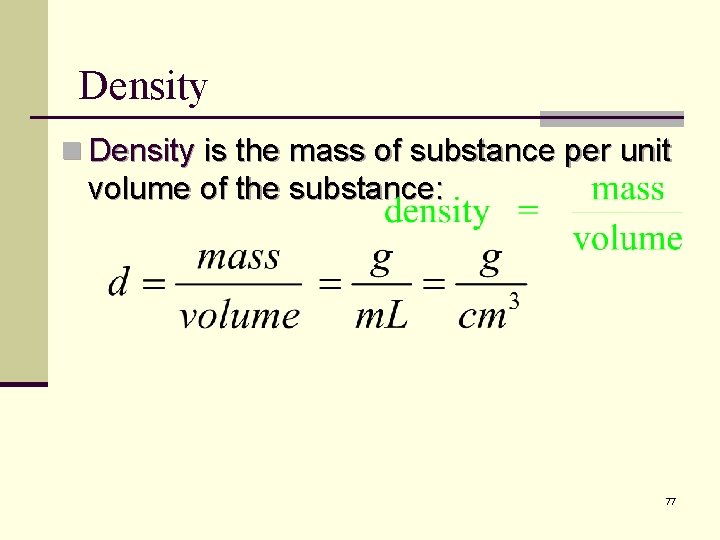
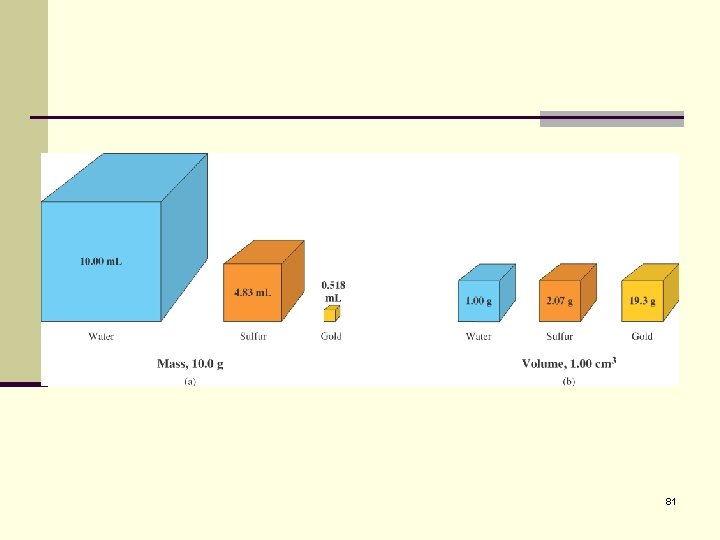
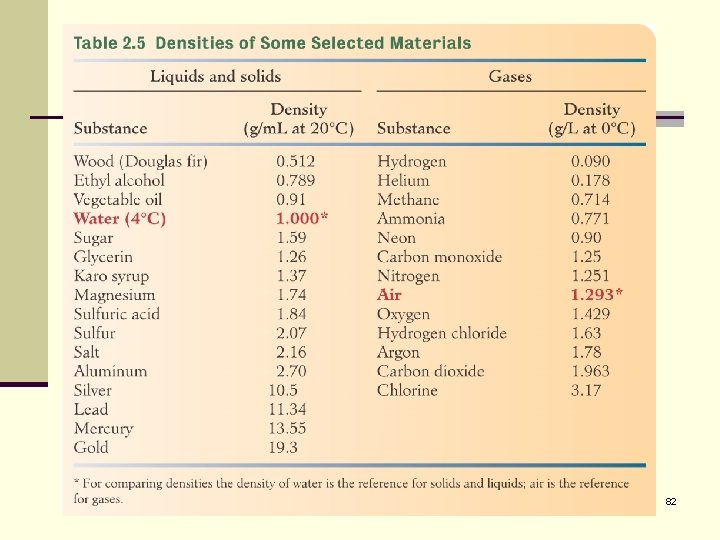
Density n Density is the mass of substance per unit volume of the substance: 77
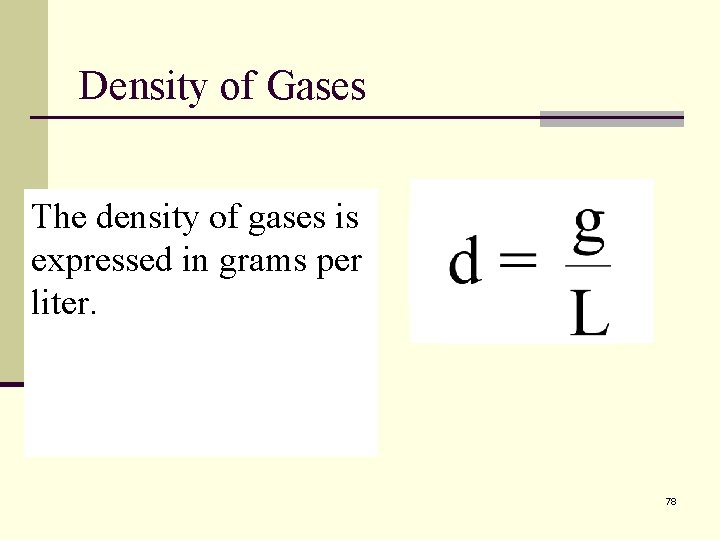
Density of Gases Massdensity is usually The of gases is expressed in in grams per expressed and volume in m. L liter. or cm 3. 78

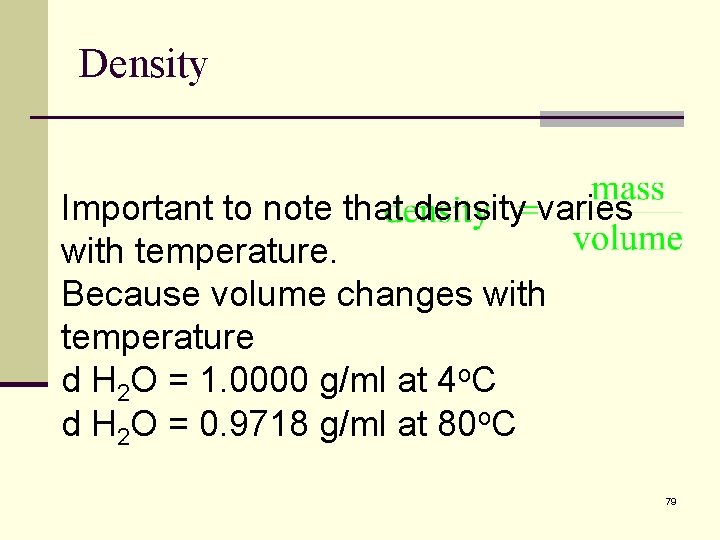
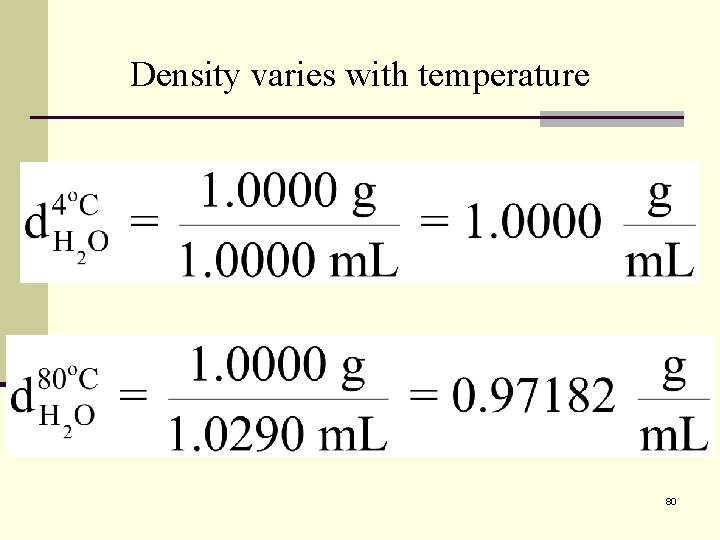
Density Important to note that density varies with temperature. Because volume changes with temperature d H 2 O = 1. 0000 g/ml at 4 o. C d H 2 O = 0. 9718 g/ml at 80 o. C 79

Density varies with temperature 80

81

82

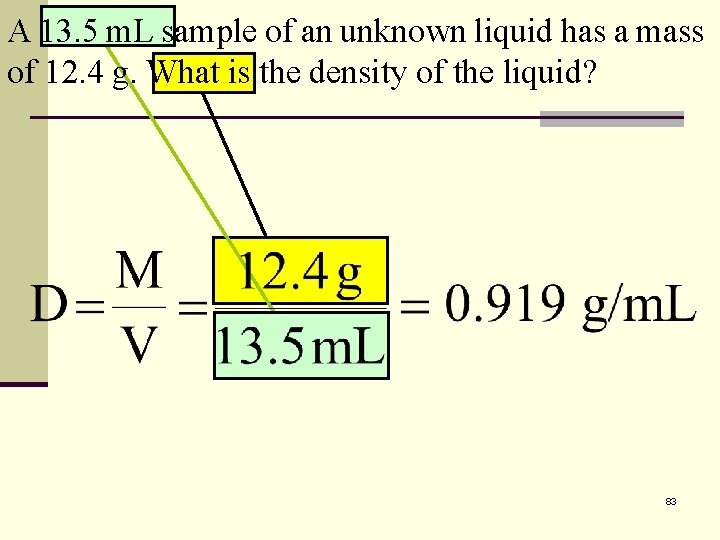
A 13. 5 m. L sample of an unknown liquid has a mass of 12. 4 g. What is the density of the liquid? 83

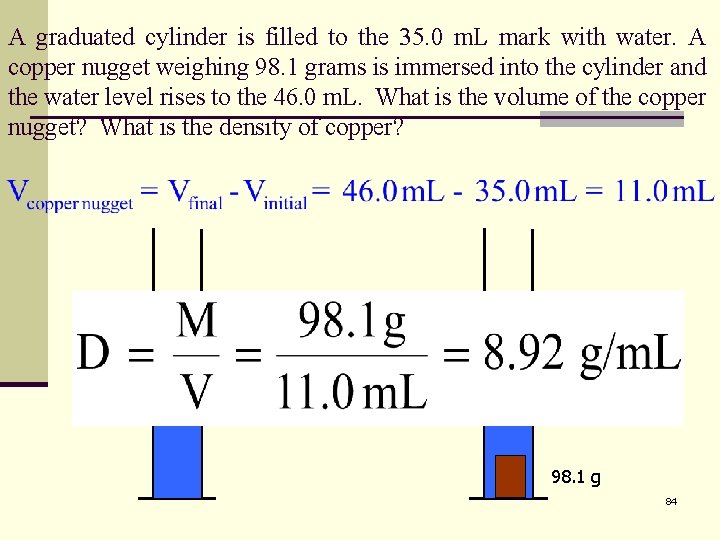
A graduated cylinder is filled to the 35. 0 m. L mark with water. A copper nugget weighing 98. 1 grams is immersed into the cylinder and the water level rises to the 46. 0 m. L. What is the volume of the copper nugget? What is the density of copper? 46. 0 m. L 35. 0 m. L 98. 1 g 84

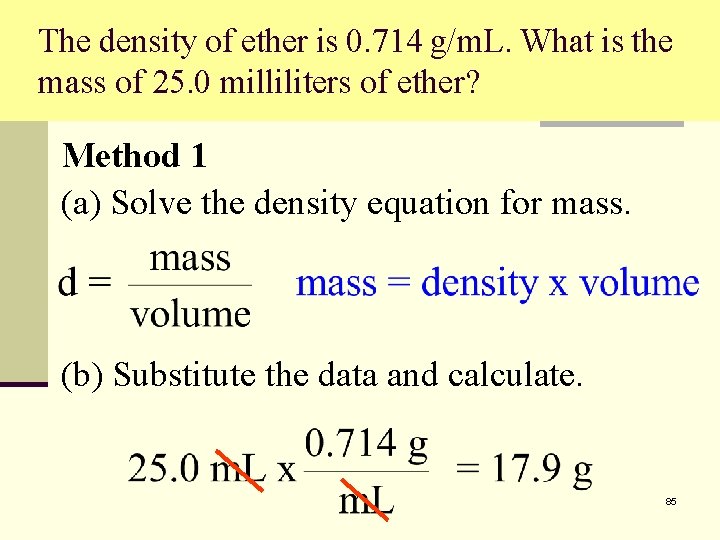
The density of ether is 0. 714 g/m. L. What is the mass of 25. 0 milliliters of ether? Method 1 (a) Solve the density equation for mass. (b) Substitute the data and calculate. 85

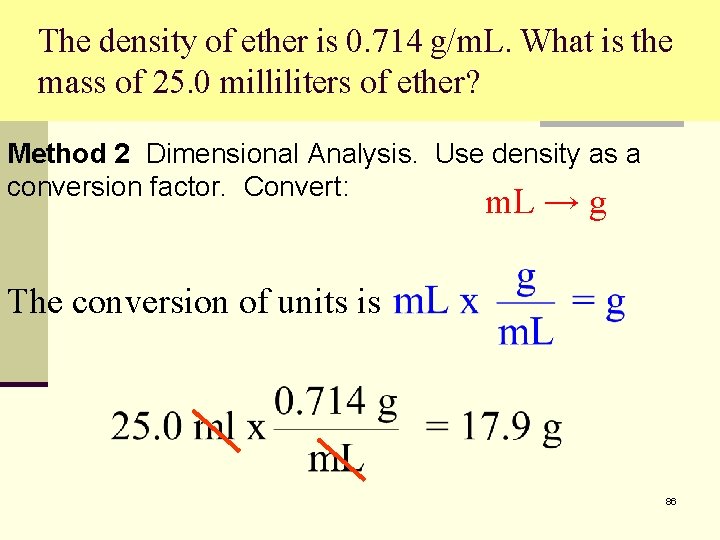
The density of ether is 0. 714 g/m. L. What is the mass of 25. 0 milliliters of ether? Method 2 Dimensional Analysis. Use density as a conversion factor. Convert: m. L → g The conversion of units is 86

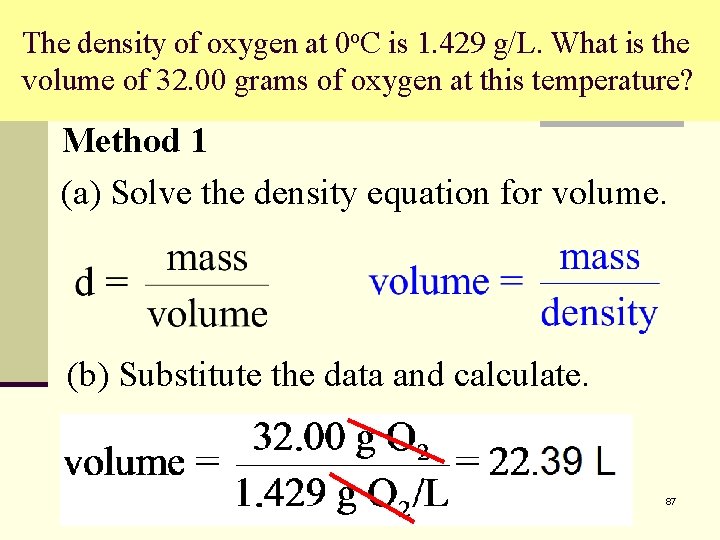
The density of oxygen at 0 o. C is 1. 429 g/L. What is the volume of 32. 00 grams of oxygen at this temperature? Method 1 (a) Solve the density equation for volume. (b) Substitute the data and calculate. 87

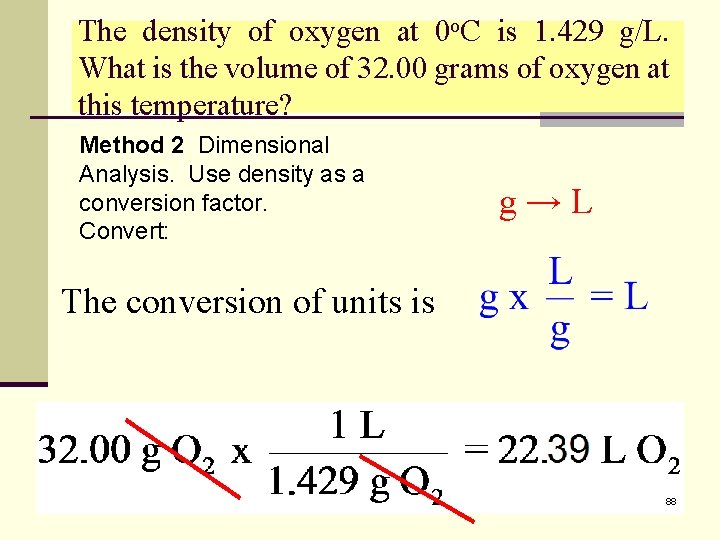
The density of oxygen at 0 o. C is 1. 429 g/L. What is the volume of 32. 00 grams of oxygen at this temperature? Method 2 Dimensional Analysis. Use density as a conversion factor. Convert: g→L The conversion of units is 88

Specific Gravity The ratio of the density of a substance with the density of another substance – usually water at 4 o. C. (unitless) 89

Chapter 2 concepts n Mass vs. Weight n Metric units of mass, length, and volume n Metric prefixes: Deci, Centi, Milli, Micro, Nano, Kilo, n n n and Mega Scientific notation Significant Figures Dimensional analysis Conversion – Length, Volume, Mass, Temeperature Heat vs. Temperature Density – Mass - Volume 90