Chapter 2 Matter Section 2 Properties of Substances

Chapter 2: Matter Section 2: Properties of Substances

Standards • Standard 1: Matter • #1. Explore matter in terms of its physical and chemical properties • #5. Evaluate pure substances and mixtures

Objectives 1. Compare and contrast physical and chemical properties 2. List the physical and chemical properties a substance can have 3. Define density and contrast it with weight 4. Calculate the density, mass, or volume of a substance using the density equation 5. Identify whether a property of a substance is physical or chemical
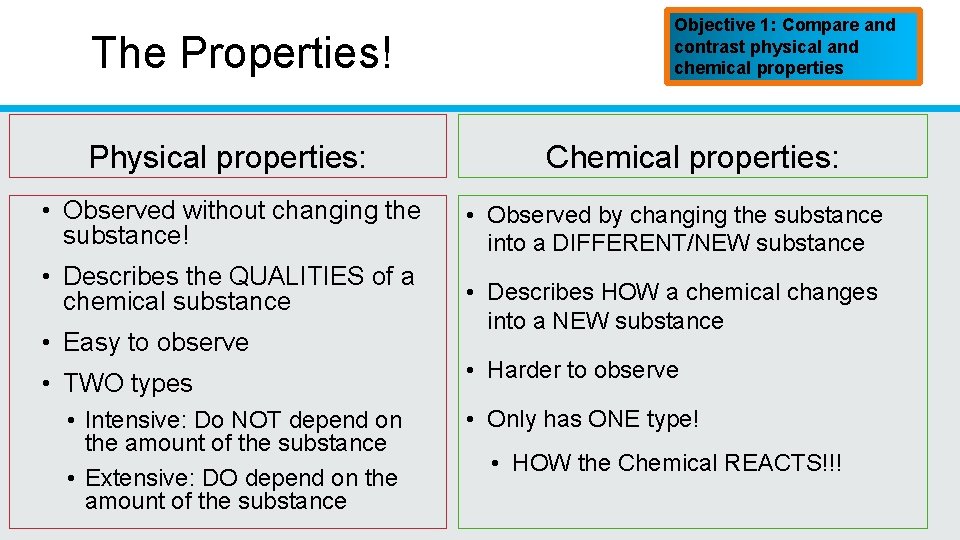
The Properties! Physical properties: • Observed without changing the substance! • Describes the QUALITIES of a chemical substance • Easy to observe • TWO types • Intensive: Do NOT depend on the amount of the substance • Extensive: DO depend on the amount of the substance Objective 1: Compare and contrast physical and chemical properties Chemical properties: • Observed by changing the substance into a DIFFERENT/NEW substance • Describes HOW a chemical changes into a NEW substance • Harder to observe • Only has ONE type! • HOW the Chemical REACTS!!!

The Properties Con’t Objective 1: Compare and contrast physical and chemical properties • BOTH physical and chemical properties help • To DEFINE a substance • To IDENTIFY a substance • To DETERMINE uses for the substance • Can be MEASURED and OBSERVED • Qualitative and Quantitative Observations!!
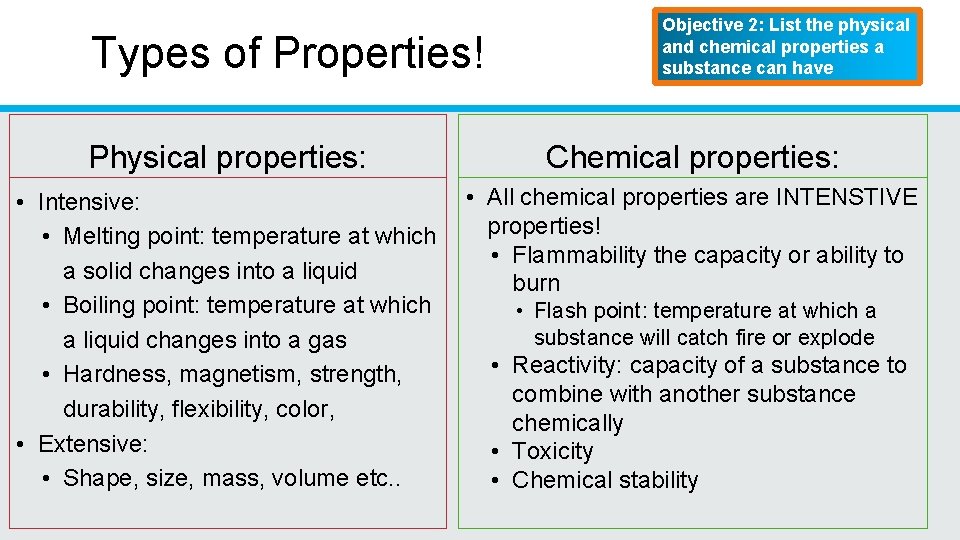
Types of Properties! Objective 2: List the physical and chemical properties a substance can have Physical properties: Chemical properties: • Intensive: • Melting point: temperature at which a solid changes into a liquid • Boiling point: temperature at which a liquid changes into a gas • Hardness, magnetism, strength, durability, flexibility, color, • Extensive: • Shape, size, mass, volume etc. . • All chemical properties are INTENSTIVE properties! • Flammability the capacity or ability to burn • Flash point: temperature at which a substance will catch fire or explode • Reactivity: capacity of a substance to combine with another substance chemically • Toxicity • Chemical stability

De. Ns. It. Y!! Objective 3: Define density and contrast it with weight • m D V

DENSITY Calculations! Objective 4: Calculate the density, mass, or volume of a substance using the density equation 1. A piece of tin (Sn) has a mass of 16. 52 g and a volume of 2. 26 m. L. What is the density of the tin? 2. A man has a bottle completely filled with 163 g of a slimy green liquid that has a density of 3. 26 g/cm 3. What is the volume of the bottle? 3. A piece of metal has a density of 11. 3 g/m. L and a volume of 6. 7 cm 3. What is the mass of the metal?

Identifying Properties! Objective 5: Identify whether a property of a substance is physical or chemical • If the property that is observed • Does NOT change the substance into a NEW substance • PHYSICIAL PROPERTY • Examples: the density of the metal is 5. 6 or the melting point of water is 0°C • DOES change the substance into a NEW substance • CHEMICAL PROPERTY • Examples: oxygen is highly flammable or 67. 4 mg of crystal meth is toxic to the liver • NOTE: melting, boiling, evaporating, or sublimation are NOT changing the substance into something NEW
- Slides: 9