Chapter 2 Matter Matter I States of Matter



















- Slides: 27

Chapter 2 Matter

Matter I. States of Matter A. Kinetic Molecular Theory B. States of Matter

A. Kinetic Molecular Theory n KMT n Particles of matter are always in motion. n The kinetic energy (speed) of these particles increases as temperature increases.

B. Four States of Matter n Solids n n n very low KE - particles vibrate but can’t move around fixed shape fixed volume

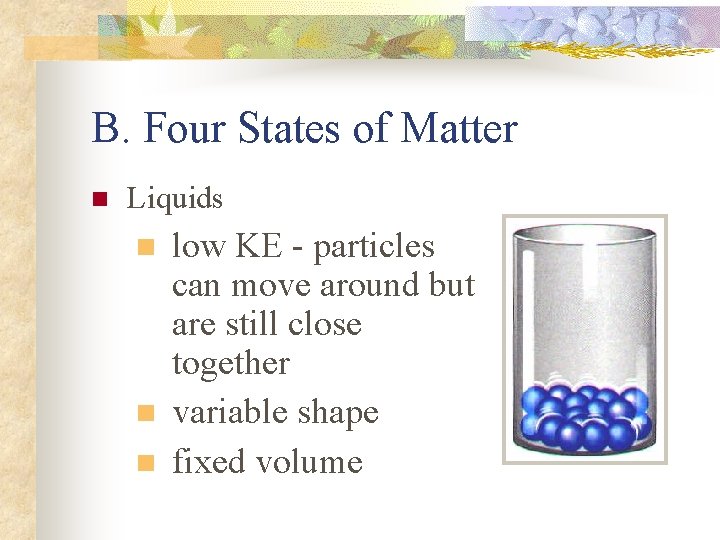
B. Four States of Matter n Liquids n n n low KE - particles can move around but are still close together variable shape fixed volume

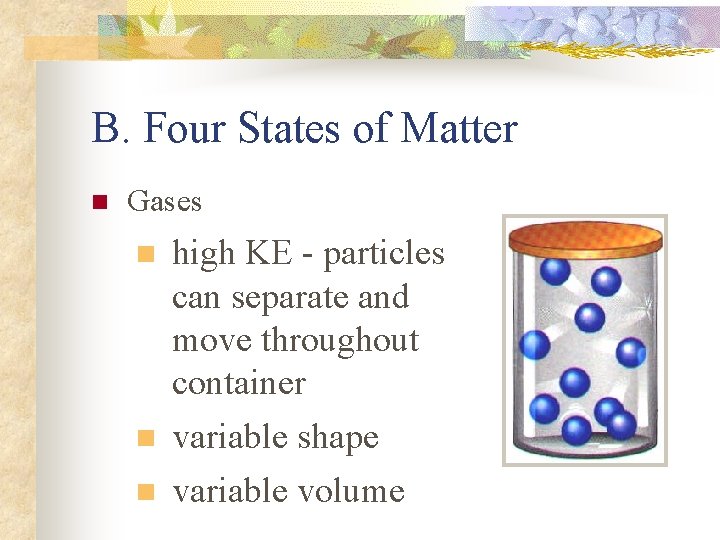
B. Four States of Matter n Gases n n n high KE - particles can separate and move throughout container variable shape variable volume

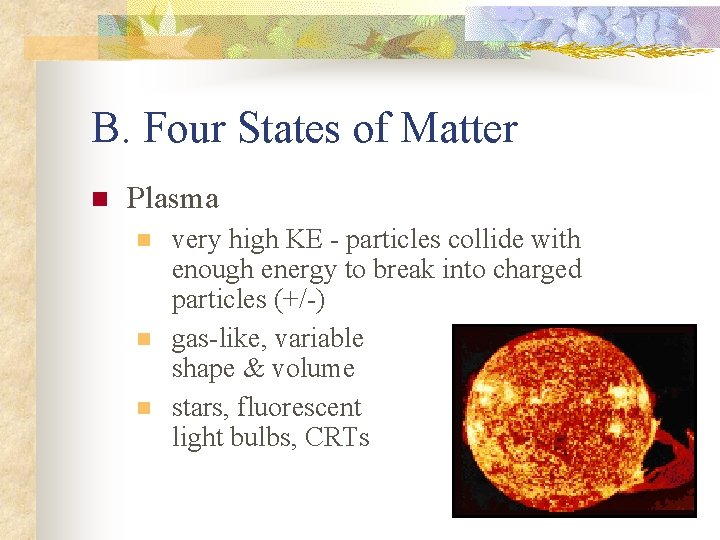
B. Four States of Matter n Plasma n n n very high KE - particles collide with enough energy to break into charged particles (+/-) gas-like, variable shape & volume stars, fluorescent light bulbs, CRTs

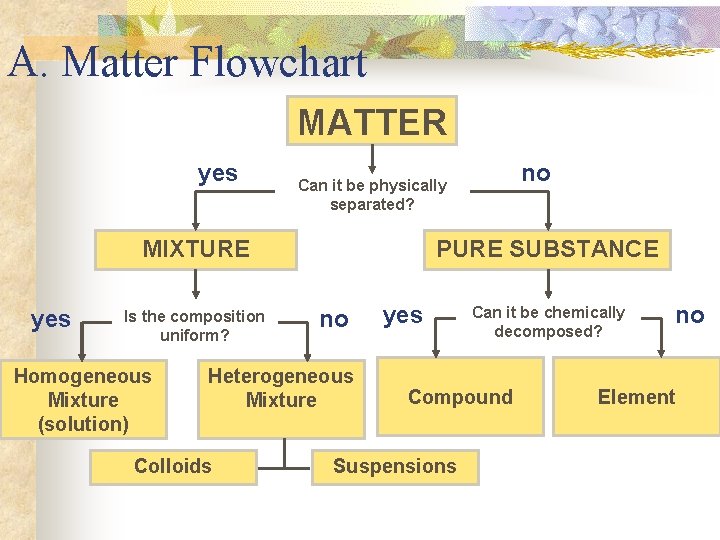
II. Classification of Matter A. Matter Flowchart B. Pure Substances C. Mixtures

A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element

B. Pure Substances n Element n composed of identical atoms n EX: copper wire, aluminum foil

B. Pure Substances n Compound n composed of 2 or more elements in a fixed ratio n properties differ from those of individual elements n EX: table salt (Na. Cl)

C. Mixtures n Variable combination of 2 or more pure substances. Heterogeneous Homogeneous

C. Mixtures n Solution n n homogeneous Tyndall Effect very small particles no Tyndall effect particles don’t settle EX: rubbing alcohol

C. Mixtures n Colloid n heterogeneous n medium-sized particles n Tyndall effect n particles don’t settle n EX: milk

C. Mixtures n Suspension n heterogeneous n large particles n Tyndall effect n particles settle n EX: fresh-squeezed lemonade

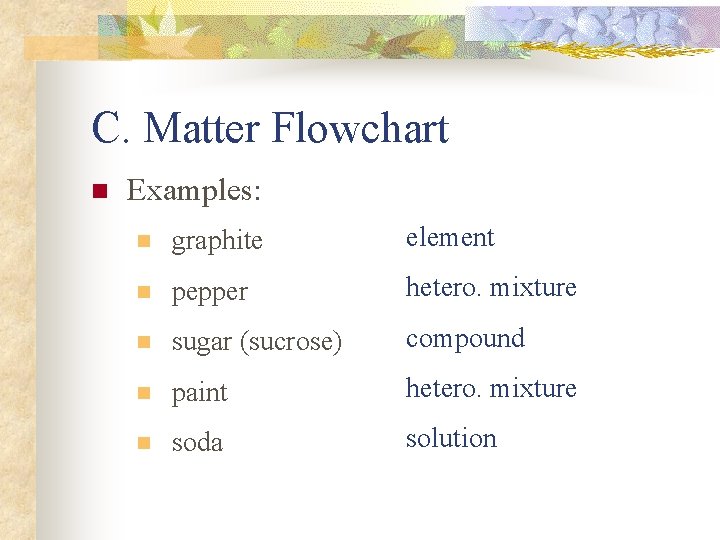
C. Matter Flowchart n Examples: n graphite element n pepper hetero. mixture n sugar (sucrose) compound n paint hetero. mixture n soda solution

C. Mixtures n Examples: n mayonnaise colloid n muddy water suspension n fog colloid n saltwater solution n Italian salad dressing suspension

III. Properties & Changes in Matter A. Extensive vs. Intensive B. Physical vs. Chemical

A. Extensive vs. Intensive n Extensive Property n n depends on the amount of matter present Intensive Property n depends on the identity of substance, not the amount

A. Extensive vs. Intensive n Examples: n boiling point intensive n volume extensive n mass extensive n density intensive n conductivity intensive

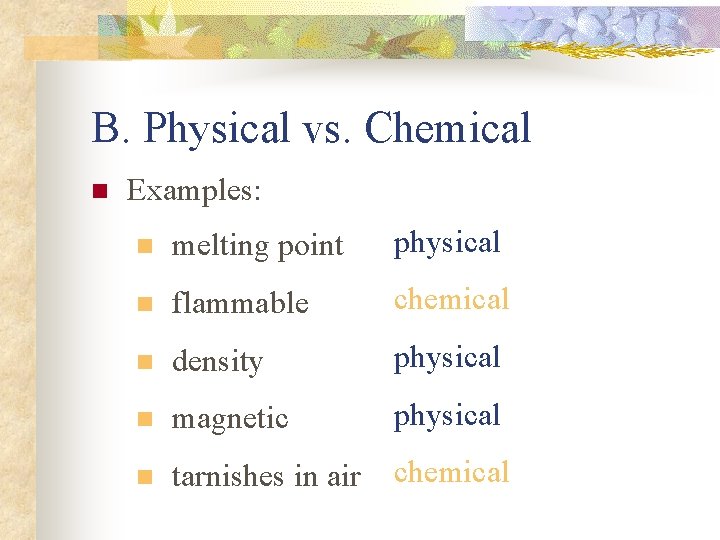
B. Physical vs. Chemical n Physical Property n n can be observed without changing the identity of the substance (can be measured) Chemical Property n describes the ability of a substance to undergo changes in identity

B. Physical vs. Chemical n Examples: n melting point physical n flammable chemical n density physical n magnetic physical n tarnishes in air chemical

B. Physical vs. Chemical n n Physical Change n changes the form of a substance without changing its identity n properties remain the same Chemical Change n changes the identity of a substance n products have different properties

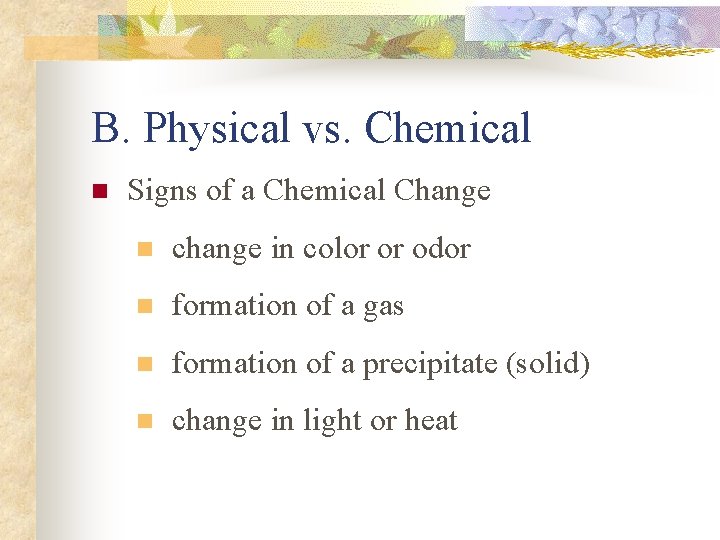
B. Physical vs. Chemical n Signs of a Chemical Change n change in color or odor n formation of a gas n formation of a precipitate (solid) n change in light or heat

B. Physical vs. Chemical n Examples: n rusting iron chemical n dissolving in water physical n burning a log chemical n melting ice physical n grinding spices physical

Choose if the following is a physical property or a chemical property: n n n Water boils at 100°C Propane burns in the air Diamonds are very hard Most roses have a sweet smell Sodium metal is very reactive with water

Decide if the following is a physical change or a chemical change: n n n Sugar dissolves in coffee Cookies burn in the oven Iron metal is melted Leaves turn color in the fall A rock is busted into small pieces