Chapter 2 Matter Change Matter vs Mass Matter







- Slides: 18

Chapter 2 --Matter & Change Matter vs. Mass • Matter is anything that has _____ mass and takes up ______. space liquids and ____. . . solids _____, gases Examples: ____, (the three phases of matter) emotions sound. . . light • Things that are NOT matter: _____, heat, ____, matter • Mass is the amount of ______ in an object. kilogram • The standard metric unit for mass is the _______.

Plasma • Plasma is a high energy electrically charged mixture of ions and electrons. _____ Stars are made of plasma. • While plasma is the most abundant phase of matter in the universe, on earth it only occurs in a few limited places: • Lightning bolts • Flames • Fluorescent lights • Aurora Borealis (Northern Lights)

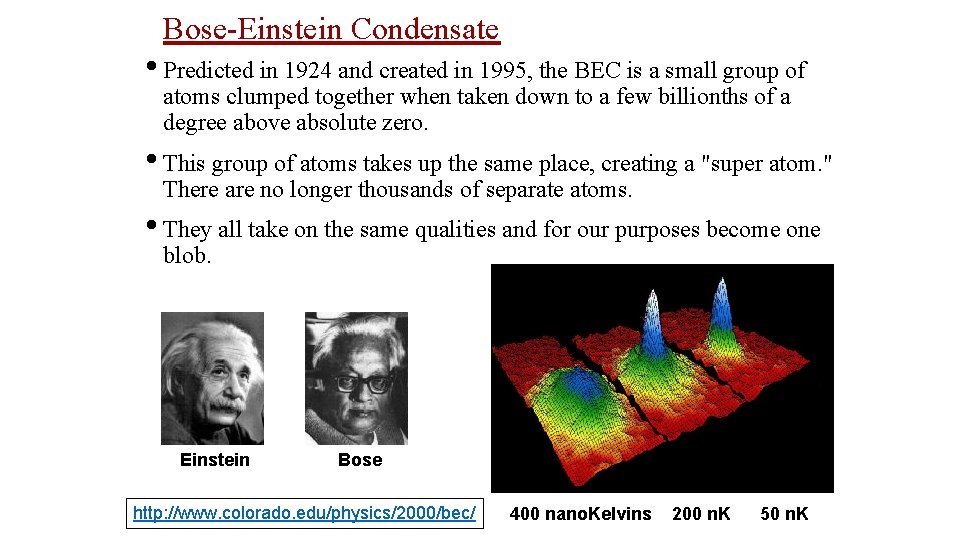
Bose-Einstein Condensate • Predicted in 1924 and created in 1995, the BEC is a small group of atoms clumped together when taken down to a few billionths of a degree above absolute zero. • This group of atoms takes up the same place, creating a "super atom. " There are no longer thousands of separate atoms. • They all take on the same qualities and for our purposes become one blob. Einstein Bose http: //www. colorado. edu/physics/2000/bec/ 400 nano. Kelvins 200 n. K 50 n. K

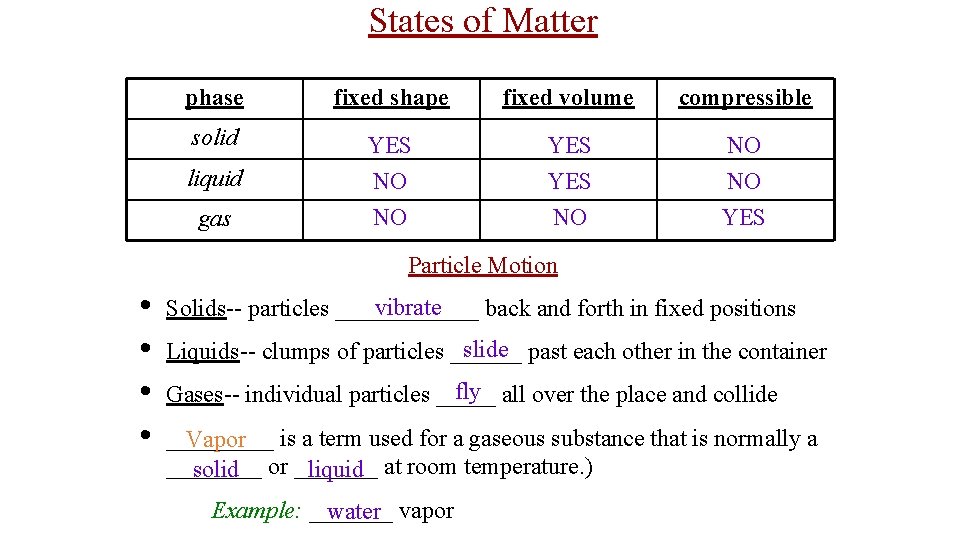
States of Matter phase fixed shape fixed volume compressible solid YES NO NO NO YES liquid gas Particle Motion • • vibrate Solids-- particles ______ back and forth in fixed positions slide past each other in the container Liquids-- clumps of particles ______ fly all over the place and collide Gases-- individual particles _________ Vapor is a term used for a gaseous substance that is normally a ____ solid or _______ liquid at room temperature. ) Example: _______ water vapor

States of Matter

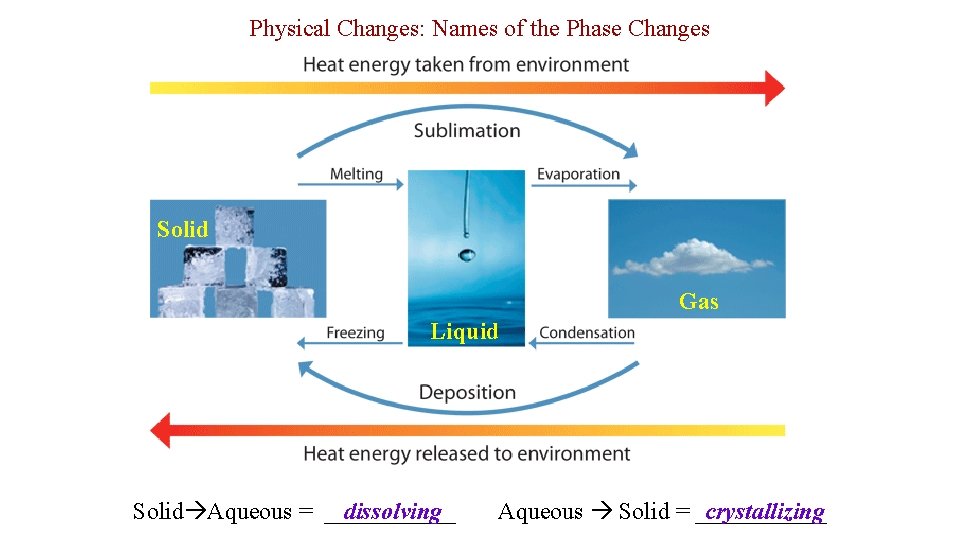
Physical Changes: Names of the Phase Changes Solid Gas Liquid Solid Aqueous = ______ dissolving Aqueous Solid = ______ crystallizing

Physical Properties and Physical Changes • Physical properties can be determined/measured without changing the substance’s composition. color odor, _____, density taste Examples: _______, mass, ____, melting _____ point, hardness, boiling point, ______ solubility, etc. • Physical Changes alter a substance without changing its composition. Examples: crushing, ripping, breaking, and any _____ phase changes…(boiling, freezing, melting, etc. ) • Most physical changes just alter the size of the particles and are usually reversible.

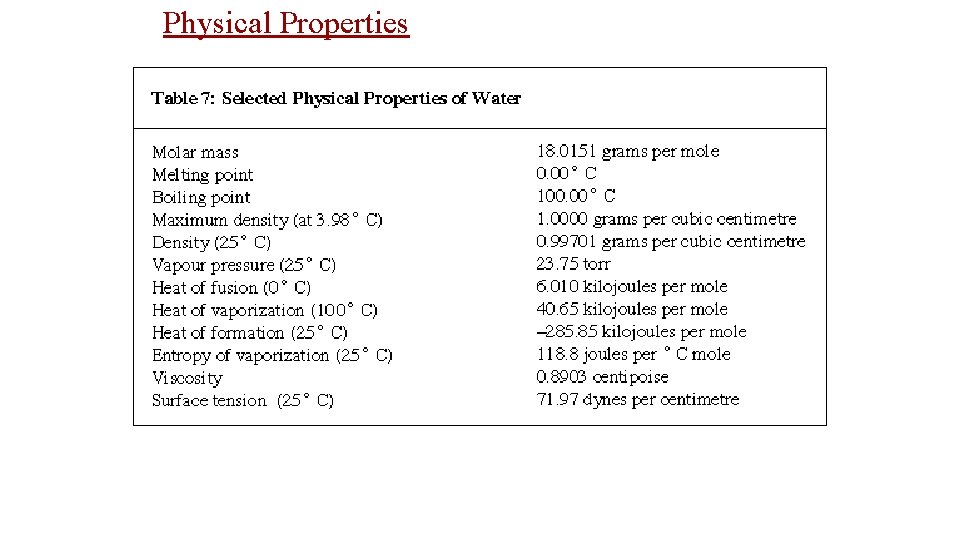
Physical Properties

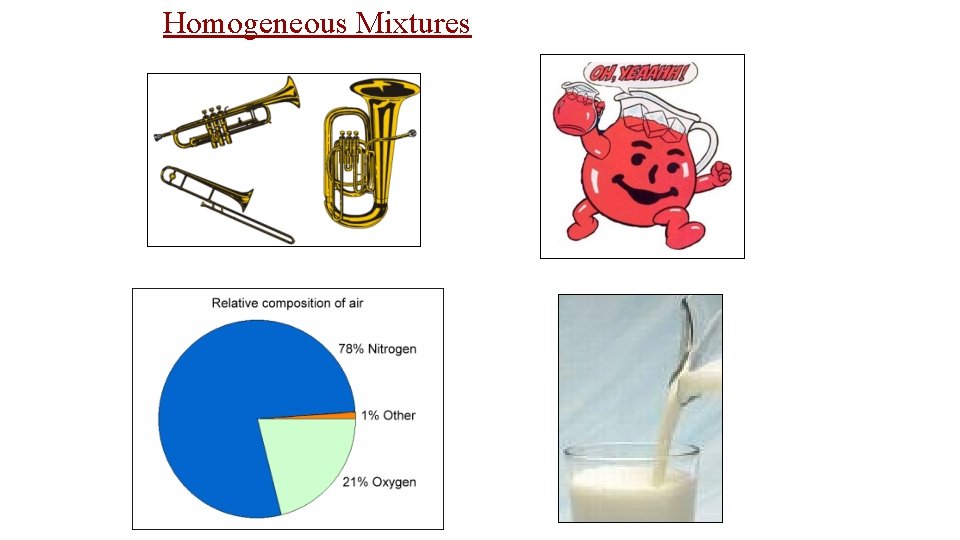
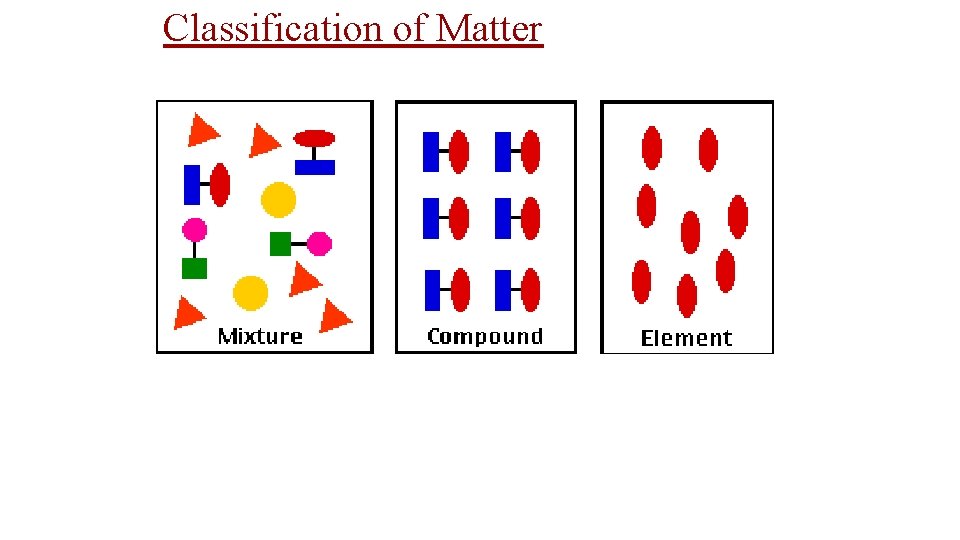
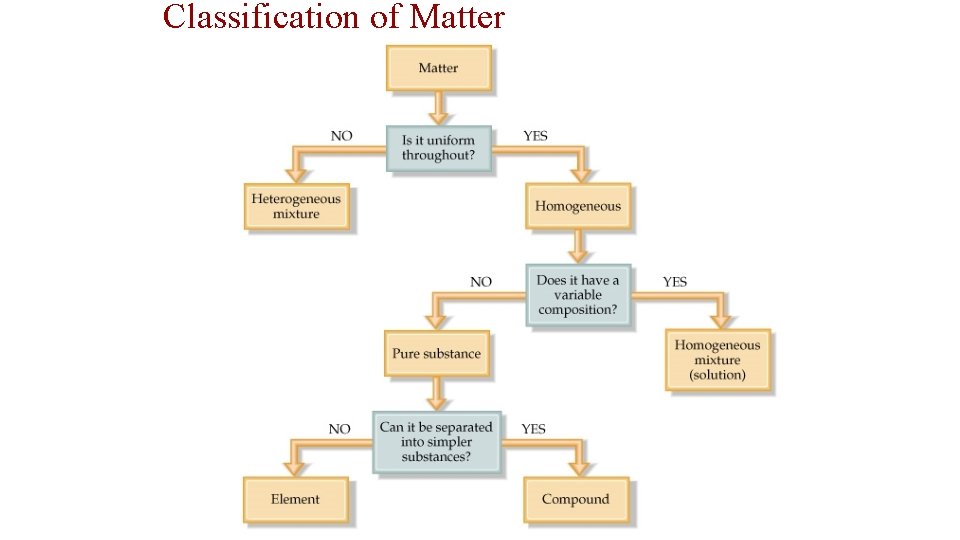
• Mixtures are a physical blend of two or more substances mixed together. ” The parts can be separated by _______ means or physical changes. ______ There are 2 types of mixtures: Heterogeneous Mixtures: the parts mixed together can still be (1) _________ distinguished from one another. . . NOT uniform in composition. dirt sand in water Examples: chicken soup, fruit salad, _____, Homogeneous (2) _________ Mixtures: the parts mixed together cannot be distinguished from one another. . . completely uniform in composition. Brass Air Kool-aid, ____, Examples: ______, salt water, milk • Another term for a homogeneous mixture is a “_______. ” solution

Heterogeneous Mixtures

Homogeneous Mixtures

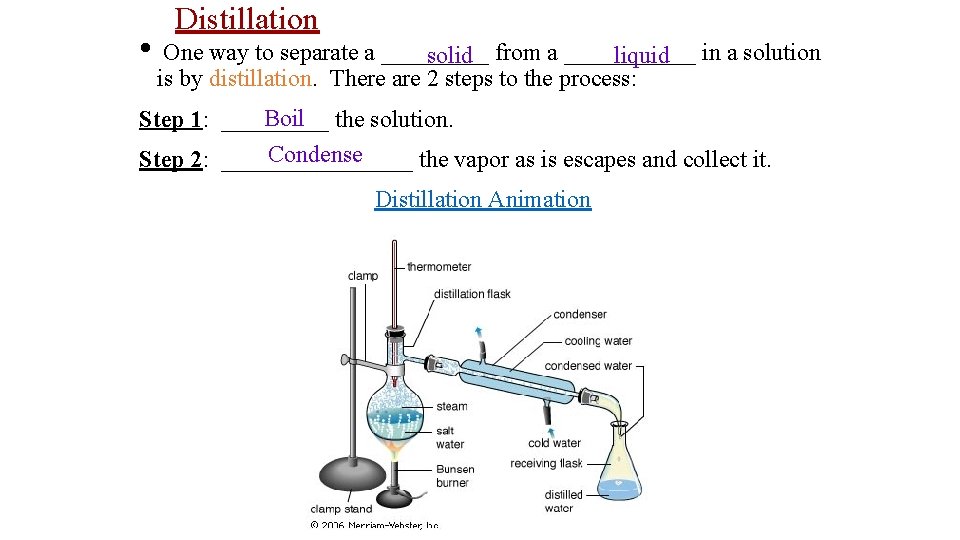
• Distillation One way to separate a _____ solid from a ______ liquid in a solution is by distillation. There are 2 steps to the process: Boil the solution. Step 1: _____ Condense Step 2: ________ the vapor as is escapes and collect it. Distillation Animation

Chemical Properties and Chemical Changes • Chemical properties cannot be determined/measured without changing the substance’s composition burning Examples: ______, whether or not it reacts with an acid or a base. Chemical Changes • Chemical changes will alter a substance and change its composition. rusting Examples: burning, ______, rotting or decomposing, _________, and other chemical fermenting reactions. • Most, but not all, chemical changes are irreversible. • You can’t “reverse” the burning of paper. Rechargeable ______ batteries use a reversible chemical reaction • _______

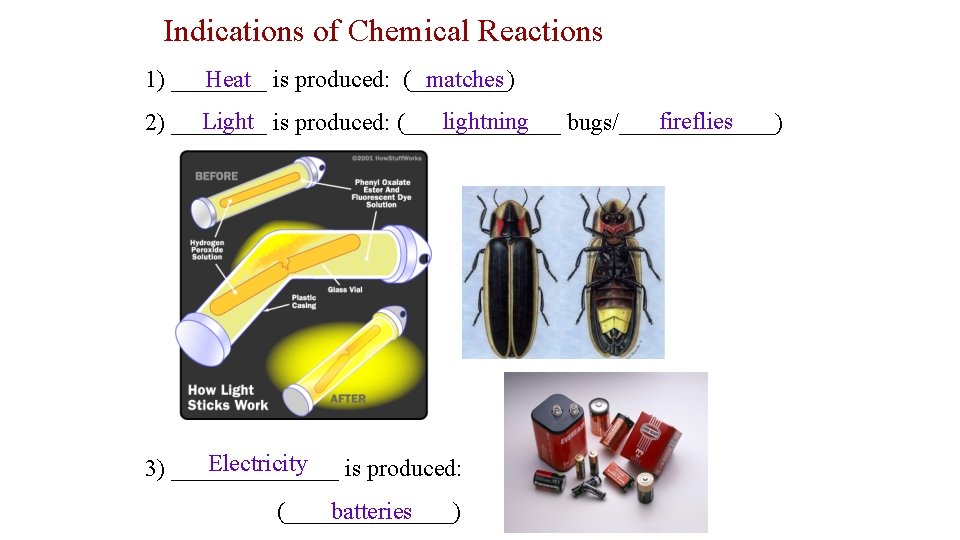
Indications of Chemical Reactions Heat is produced: (____) matches 1) ____ Light is produced: (_______ lightning fireflies 2) ____ bugs/_______) Electricity 3) _______ is produced: (_______) batteries

Indications of Chemical Reactions Precipitate soap ____) scum 4) __________ forms: (_______ Two liquids chemically react to form a solid. 5) gas/smoke/odor/bubbles produced: soda fizz (______)

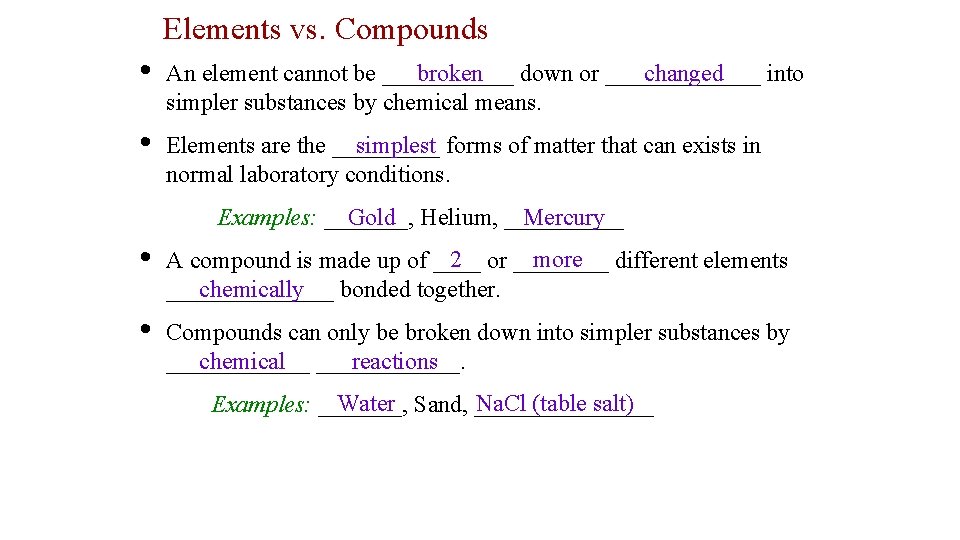
Elements vs. Compounds • broken changed An element cannot be ______ down or _______ into simpler substances by chemical means. • simplest forms of matter that can exists in Elements are the _____ normal laboratory conditions. Gold Helium, _____ Mercury Examples: _______, • 2 or ____ more different elements A compound is made up of ____ chemically bonded together. _______ • Compounds can only be broken down into simpler substances by ______ chemical ______. reactions Water Sand, ________ Na. Cl (table salt) Examples: _______,

Classification of Matter

Classification of Matter