Chapter 2 Matter and Energy Table of Contents

Chapter 2 Matter and Energy Table of Contents Section 1 Energy Section 2 Studying Matter and Energy Section 3 Measurements and Calculations in Chemistry Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Bellringer • Work in small groups to brainstorm ideas relating to energy. • List different types of energy, list why energy is important, and when energy is released or absorbed. • After brainstorming for five minutes, examine your lists and write your own definition for energy. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 1 Energy Objectives • Explain that physical and chemical changes in matter involve transfers of energy. • Apply the law of conservation of energy to analyze changes in matter. • Distinguish between heat and temperature. • Convert between the Fahrenheit, Celsius and Kelvin temperature scales. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change • Energy is the capacity to do some kind of work, such as moving an object, forming a new compound, or generating light. • Energy is always involved when there is a change in matter. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Changes in Matter Can Be Physical or Chemical • Ice melting and water boiling are examples of physical changes. • A physical change is a change of matter that affects only the physical properties of the matter. • For example, when ice melts and turns into liquid water, you still have the same substance represented by the formula H 2 O. Only the physical state of the substance changed. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Changes in Matter Can Be Physical or Chemical, continued • In contrast, the reaction of hydrogen and oxygen to produce water is an example of a chemical change. • A chemical change is a change that occurs when one or more substances change into entirely new substances with different properties. • A chemical change occurs whenever a new substance is made. • The water, H 2 O, is a different substance than the hydrogen, H 2, and oxygen, O 2. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Every Change in Matter Involves a Change in Energy • All physical and chemical changes involve a change in energy. • Sometimes energy must be supplied for the change in matter to occur. • For example, for ice to melt, energy must be supplied so that the particles can move past one another. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Every Change in Matter Involves a Change in Energy, continued • Another example of a change in which energy must be added is when liquid water changes to form gaseous water vapor. • Evaporation is the change of a substance from a liquid to a gas. • Imagine a pot of water on the stove. When energy is supplied by the stove, the water evaporates, or turns into water vapor. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Every Change in Matter Involves a Change in Energy, continued • Some changes in matter release energy. • For example, the explosion that occurs when hydrogen and oxygen react to form water is a release of energy. • Heat energy and light energy are released as the reaction takes place. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Endothermic and Exothermic Processes, continued • Any change in matter in which energy is released is an exothermic process. • The freezing of water and the condensation of water vapor are two examples of physical changes that are exothermic processes. • The burning of paper and the explosive reaction between hydrogen and oxygen to form water are examples of chemical changes that are exothermic processes. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Endothermic and Exothermic Processes • Any change in matter in which energy is absorbed from the surroundings is an endothermic process. • The melting of ice and the boiling of water are examples of physical changes that are endothermic. • The reaction that occurs when barium hydroxide and ammonium nitrate are mixed is an example of a chemical change that is endothermic. • As the chemicals react, energy is absorbed. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Endothermic and Exothermic Processes, continued • Energy can be absorbed by the surroundings or released to the surroundings, but it cannot be created or destroyed. • The law of conservation of energy states that during any physical or chemical change, the total quantity of energy remains constant. • In other words, energy cannot be destroyed or created. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Visual Concepts Law of Conservation of Energy PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Energy Is Often Transferred • To keep track of energy changes, chemists use the terms system and surroundings. • A system consists of all the components that are being studied at any given time. • In the reaction between water and ammonium nitrate, the system consists of the mixture inside the flask. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Energy Is Often Transferred, continued • The surroundings include everything outside the system. • In the reaction between water and ammonium nitrate, the surroundings consist of everything else including the air both inside and outside the flask and the flask itself. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Energy Is Often Transferred, continued • Energy is often transferred back and forth between a system and its surroundings. • An exothermic process involves a transfer of energy from a system to its surroundings. • An endothermic process involves a transfer of energy from the surroundings to the system. • In every case, the total energy of the systems and their surroundings remains the same. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
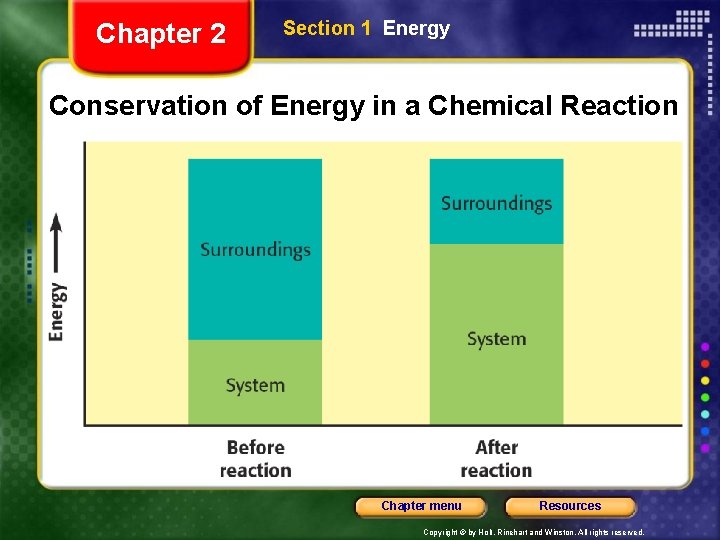
Chapter 2 Section 1 Energy Conservation of Energy in a Chemical Reaction Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy and Change, continued Energy Can Be Transferred in Different Forms • Energy exists in different forms, including • • • chemical mechanical light heat electrical sound • The transfer of energy between a system and its surroundings can involve any one of these forms of energy. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat • Heat is the energy transferred between objects that are at different temperatures. • Heat energy is always transferred from a warmer object to a cooler object. • For example, when ice cubes are placed in water, heat energy is transferred from the water to the ice. • Energy is also transferred as heat during chemical changes. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Energy Can Be Released As Heat • An explosion provides an example of energy being released during an exothermic reaction. • All of the energy that is released comes from the energy that is stored within the reacting chemicals. • When a chemical reaction involves an explosion, it can make a loud sound, generate great amounts of heat, or send objects flying. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Energy Can Be Released As Heat, continued • During the explosion, the sound results from the generation of sound energy. • The movement of the flying objects results from kinetic energy. • Kinetic energy is the energy of an object that is due to the object’s motion. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Energy Can Be Absorbed As Heat • In an endothermic reaction, energy is absorbed by the chemicals that are reacting. • For example, sodium bicarbonate, a chemical in baking soda forms three substances when heated. • It becomes sodium carbonate, water vapor, and carbon dioxide gas, in the following endothermic reaction: 2 Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Heat Is Different from Temperature • Temperature indicates how hot or cold something is. • Scientists define temperature as a measurement of the average kinetic energy of the random motion of particles in a substance. • The transfer of energy as heat can be measured by calculating changes in temperature. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Heat Is Different from Temperature, continued • Imagine that you are heating water on a stove. The water molecules have kinetic energy as they move. • Energy transferred as heat from the stove causes these water molecules to move faster. • The more rapidly the water molecules move, the greater their average kinetic energy. • As the average kinetic energy of the water molecules increases, the temperature of the water increases. • The temperature change of the water is a measure of the energy transferred from the stove as heat. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales • Thermometers are usually marked with the Fahrenheit or Celsius temperature scales. • A third temperature scale, uses the unit Kelvin, K. • The zero point on the Celsius scale is designated as the freezing point of water. • The zero point on the Kelvin scale is designated as absolute zero, the temperature at which the minimum average kinetic energies of all particles occur. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
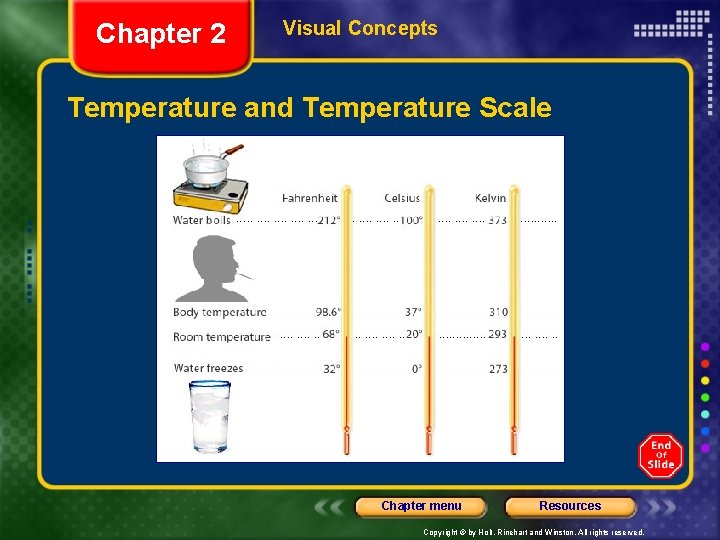
Chapter 2 Visual Concepts Temperature and Temperature Scale Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Fahrenheit scale expressed as F water boils at 212 F water freezes at 32 F lowest temperature -459ºF Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Celsius scale expressed as C water boils at 100 C water freezes at 0 C lowest temperature -273ºC Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Kelvin scale also called Absolute Temperature Scale expressed as K water boils at 373 K water freezes at 273 K àlowest temperature 0 K Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales absolute zero the lowest, coldest possible temperature (-459 F, -273 C, 0 K) the temperature at which the motion of particles of matter ceases. the temperature at which atoms and molecules stop moving and have zero kinetic energy Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
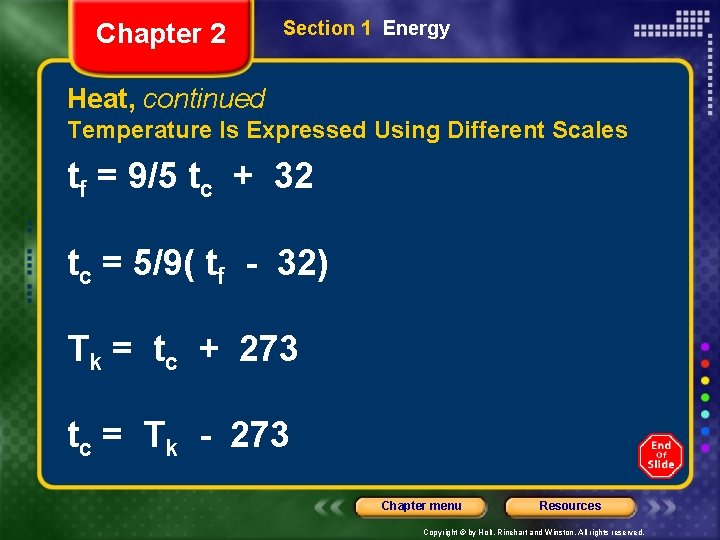
Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales tf = 9/5 tc + 32 tc = 5/9( tf - 32) Tk = tc + 273 tc = Tk - 273 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
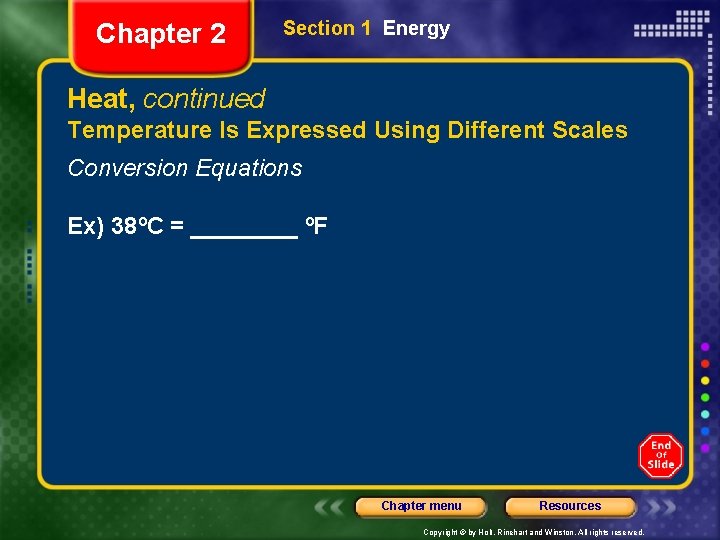
Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Conversion Equations Ex) 38ºC = ____ ºF Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Conversion Equations Ex) 77ºF = ____ ºC Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
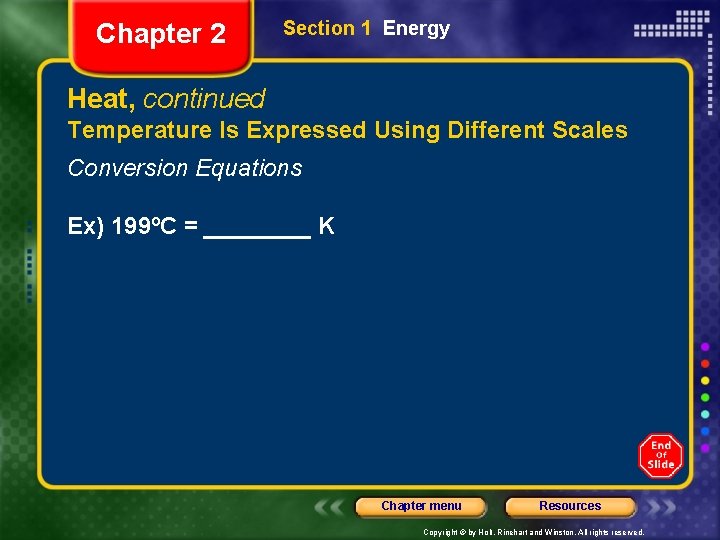
Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Conversion Equations Ex) 199ºC = ____ K Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
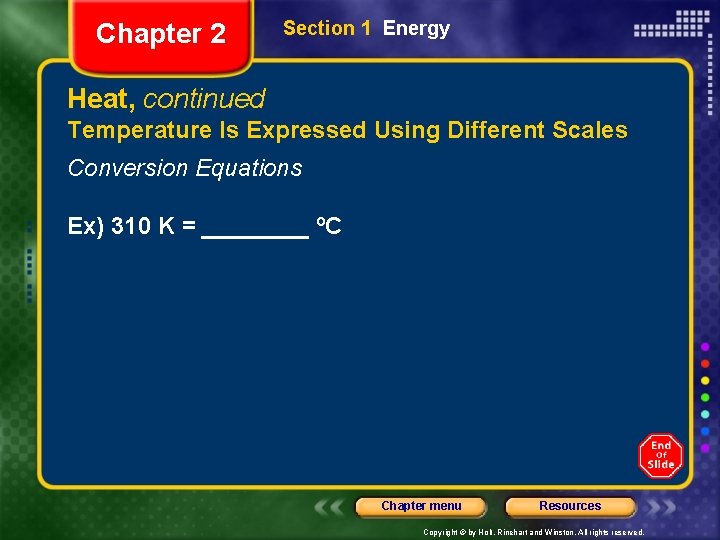
Chapter 2 Section 1 Energy Heat, continued Temperature Is Expressed Using Different Scales Conversion Equations Ex) 310 K = ____ ºC Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy SOLVE 1. 91. 5 °C = ____°F 6. 79. 75 °C = ____K 2. 25 °C = ______°F 7. 548. 83 K = ____°C 3. 180. 72 °F =____°C 8. 152. 6 K =______°C 4. 1219 °F =______°C 9. 189. 69 °F =______K 5. 137. 63 °C = ____ K 10. 373 K =____°F Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Transfer of Heat May Not Affect the Temperature • The transfer of energy as heat does not always result in a change of temperature. • For example, consider what happens when energy is transferred to a mixture of ice and water. • As energy is transferred as heat to the ice-water mixture, the ice cubes will start to melt. • The temperature of the mixture remains at 0°C until all of the ice has melted. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Transfer of Heat May Not Affect the Temperature, continued • Once all the ice has melted, the temperature of the water will start to increase until it reaches 100°C. • As the water boils, the temperature remains at 100°C until all the water has turned into a gas. • The temperature remains constant during the physical changes in this system. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Transfer of Heat May Not Affect the Temperature, continued • During these physical changes, the energy that is transferred as heat is actually being used to move molecules past one another or away from one another. • This energy causes the molecules in the solid ice to move more freely so that they form a liquid. • This energy also causes the water molecules to move farther apart so that they form a gas. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
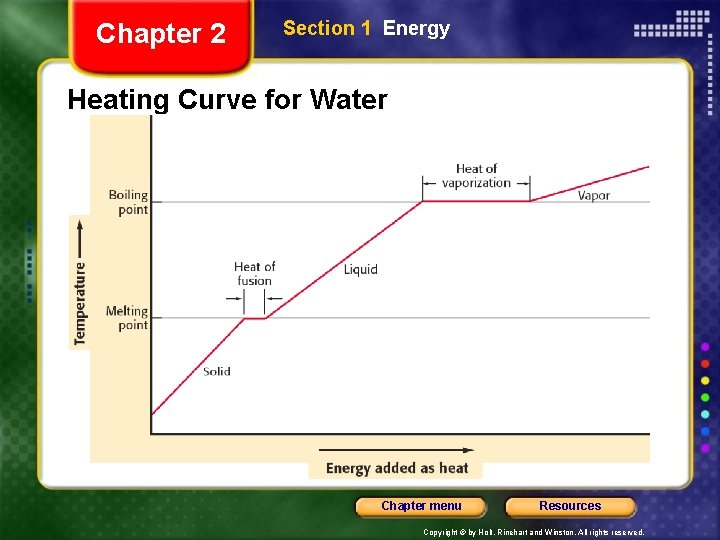
Chapter 2 Section 1 Energy Heating Curve for Water Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

heat of fusion is the amount of thermal energy which must be absorbed or evolved for 1 mole of a substance to change states from a solid to a liquid or vice versa The energy required to change a gram of a liquid into the gaseous state at the boiling point is called the "heat of vaporization Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Transfer of Heat Affects Substances Differently • If you transfer the same quantity of heat to similar masses of different substances, they do not show the same increase in temperature. • This relationship between energy transferred as heat to a substance and the substance’s temperature change is called the specific heat. • The specific heat of a substance is the quantity of energy as heat that must be transferred to raise the temperature of 1 g of a substance 1 K. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 1 Energy Heat, continued Transfer of Heat Affects Substances Differently, continued • The SI unit for energy is the joule (J). • Specific heat is expressed in joules per gram kelvin (J/g • K). • Metals tend to have low specific heats. • Water has an extremely high specific heat. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Visual Concepts Specific Heat Capacity PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Bellringer • Write a brief paragraph describing your concept of the scientific method. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Objectives • Describe how chemists use the scientific method. • Explain the purpose of controlling the conditions of an experiment. • Explain the difference between a hypothesis, a theory, and a law. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy The Scientific Method • The scientific method is a series of steps followed to solve problems, including • Observe stuff and ask “WHY” (collecting data) • formulating a hypothesis (predict) • Testing (experiment) the hypothesis • stating conclusions • A scientist chooses which set of steps to use depending on the nature of the investigation. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
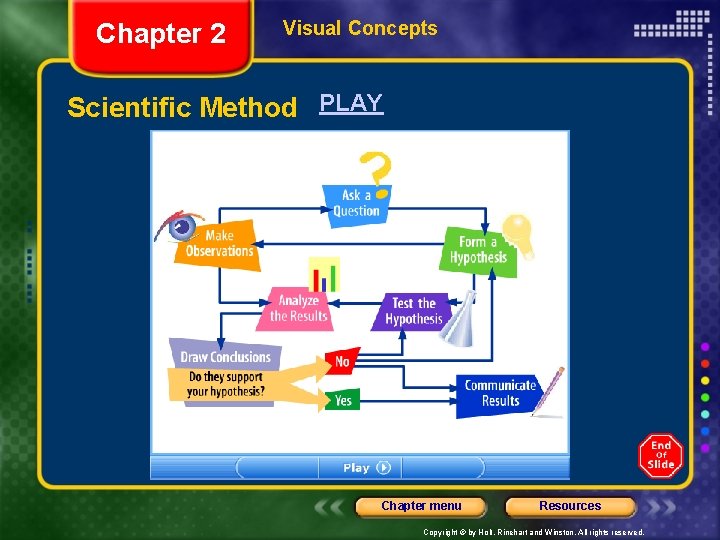
Chapter 2 Visual Concepts Scientific Method PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
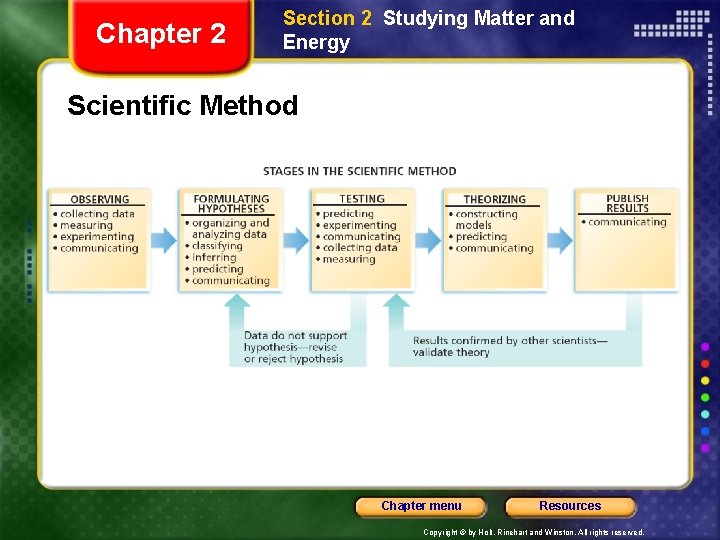
Chapter 2 Section 2 Studying Matter and Energy Scientific Method Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Experiments Are Part of the Scientific Method • The success of the scientific method depends on publishing the results so that others can repeat the procedures and verify the results. • An experiment is the process by which scientific ideas are tested. • For example, both manganese dioxide and beef liver cause a solution of hydrogen peroxide to bubble. • You might conclude that the liver contains manganese dioxide. • To support your conclusion, you would test for the presence of manganese dioxide in the liver. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Experiments May Not Turn Out As Expected • Your tests would reveal that liver does not contain any manganese dioxide. • Scientists often find that their results do not turn out as expected. Such cases are not failures. • Rather, scientists analyze these results and continue with the scientific method. • Unexpected results often give scientists as much information as expected results do. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Scientific Discoveries Can Come from Unexpected Observations • Some discoveries have been made by accident, such as the discovery of a compound known as Teflon®. • Teflon is the nonstick coating used on pots and pans. • Its properties of very low chemical reactivity and very low friction make it valuable in many applications. • Teflon was discovered when a scientist made a simple observation. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Teflon Was Discovered by Chance • In 1938, Du. Pont chemist Dr. Roy Plunkett, was trying to produce a new coolant gas to use as a refrigerant. • He wanted to react a gas called tetrafluoroethene (TFE) with hydrochloric acid. • He recorded the mass of a cylinder of TFE. • He then opened the cylinder to let the TFE flow into a container filled with hydrochloric acid, but no TFE came out of the cylinder. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Teflon Was Discovered by Chance, continued • Because the cylinder had the same mass as it did when it was filled with TFE, Plunkett knew that none of the TFE had leaked out. • When he opened it, he found only a few white flakes. • Plunkett analyze the white flakes and discovered that the TFE molecules joined together to form a long chain of polytetrafluoroethene (PTFE). • These long-chained molecules were very slippery. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Synthetic Dyes Were Also Discovered by Chance • In London, England in 1856, the discovery of the dye aniline purple was made unintentionally by an 18 -year-old student named William Perkin. • Perkin was experimenting to a way to make quinine, a drug used to treat malaria. • One of his experiments resulted in a product that was a thick, sticky, black substance. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy The Scientific Method, continued Synthetic Dyes Were Also Discovered by Chance, continued • Perkin could tell that the substance was not quinine. • In trying to wash away the substance, he made an unexpected discovery. Analyzing an Unexpected Discovery • Pouring alcohol on the substance turned it purple. • He named his accidental discovery “aniline purple, ” but the people of Paris soon renamed it mauve. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations • Questions that scientists hope to answer often come after they observe something carefully in the natural world or in a laboratory. • Once observations are made, they must be analyzed. • Scientists look at the data they have gathered for patterns that might suggest an explanation for the observations. • A hypothesis is a reasonable and testable explanation for observations. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Chemists Use Experiments to Test a Hypothesis • Once a scientist has developed a hypothesis, the next step is to test the validity of the hypothesis. • The testing is often done by carrying out experiments. • Imagine that you have observed that your family car has been getting better mileage. • You state that a new brand of gasoline is the cause. • You’ve made a hypothesis to explain an observation. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Scientists Must Identify the Possible Variables • To test the validity of your hypothesis, your next step is to plan your experiments. • You must begin by identifying as many factors as possible that could account for your observations. • A factor that could affect the results of an experiment is called a variable. • A scientist changes variables one at a time to see which variable affects the outcome of an experiment. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Scientists Must Identify the Possible Variables, continued • Variables that affect mileage might include: the use of a new brand of gasoline, driving more on highways, making fewer short trips, having the car’s engine serviced, and avoiding quick accelerations. • To know if your hypothesis is right, the experiment must be designed to test each variable separately. • Ideally, the experiments will eliminate all but one variable so that the exact cause can be identified. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Each Variable Must Be Tested Individually • When a variable is kept constant from one experiment to the next, the variable is called a control. • The procedure is called a controlled experiment. • Think about how you might carry out a controlled experiment to test your hypothesis. • First, you might fill the car with a new brand of gasoline and keep an accurate record of the mileage. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Each Variable Must Be Tested Individually, continued • Then, you might fill the car with the old brand of gasoline and drive the car under the same conditions, again keeping record of the mileage. • You then have designed the experiment so that only one variable—the brand of gasoline—is being tested. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Data from Experiments Can Lead to a Theory • Any hypothesis that withstands repeated testing may become part of a theory. • In science, a theory is a well-tested explanation of observations. • (This is different from common use of the term, which means “a guess. ”) • Theories are explanations, not facts, so they can be disproved but can never be completely proven. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Theories and Laws Have Different Purposes • Some facts in science always hold true. These facts are called laws. • A law is a statement or mathematical expression that reliably describes a behavior of the natural world. • While a theory is an attempt to explain the cause of certain events in the natural world, a scientific law describes the events. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Comparing Theories and Laws PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Theories and Laws Have Different Purposes, continued • For example, the law of conservation of mass states that the products of a chemical reaction have the same mass as the reactants have. • This law does not explain why matter in chemical reactions behaves this way; the law simply describes this behavior. • Keep in mind that a hypothesis predicts an event, a theory explains it, and a law describes it. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Models Can Illustrate the Microscopic World of Chemistry • A model represents an object, a system, a process, or an idea. • A model is also simpler than the actual thing that is modeled. • In chemistry, models can be most useful in understanding what is happening at the microscopic level. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
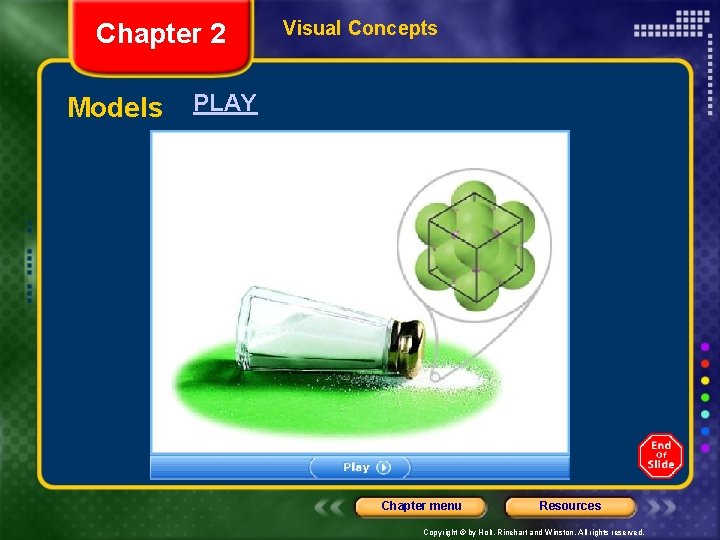
Chapter 2 Models Visual Concepts PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Models Can Illustrate the Microscopic World of Chemistry, continued • This model shows what happens during a reaction between a hydrogen molecule and an oxygen atom. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Studying Matter and Energy Scientific Explanations, continued Models Can Illustrate the Microscopic World of Chemistry, continued • Keep in mind that models are simplified representations. • For example, the actual particles of the chemical substances represented in the models do not have the shapes, sizes, or colors shown. • These models do show the geometric arrangement of the units, their relative sizes, and how they interact. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 1 Measurements and Calculations in Chemistry Objectives • Distinguish between accuracy and precision in measurements. • Determine the number of significant figures in a measurement, and apply rules for significant figures in calculations. • Write very large and very small numbers in scientific notation. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Measurements and Calculations in Chemistry Accuracy and Precision • To reduce the impact of error, scientists repeat their measurements and calculations many times. • If their results are not consistent, they will try to identify and eliminate the source of error. Measurements Must Involve the Right Equipment • Selecting the right equipment to make measurements is the first step to reducing errors in an experiment. • For example, think about a beaker, a buret, and a graduated cylinder. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Accuracy and Precision, continued Measurements Must Involve the Right Equipment, continued • If an experimental procedure calls for measuring 8. 6 m. L of a liquid, you would use the buret. • The buret allows you to measure a volume of liquid that is as close to 8. 6 m. L as possible. • If a procedure calls for measuring 98 m. L of a liquid, you would use a 100 m. L graduated cylinder. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Accuracy and Precision, continued Accuracy Is How Close a Measurement Is to the True Value • When scientists make and report measurements, one factor they consider is accuracy. • The accuracy of a measurement is how close the measurement is to the true or actual value. • To understand what accuracy is, imagine that you throw four darts separately at a dartboard. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Accuracy and Precision Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
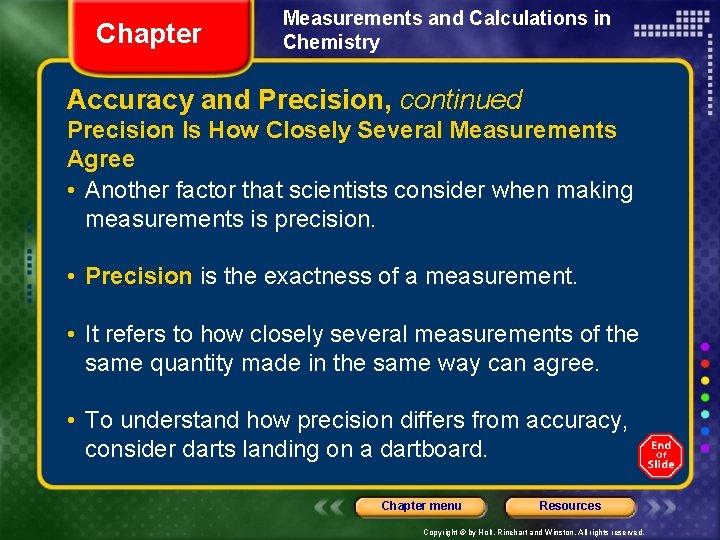
Chapter Measurements and Calculations in Chemistry Accuracy and Precision, continued Precision Is How Closely Several Measurements Agree • Another factor that scientists consider when making measurements is precision. • Precision is the exactness of a measurement. • It refers to how closely several measurements of the same quantity made in the same way can agree. • To understand how precision differs from accuracy, consider darts landing on a dartboard. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Accuracy and Precision, continued Accuracy Is How Close a Measurement Is to the True Value, continued • Suppose the procedure for a chemical reaction calls for adding 36 m. L of a solution. • The experiment is done twice. • The first time 35. 8 m. L is added, and the second time 37. 2 m. L is added. • The first measurement was more accurate because 35. 8 m. L is closer to the true value of 36 m. L. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Visual Concepts Accuracy and Precision PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

The percentage error (% error) is calculated according to the following equation error = observed value – accepted value x 100 accepted value The sign of the experimental error and the percentage error may be either positive (the experimental result is too high) or negative (the experimental result is too low). Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
UNITED STREAMING VIDEO Accuracy /Precision/Sig Fig Intro Video Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Measurements and Calculations in Chemistry Significant Figures • When you make measurements or perform calculations, the way you report a value tells about how you got it. • Scientists report values using significant figures. • The significant figures of a measurement or a calculation consist of all the digits known with certainty as well as one estimated, or uncertain, digit. • The last digit or significant figure reported after a measurement is uncertain or estimated. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Visual Concepts Significant Figures Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

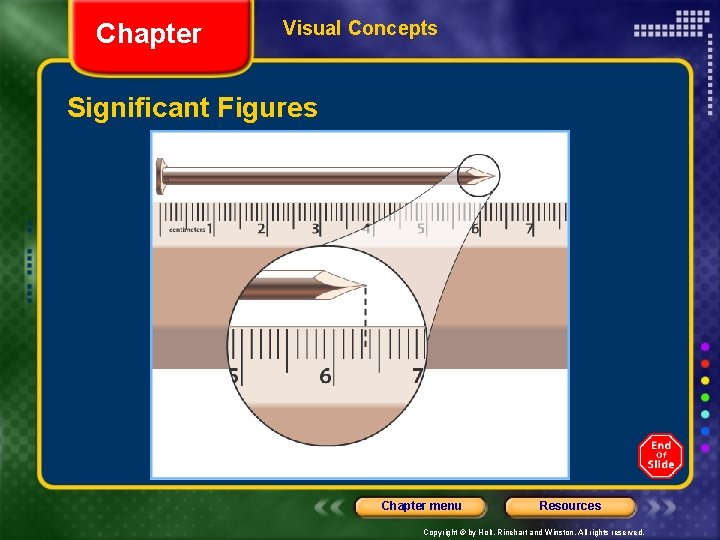
Chapter Measurements and Calculations in Chemistry Significant Figures, continued Significant Figures Are Essential to Reporting Results • Reporting all measurements in an experiment to the correct number of significant figures is necessary to be sure the results are true. • Imagine you are measuring temperature with a thermometer calibrated in one-tenth degree increments. • For a reading of 36. 5°C, the three digits in the value are all significant figures. The first two digits are known with certainty, while the last is estimated. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
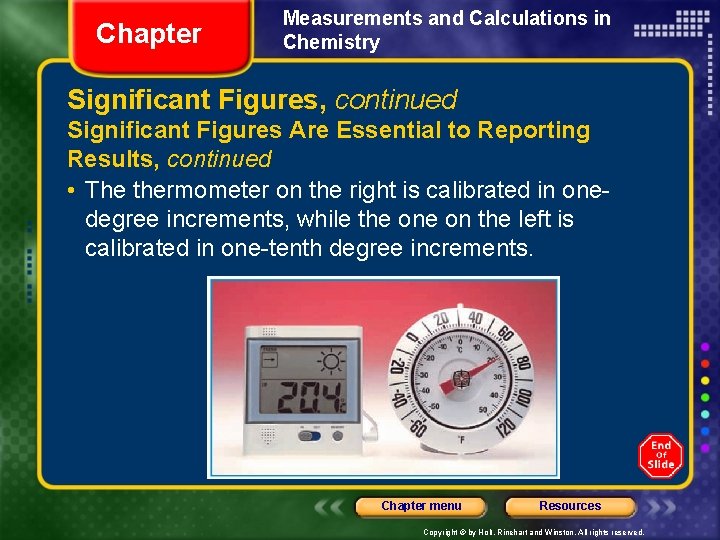
Chapter Measurements and Calculations in Chemistry Significant Figures, continued Significant Figures Are Essential to Reporting Results, continued • The thermometer on the right is calibrated in onedegree increments, while the on the left is calibrated in one-tenth degree increments. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Significant Figures, continued Rules for Determining Significant Figures 1. Nonzero digits are always significant. Ex) 127. 34 contains 5 s. f. 2. Leading zeros Zeros in front of nonzero digits are not significant. Ex) 0. 00476 contains 3 s. f. 3. Captive Zeros between nonzero digits are significant. Ex) 120. 007 contains 6 s. f. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Measurements and Calculations in Chemistry Significant Figures, continued Rules for Determining Significant Figures, continued 4. Trailing zeros at the right end of the number. They are significant only if the number contains a decimal point. . Ex) 109, 000 contains 3 s. f. Ex) 25, 100. 00 contains 7 s. f. Ex) 0. 1000 contains 4 s. f. 5. Exact Numbers. Any number that represents a numerical count or an exact definition can be assumed to have an unlimited number of significant figures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Section 3 Measurements and Calculations in Chemistry Determine the number of significant figures: 1) 29. 625 2) 0. 02006 3) 3. 017 4) 10. 082 5) 0. 00011 6) 0. 000009 7) 3. 000009 8) 2, 690 9) 6. 50 10)900. 00 11)60, 013 12)21. 040 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Measurements and Calculations in Chemistry Significant Figures, continued Calculators Do Not Identify Significant Figures • When you use a calculator to find a result, you must pay special attention to significant figures. • A calculator does not round the result to the correct number of significant figures. • To determine the number of significant figures in the answer, you must first find the number of significant figures in the values used to calculate the answer. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Significant Figures, continued Rules for Using Significant Figures in Calculations 1. With multiplication and division, the answer cannot have more significant figures than the measurement with the smallest number of significant figures. Ex) V = lwh = (3. 05 cm)(2. 10 cm) (0. 75 cm) = 4. 80375 cm 3 = 4. 8 cm 3 Additional examples see page 58 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
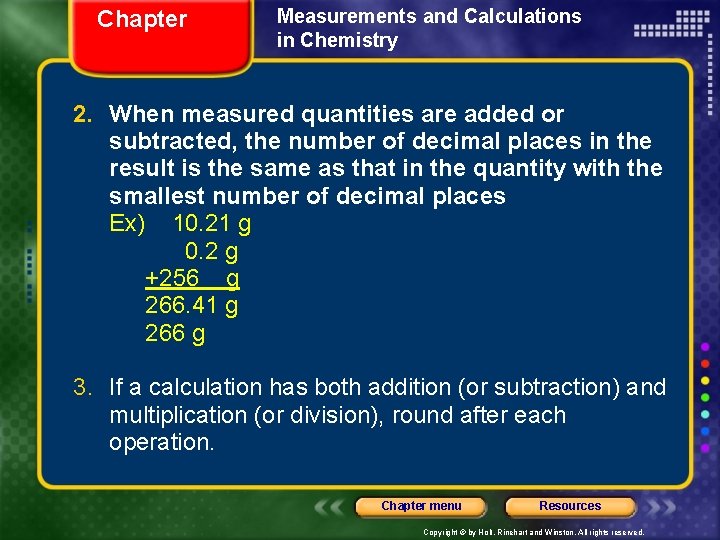
Chapter Measurements and Calculations in Chemistry 2. When measured quantities are added or subtracted, the number of decimal places in the result is the same as that in the quantity with the smallest number of decimal places Ex) 10. 21 g 0. 2 g +256 g 266. 41 g 266 g 3. If a calculation has both addition (or subtraction) and multiplication (or division), round after each operation. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
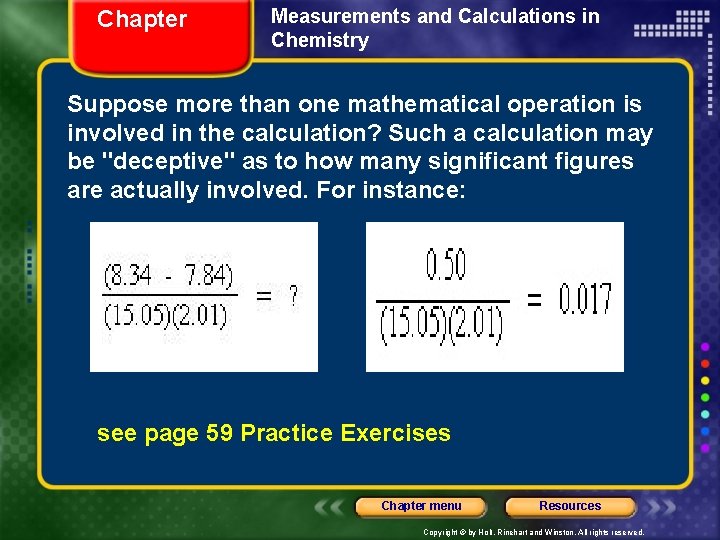
Chapter Measurements and Calculations in Chemistry Suppose more than one mathematical operation is involved in the calculation? Such a calculation may be "deceptive" as to how many significant figures are actually involved. For instance: see page 59 Practice Exercises Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Perform the following calculations, and express the results in the correct units and with the proper number of significant figures. 1) (0. 054 kg +1. 33 kg) x 5. 4 m 2 7. 5 kg · m 2 2) 67. 35 cm 2 / (1. 401 cm - 0. 399 cm) 67. 22 cm 3) 4. 198 kg x (1019 m 2 - 40 m 2 ) / (54. 2 s X 31. 3 s) 2. 42 kg· m 2/s 2 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
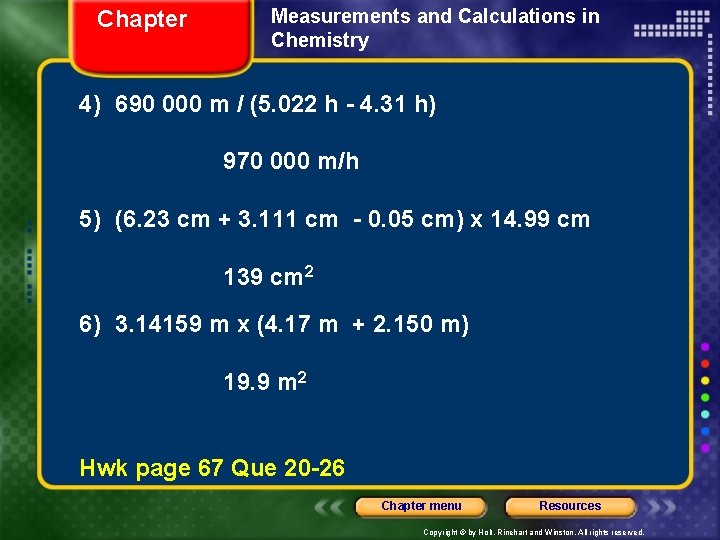
Chapter Measurements and Calculations in Chemistry 4) 690 000 m / (5. 022 h - 4. 31 h) 970 000 m/h 5) (6. 23 cm + 3. 111 cm - 0. 05 cm) x 14. 99 cm 139 cm 2 6) 3. 14159 m x (4. 17 m + 2. 150 m) 19. 9 m 2 Hwk page 67 Que 20 -26 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Significant Figures, continued Exact Values Have Unlimited Significant Figures • Some values have an unlimited number of significant figures. These exact values have no uncertainty. • One type of exact value, a count value, is determined by counting, not by measuring. • Another value that can have an unlimited number of significant figures is a conversion factor. • Ignore both count values and conversion factors when determining the number of significant figures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Determining the Number of Significant Figures Sample Problem A A student heats 23. 62 g of a solid and observes that its temperature increases from 21. 6°C to 36. 79°C. Calculate the temperature increase per gram of solid. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
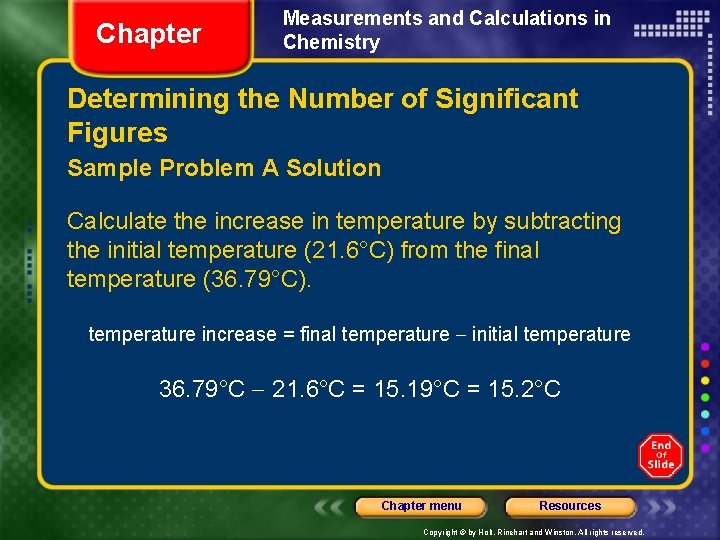
Chapter Measurements and Calculations in Chemistry Determining the Number of Significant Figures Sample Problem A Solution Calculate the increase in temperature by subtracting the initial temperature (21. 6°C) from the final temperature (36. 79°C). temperature increase = final temperature initial temperature 36. 79°C 21. 6°C = 15. 19°C = 15. 2°C Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
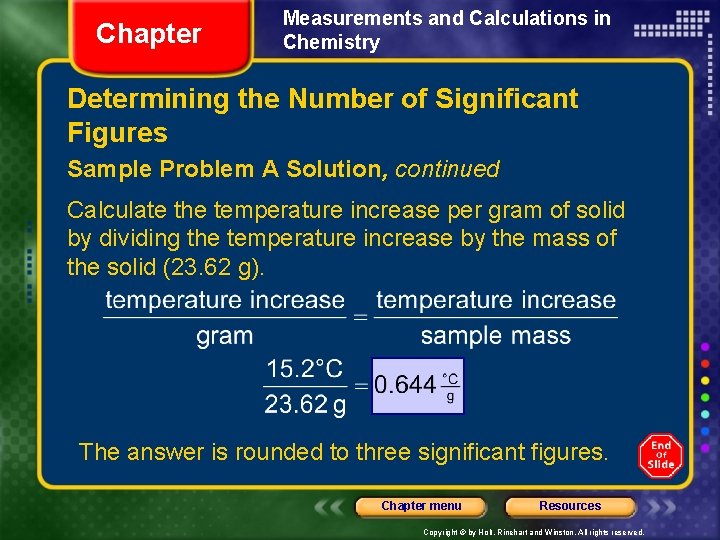
Chapter Measurements and Calculations in Chemistry Determining the Number of Significant Figures Sample Problem A Solution, continued Calculate the temperature increase per gram of solid by dividing the temperature increase by the mass of the solid (23. 62 g). The answer is rounded to three significant figures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Scientific Notation • Very large and very small numbers are often written in scientific notation. • A number in scientific notation has two parts. • The first part is a number that is between 1 and 10. • The second part consists of a power of 10. • To write the first part of the number, move the decimal to the right or the left so that only one nonzero digit is to the left of the decimal. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Scientific Notation, continued • Write the second part of the value as an exponent. This part is determined by counting the number of decimal places the decimal point is moved. • If the decimal is moved to the right, the exponent is negative. • If the decimal is moved to the left, the exponent is positive. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Visual Concepts Scientific Notation PLAY Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Scientific Notation, continued Scientific Notation in Calculations 1. In scientific notation, exponents are count values. 2. In addition and subtraction problems, all values must have the same exponent before they can be added or subtracted. The result is the sum of the difference of the first factors multiplied by the same exponent of 10. 3. In multiplication problems, the first factors of the numbers are multiplied and the exponents of 10 are added. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Scientific Notation, continued Scientific Notation in Calculations, continued 4. In division problems, the first factors of the numbers are divided and the exponent of 10 in the denominator is subtracted from the exponent of 10 in the numerator. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter Measurements and Calculations in Chemistry Scientific Notation, continued Scientific Notation with Significant Figures 1. Use scientific notation to eliminate all place-holding zeros. 2. Move the decimal in an answer so that only one digit is to the left, and change the exponent accordingly. The final value must contain the correct number of significant figures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
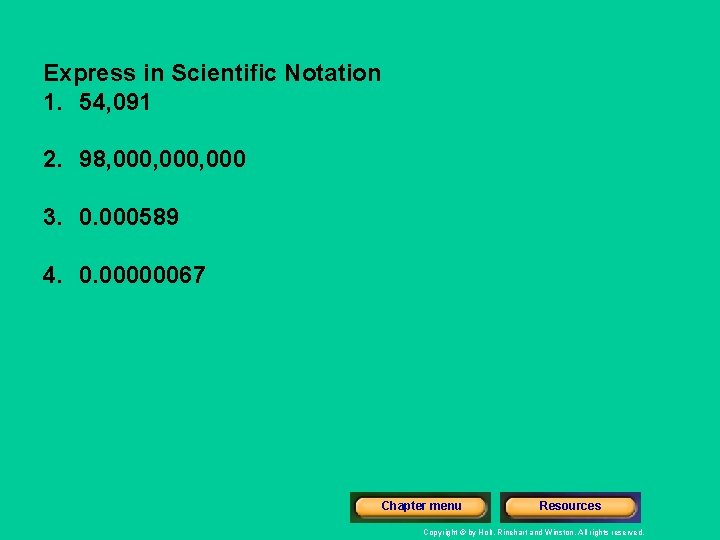
Express in Scientific Notation 1. 54, 091 2. 98, 000, 000 3. 0. 000589 4. 0. 00000067 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
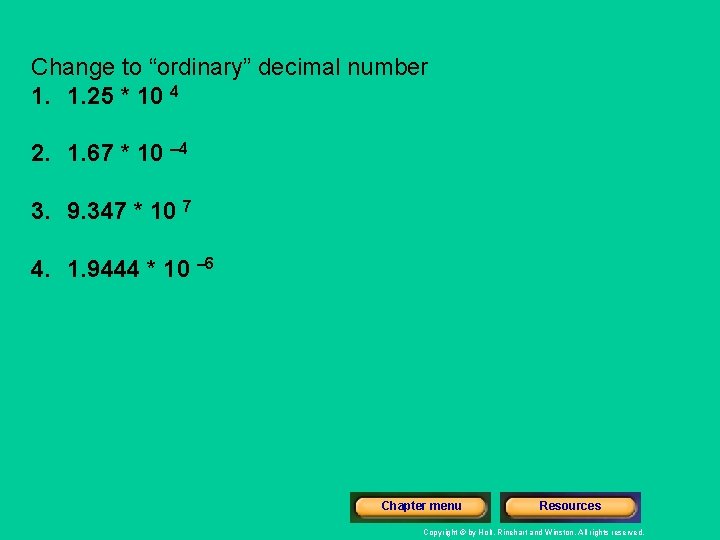
Change to “ordinary” decimal number 1. 1. 25 * 10 4 2. 1. 67 * 10 – 4 3. 9. 347 * 10 7 4. 1. 9444 * 10 – 6 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Sample Problems 1) 4. 68 * 10 – 8 + 1. 23 * 10 – 9 2) 7. 62 * 10 10 - 9. 80 * 10 9 3) ( 2. 3 * 10 – 6 ) ( 4. 6 * 10 – 7 ) ( 5. 2 * 10 – 3 ) 4) 9. 72 * 10 10 3. 45 * 10 – 4 5) ( 3. 5 * 10 8 ) ( 1. 72 * 10 – 2 ) (2. 3 * 10 4 ) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
SCIENTIFIC NOTATION WORKSHEET Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 3 Measurements and Calculations in Chemistry Objectives • Calculate changes in energy using the equation for specific heat, and round the results to the correct number of significant figures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter Measurements and Calculations in Chemistry Significant Figures, continued Specific Heat Depends on Various Factors • Recall that the specific heat is the quantity of energy that must be transferred as heat to raise the temperature of 1 g of a substance by 1 K. • Specific heat depends on the nature of the material that is changing temperature, the mass of the material, and the size of the temperature change. • For example, iron has a larger specific heat than silver. Therefore, more energy as heat can be transferred to iron than to silver. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Measurements and Calculations in Chemistry Significant Figures, continued Calculating the Specific Heat of a Substance • Specific heats can be used to compare how different materials absorb energy as heat under the same conditions. • The specific heat (cp) of a substance at a given pressure (p) is calculated by the following formula: • Note that q is the energy transferred as heat, m is the mass of the substance, and ∆T is the difference between the initial and final temperatures. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Measurements and Calculations in Chemistry Calculating the Specific Heat of a Substance Q=cpmΔT where Q= energy (heat) required cp= specific heat capacity m= mass of sample in grams ΔT= change in temperature in Kelvin Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Measurements and Calculations in Chemistry Calculating Specific Heat Sample Problem B A 4. 0 g sample of glass was heated from 275 K to 314 K and was found to absorb 32 J of energy as heat. Calculate the specific heat of this glass. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
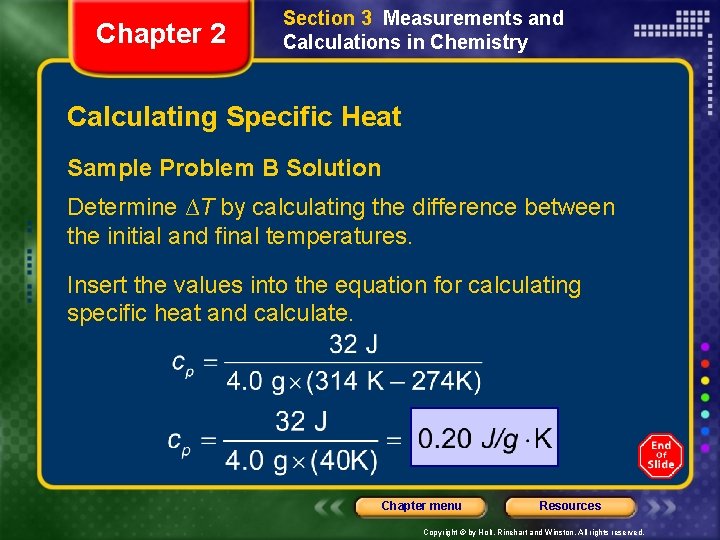
Chapter 2 Section 3 Measurements and Calculations in Chemistry Calculating Specific Heat Sample Problem B Solution Determine ∆T by calculating the difference between the initial and final temperatures. Insert the values into the equation for calculating specific heat and calculate. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Ex) Calculate the specific heat of a substance if a 35 g sample absorbs 48 J as the temperature is raised from 294 K to 313 K. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Ex) The temperature of a piece of copper with a mass of 95. 4 g increases from 298. 0 K to 321. 1 K when the metal absorbs 849 J of energy as heat. What is the specific heat of copper? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Ex) If 980 k. J of energy as heat are transferred to 6. 2 L of water at 291 K, what will the final temperature of the water be? The specific heat of water is 4. 18 J/g·K. Assume that 1. 0 m. L of water equals 1. 0 g of water. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Ex) How much energy as heat must be transferred to raise the temperature of a 55 g sample of aluminum from 22. 4°C to 94. 6°C? The specific heat of aluminum is 0. 897 J/g·K. Note that a temperature change of 1°C is the same as a temperature change of 1 K because the sizes of the degree divisions on both scales are equal. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Homework 1. Calculate the specific heat of a substance when 63 J of energy are transferred as heat to an 8. 0 g sample to raise its temperature from 314 K to 340 K. 2. Determine the specific heat of a material if a 35 g sample of the material absorbs 48 J as it is heated from 298 K to 313 K. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

3. How much energy is needed to raise the temperature of a 75 g sample of aluminum from 295. 55 K to 367. 75 K? Refer to Table 1. 4. Energy in the amount of 420 J is added to a 35 g sample of water at a temperature of 283. 15 K. What is the final temperature of the water? Refer to Table 1. 5. How much heat is lost when a solid aluminum ingot with a mass of 4, 110 grams cools from 933 k to 298 K? Refer to Table 1. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

TEST Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
- Slides: 126