Chapter 2 Matter and Change 2 1 Matter






- Slides: 9

Chapter 2: Matter and Change

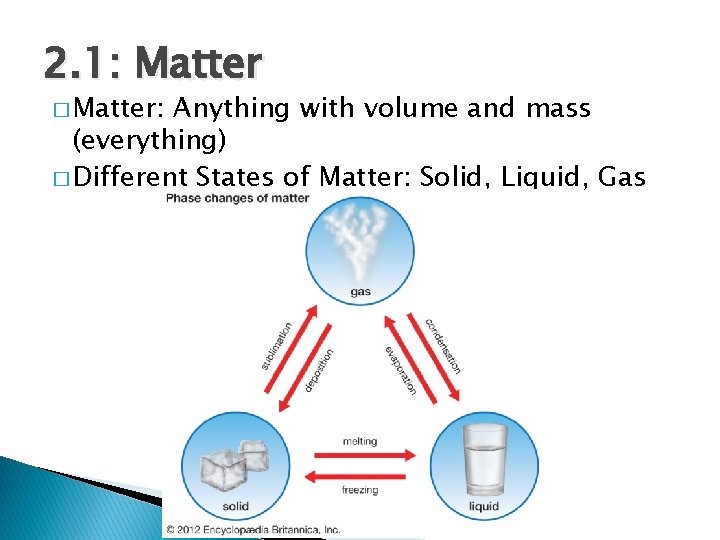
2. 1: Matter � Matter: Anything with volume and mass (everything) � Different States of Matter: Solid, Liquid, Gas

2. 1: Matter � Physical Change: Change in matter that does not change substance itself at chemical level ◦ Phase changes are always physical changes! � Chemical Change: Change in matter that does change the substance itself ◦ Clues: Color change, gas production (bubbles), precipitate (solid forms) � Are these changes chemical or physical? ◦ Melting ice? ◦ Burning magnesium? ◦ Vinegar and baking soda?

2. 2+2. 3 Elements, Compounds, and Mixtures � Homogenous Mixture: A MIXTURE where substance is same throughout. ◦ Wherever I take sample, it is the same � Heterogeneous Mixture: A MIXTURE where is not same throughout ◦ Sample will be different depending on where I take it � Pure Substance: Only one thing (a element or a compound)

2. 2+2. 3 Elements, Compounds, and Mixtures � Element: Simplest form of matter (can’t be broken down). ◦ All elements are found on the periodic table. ◦ Gold (Au), Silver (Ag), Helium (He) � Compounds: Matter that can be broken down further into elements ◦ Water (H 2 O), Carbon dioxide (CO 2), Salt (Na. Cl), Sugar C 6 H 12 O 6 � Mixture: Multiple types of matters mixed ◦ Air (mixture of oxygen and carbon dioxide), Kool. Aid (mixture of water and Sugar (H 2 O and C 6 H 12 O 6)

Do on your own! Will help you on quiz. �Pure 1) 2) 3) 4) 5) 6) substance or mixture? Oxygen (O 2) gas: An Aluminum (Al) can: Methane gas (CH 4): Soda: Helium (He) Gas: A hamburger:

Do on your own! Will help you on quiz. � Are the following either a homogenous mixture of a heterogeneous mixture? 7) A hamburger 8) Salt water 9) Concrete 10) Cereal

2. 4: Elements, Compounds, Mixtures � Parts of a chemical reaction ◦ 2 H 2 + O 2 2 H 20 ◦ 2 H 2 + O 2 = Reactant ◦ 2 H 2 O = Products � Physical Property (Different than physical changes) ◦ Property that can be observed without changing substances (often measured with senses) � Chemical change) Property (different than chemical ◦ Property that can only be determined by a chemical reaction or attempted chemical reaction.

Examples: Chemical and Physical Properties �Are the following CHEMICAL or PHYSICAL properties ◦ Blue-gray color ◦ Brittle (breaks easy) ◦ Rusts in rain ◦ Insoluble in water ◦ Melts at 1410 degrees ◦ Reacts with fluorine