Chapter 2 Heat Capacity and Specific Heat Rice











- Slides: 30

Chapter 2 Heat Capacity and Specific Heat Rice

Some Basic Concepts

Kinds of Energy l l l Heat Light Chemical Nuclear Electrical Mechanical Any one of these can be converted into any of the other forms.

Types of Energy 1) Kinetic Energy – energy of motion KE = ½ mv 2 m= mass in kg v = velocity in m/s 2) Potential Energy – stored energy 1. Gravitational Potential Energy 2. Electrostatic Attraction Potential Energy 3) Total Energy of a Substance is equal to: ETotal = PE + KE

How does KE & PE relate to Chemistry? l KE = the motion of the molecules in chemical substances. l PE = the sum of all the attractions in a chemical substance, including all the covalent bonds, ionic bonds, and electrostatic attractions.

Temperature l l A measure of the average kinetic energy of the particles in a sample of matter. It is a description of one characteristic of the individual particles in an object. How hot or cold something is. K. E Temp

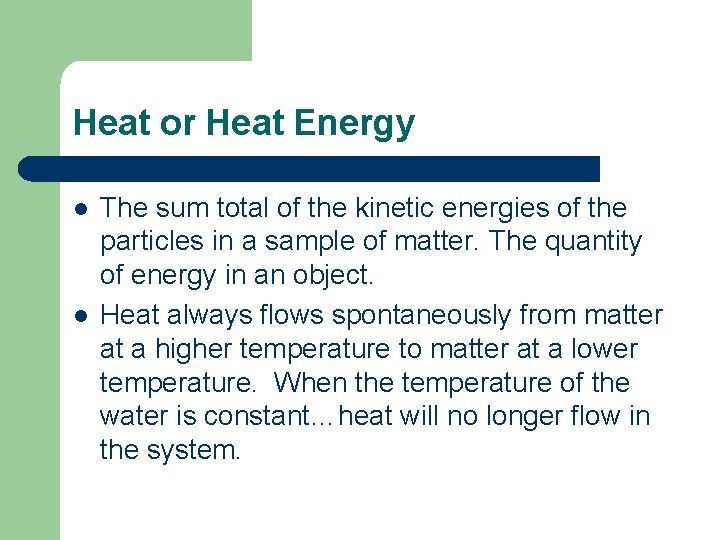
Heat or Heat Energy l l The sum total of the kinetic energies of the particles in a sample of matter. The quantity of energy in an object. Heat always flows spontaneously from matter at a higher temperature to matter at a lower temperature. When the temperature of the water is constant…heat will no longer flow in the system.

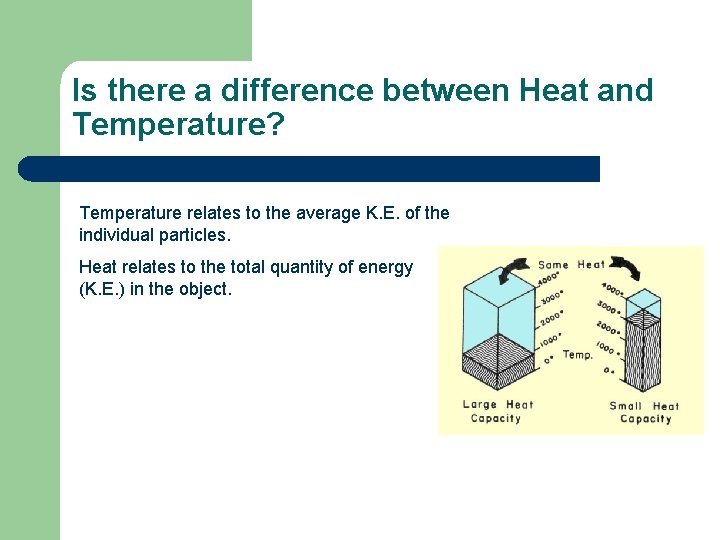
Is there a difference between Heat and Temperature? Temperature relates to the average K. E. of the individual particles. Heat relates to the total quantity of energy (K. E. ) in the object.

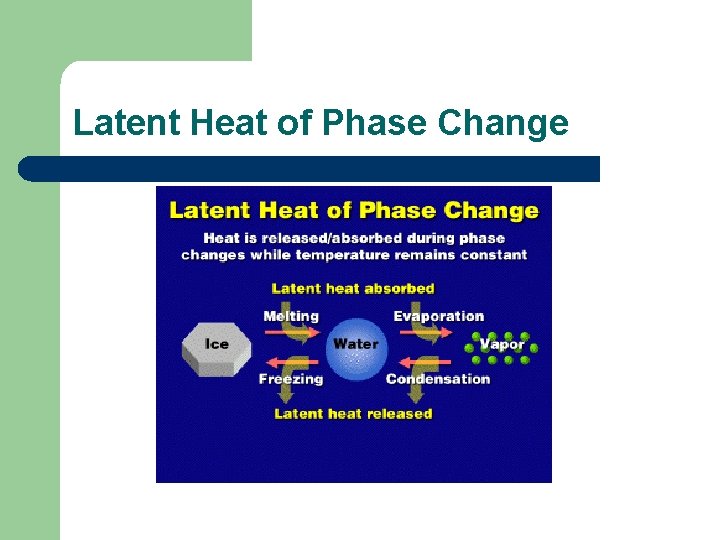
Latent Heat of Phase Change

Heat Transfer l Heat flows from regions of high heat content to regions of low heat content. – – – Conduction: heat spread by collisions between molecules. Convection: heat spread by movement of air Radiation: energy moving through space in the form of electromagnetic radiation

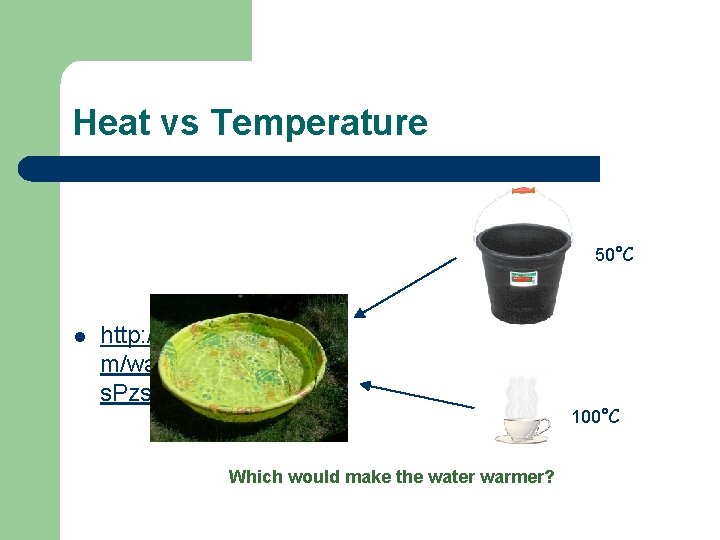
Heat vs Temperature 50°C l http: //www. youtube. co m/watch? v=r. Us. Pzsh. Vn. M 100°C Which would make the water warmer?

The Measurement of Energy l l Energy cannot be measured directly. Heat is measured by determining temperature changes caused by the release or absorbtion of heat in a chemical process. Heat energy was originally defined in terms of the calorie (the amount of heat needed to raise the temperature of 1 g of water 1°C). 1 cal = 4. 184 J

Heat Capacity l The amount of heat energy needed to raise the temperature of a given sample of matter by 1°C

Specific Heat The amount of energy needed to raise the temperature of 1 g of water 1°C. Ø The specific heat of water is 4. 184 J/g°C or 1 cal/g°C.

Once the specific heat of water is defined, the specific heat of any other substance can be determined. Ø One method is to immerse a hot object in a known quantity of H 2 O and then measure the temperature change. q (gained by water) = q (lost by metal)

Units of Temperature vs Units of Heat l Temperature °C, °F, K l Heat l cal (calorie) or Cal (food calorie) 1 Cal = 1000 cal J (joule) 1 cal = 4. 184 J

Convert the following: 1) 110 cal to J 2) 22 k. J to cal

Solutions 1) 110 cal to J 110 cal x 1 cal = 26 J 1 4. 184 J 2) 22 k. J to cal 22 k. J x 1000 J 1 1 k. J x 4. 184 J = 92000 cal 1 cal

Calorimetry l Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry involves the use of a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat.

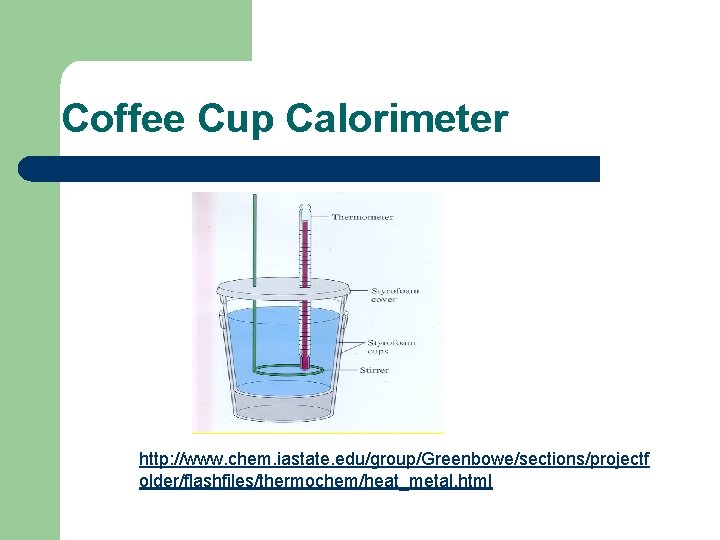
Coffee Cup Calorimeter http: //www. chem. iastate. edu/group/Greenbowe/sections/projectf older/flashfiles/thermochem/heat_metal. html

Coffee Cup Calorimeter Constant pressure calorimeter is called a coffee cup calorimeter. Ø Water is put in the cup and the temperature is taken, the temperature of heated metal is taken. The heated metal is then placed in the calorimeter and the cover is placed on the calorimeter. When the temperature no longer changes, it is recorded. qlost = qgained

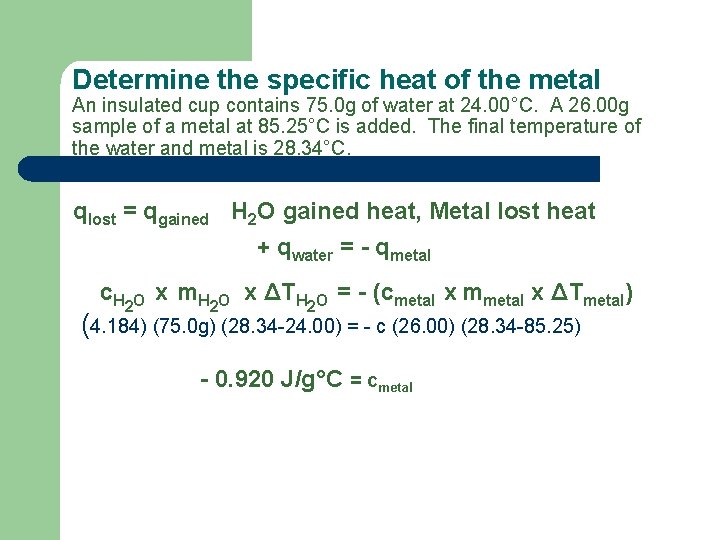
Example An insulated cup contains 75. 0 g of water at 24. 00°C. A 26. 00 g sample of a metal at 85. 25°C is added. The final temperature of the water and metal is 28. 34°C. – What is the specific heat of the metal?

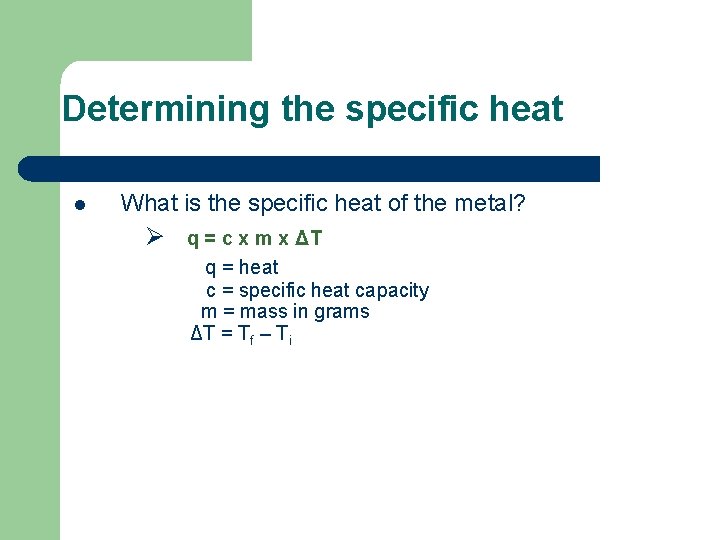
Determining the specific heat l What is the specific heat of the metal? Ø q = c x m x ΔT q = heat c = specific heat capacity m = mass in grams ΔT = Tf – Ti

Determine the specific heat of the metal An insulated cup contains 75. 0 g of water at 24. 00°C. A 26. 00 g sample of a metal at 85. 25°C is added. The final temperature of the water and metal is 28. 34°C. qlost = qgained H 2 O gained heat, Metal lost heat + qwater = - qmetal c. H 2 O x m. H 2 O x ΔTH 2 O = - (cmetal x mmetal x ΔTmetal) (4. 184) (75. 0 g) (28. 34 -24. 00) = - c (26. 00) (28. 34 -85. 25) - 0. 920 J/g°C = cmetal

Your Turn An insulated cup contains 250 g of water at 25. 0°C. A 5. 0 g sample of a metal at 100. 0°C is added. The final temperature of the water and metal is 25. 2°C. – What is the specific heat of the metal?

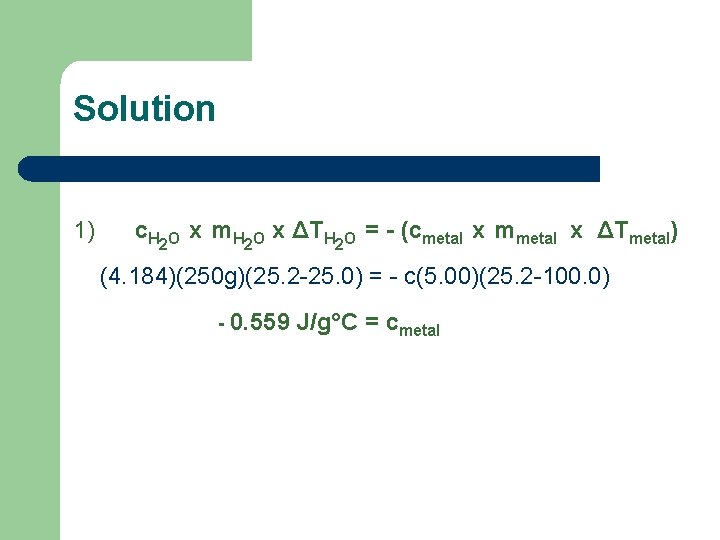
Solution 1) c. H 2 O x m. H 2 O x ΔTH 2 O = - (cmetal x mmetal x ΔTmetal) (4. 184)(250 g)(25. 2 -25. 0) = - c(5. 00)(25. 2 -100. 0) - 0. 559 J/g°C = cmetal

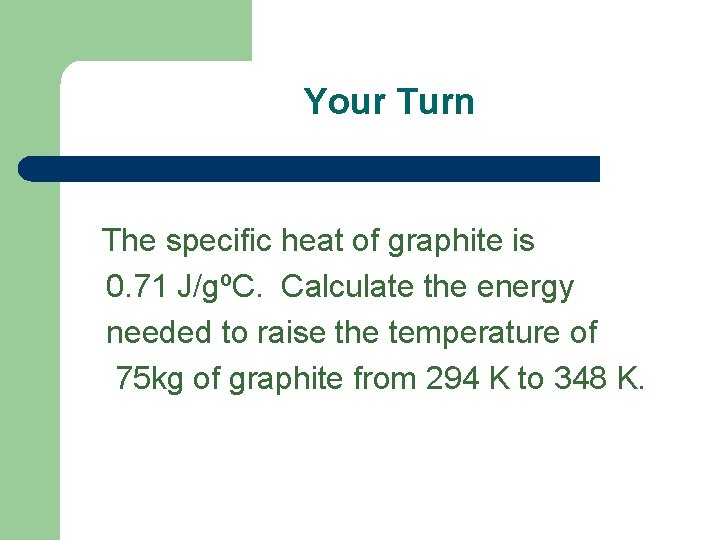
Your Turn The specific heat of graphite is 0. 71 J/gºC. Calculate the energy needed to raise the temperature of 75 kg of graphite from 294 K to 348 K.

Solution First convert: Ø 75 kg x 1000 g = 75, 000 g 1 1 kg Tf = 348 K - 273 = 75ºC Need ºC for Units Ti = 294 K - 273 = 21ºC q = cmΔT q = (0. 71 J/gºC) (75, 000 g)(75 -21) q = 2, 875, 500 J or 2, 875. 5 k. J

Your Turn A 46. 2 g sample of copper is heated to 95. 4ºC and then placed in a calorimeter containing 75. 0 g of water at 19. 6ºC. The final temperature of both the water and the copper is 21. 8ºC. What is the specific heat of copper?

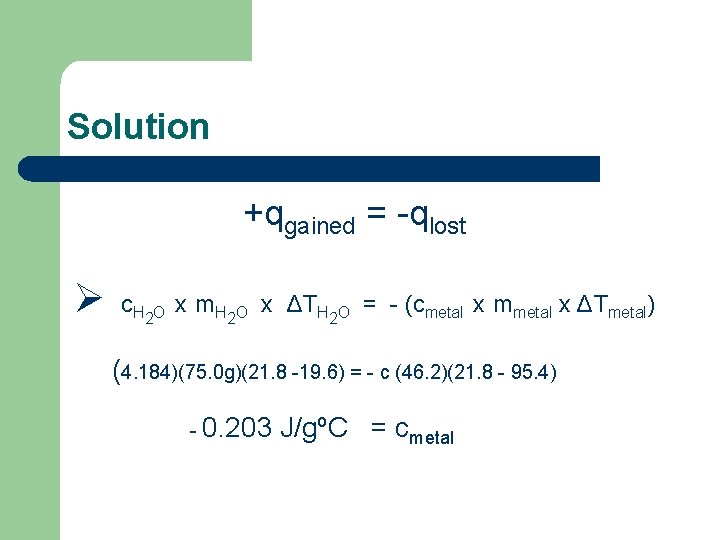
Solution +qgained = -qlost Ø c. H 2 O x m. H 2 O x ΔTH 2 O = - (cmetal x mmetal x ΔTmetal) (4. 184)(75. 0 g)(21. 8 -19. 6) = - c (46. 2)(21. 8 - 95. 4) - 0. 203 J/gºC = cmetal