Chapter 2 Elements Compounds Mixtures An Overview Introducing














- Slides: 45

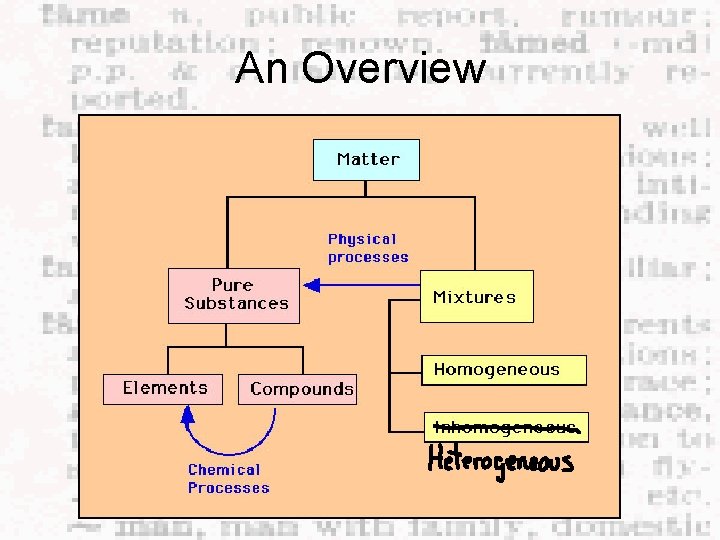
Chapter 2 Elements, Compounds, Mixtures

An Overview

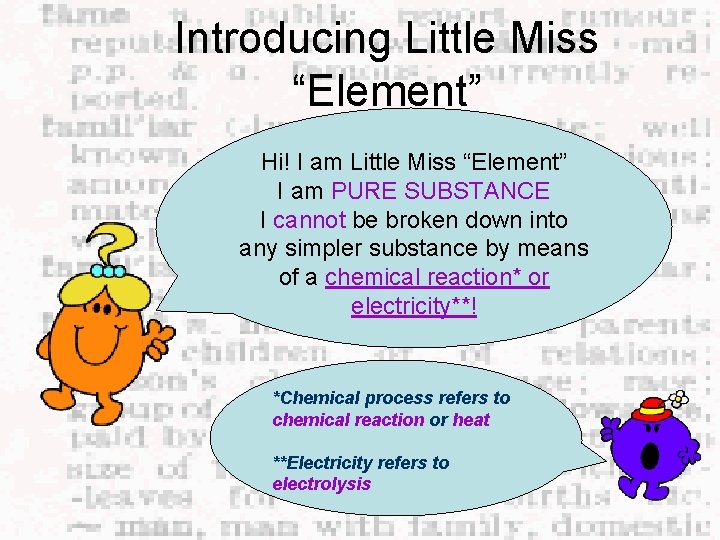
Introducing Little Miss “Element” Hi! I am Little Miss “Element” I am PURE SUBSTANCE I cannot be broken down into any simpler substance by means of a chemical reaction* or electricity**! *Chemical process refers to chemical reaction or heat **Electricity refers to electrolysis

Elements Definition of an element: An element is a pure substance which cannot be split up into two or more simpler substances by chemical means. Sugar is not an element as it can be broken down into carbon and water.

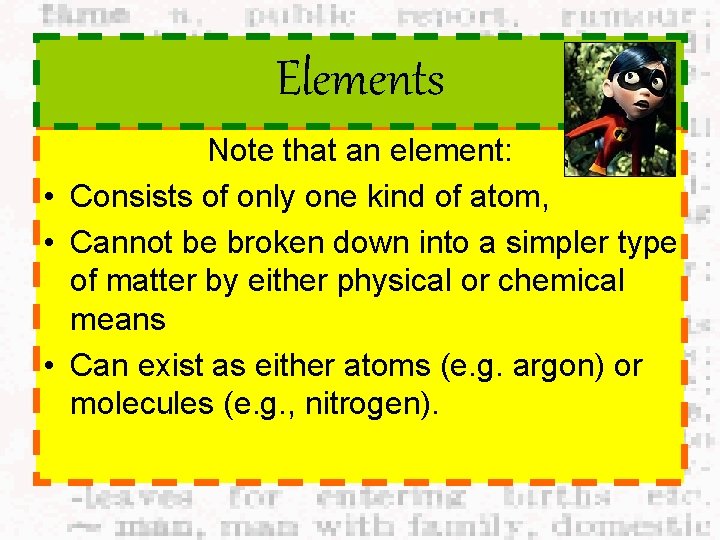
Elements Note that an element: • Consists of only one kind of atom, • Cannot be broken down into a simpler type of matter by either physical or chemical means • Can exist as either atoms (e. g. argon) or molecules (e. g. , nitrogen).

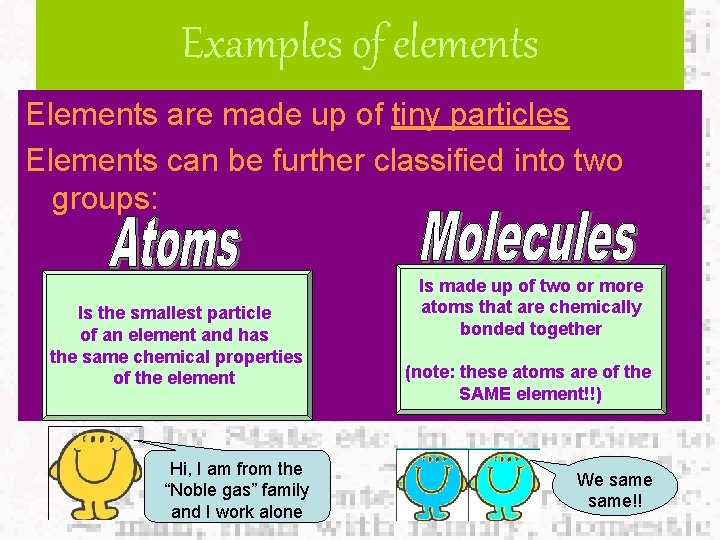
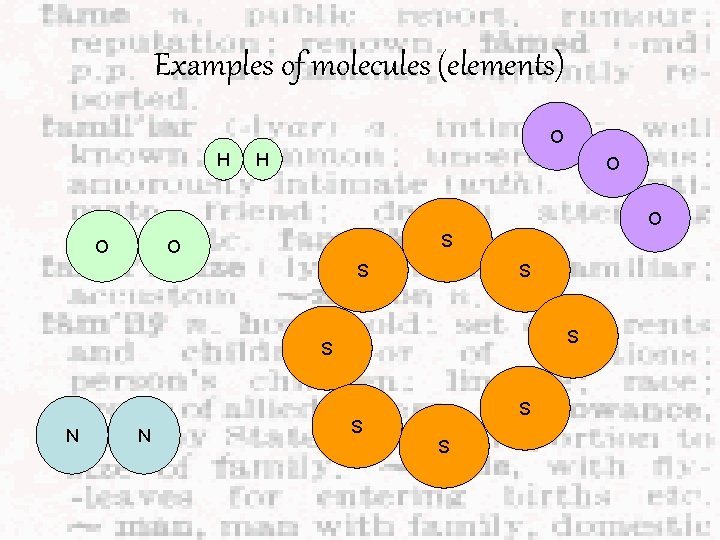
Examples of elements Elements are made up of tiny particles Elements can be further classified into two groups: Is the smallest particle of an element and has the same chemical properties of the element Hi, I am from the “Noble gas” family and I work alone Is made up of two or more atoms that are chemically bonded together (note: these atoms are of the SAME element!!) We same!!

These are elements! Atoms of same element Cu copper element Na sodium element He Molecules of same element H helium element H Hydrogen gas element O O ozone O

Atoms An element is made of tiny particles called atoms. The atoms of an element is different from that of another element.

Elements- Atoms Consists of only one kind of atom Microscopic view of the atoms of the element argon (gas phase).

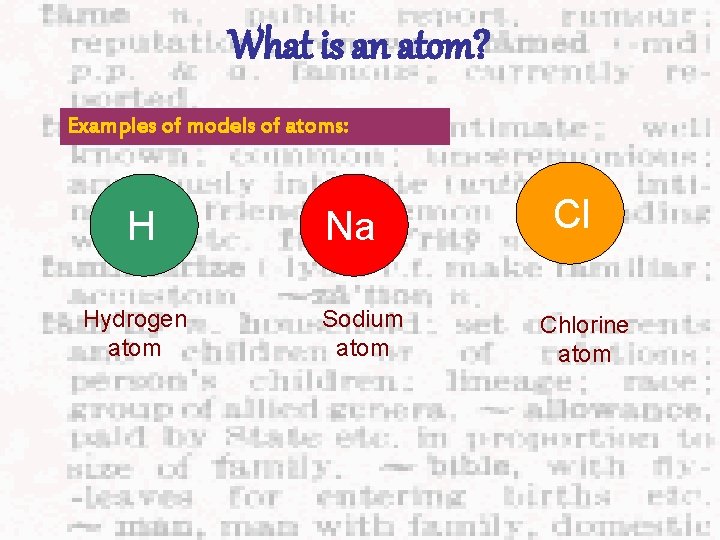
What is an atom? Examples of models of atoms: H Hydrogen atom Na Sodium atom Cl Chlorine atom

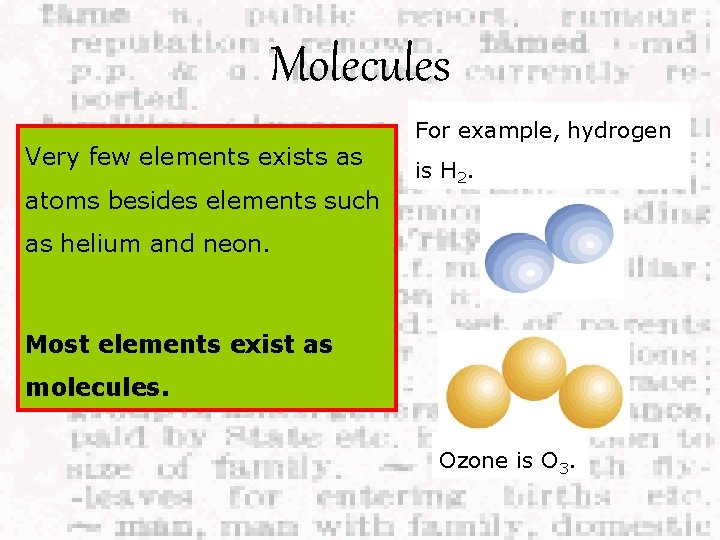
Molecules Very few elements exists as atoms besides elements such For example, hydrogen is H 2. as helium and neon. Most elements exist as molecules. Ozone is O 3.

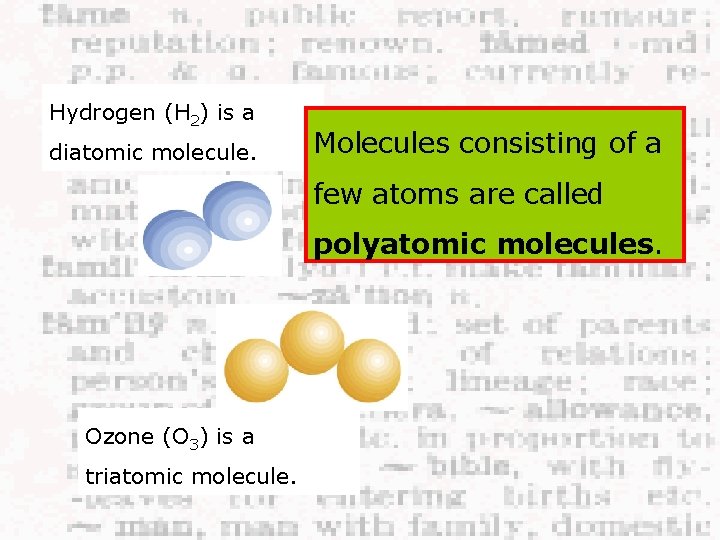
Hydrogen (H 2) is a diatomic molecule. Molecules consisting of a few atoms are called polyatomic molecules. Ozone (O 3) is a triatomic molecule.

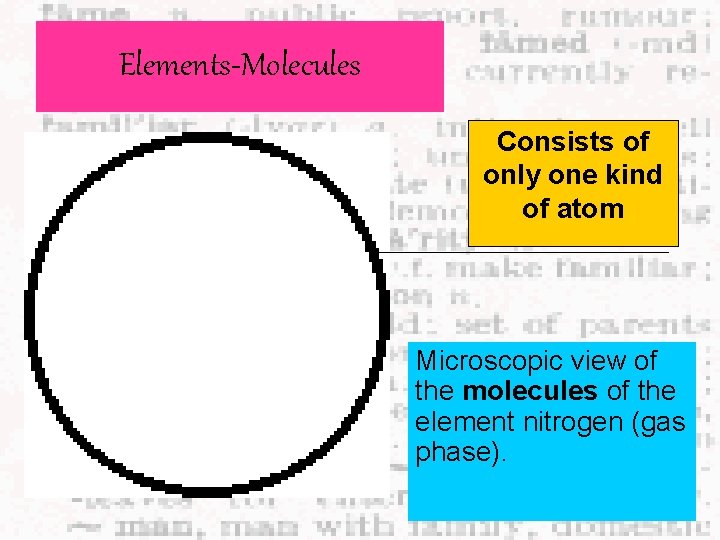
Elements-Molecules Consists of only one kind of atom Microscopic view of the molecules of the element nitrogen (gas phase).

Examples of molecules (elements) O H H O O O S S S S N N S S S

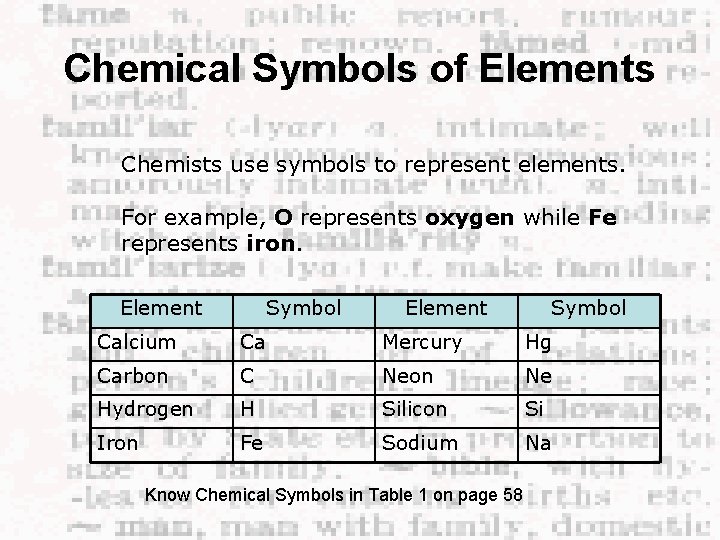
Chemical Symbols of Elements Chemists use symbols to represent elements. For example, O represents oxygen while Fe represents iron. Element Symbol Calcium Ca Mercury Hg Carbon C Neon Ne Hydrogen H Silicon Si Iron Fe Sodium Na Know Chemical Symbols in Table 1 on page 58

Classification of Elements – Metals and Non-metals There are two major groups of elements – metals and non-metals. Iron is a metal. Oxygen is a non-metal. Metals and non-metals are grouped separately on the Periodic Table. There are some elements called metalloids which behave like both metals and non-metals.

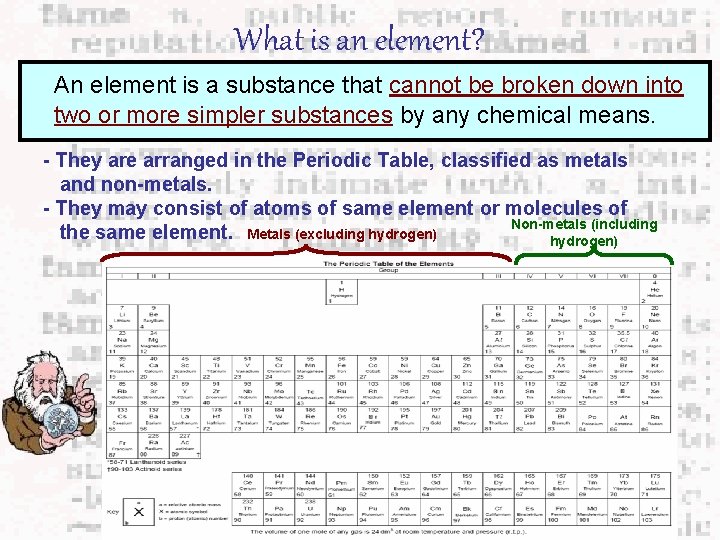
What is an element? An element is a substance that cannot be broken down into two or more simpler substances by any chemical means. - They are arranged in the Periodic Table, classified as metals and non-metals. - They may consist of atoms of same element or molecules of Non-metals (including the same element. Metals (excluding hydrogen)

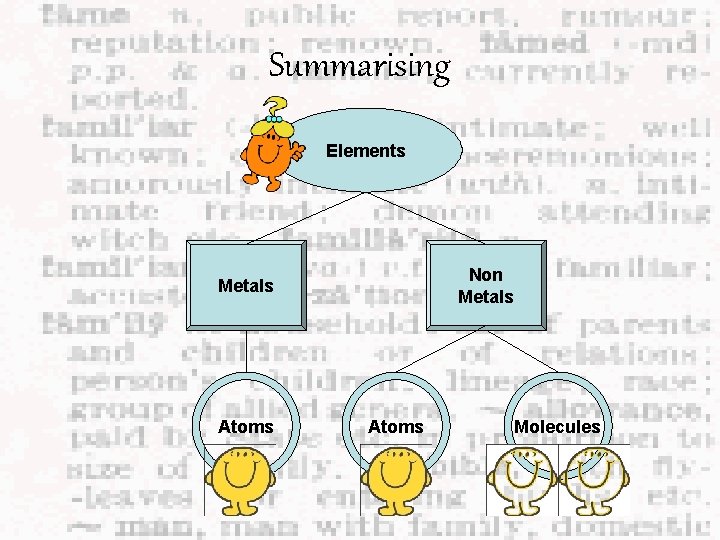
Summarising Elements Non Metals Atoms Molecules

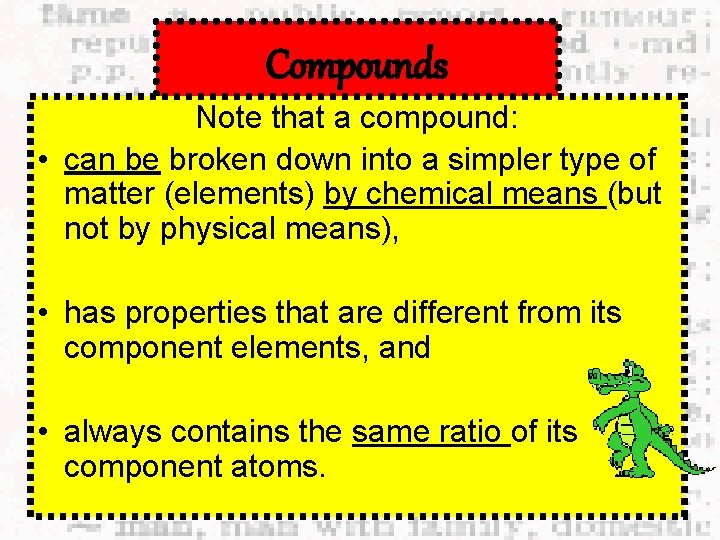
Compounds Note that a compound: • can be broken down into a simpler type of matter (elements) by chemical means (but not by physical means), • has properties that are different from its component elements, and • always contains the same ratio of its component atoms.

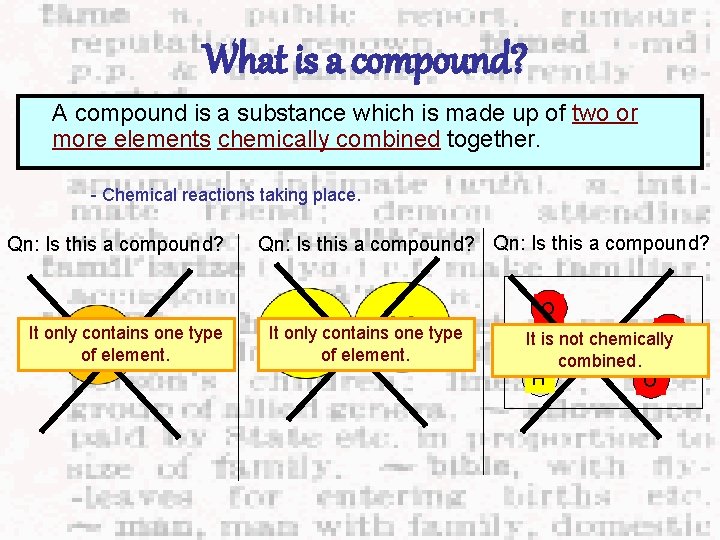
What is a compound? A compound is a substance which is made up of two or more elements chemically combined together. - Chemical reactions taking place. Qn: Is this a compound? O It only contains one type of element. O It is not chemically H combined. H O

So, what is a compound then? H Water Ammonia gas Consists of two or more elements And They are chemically combined together!

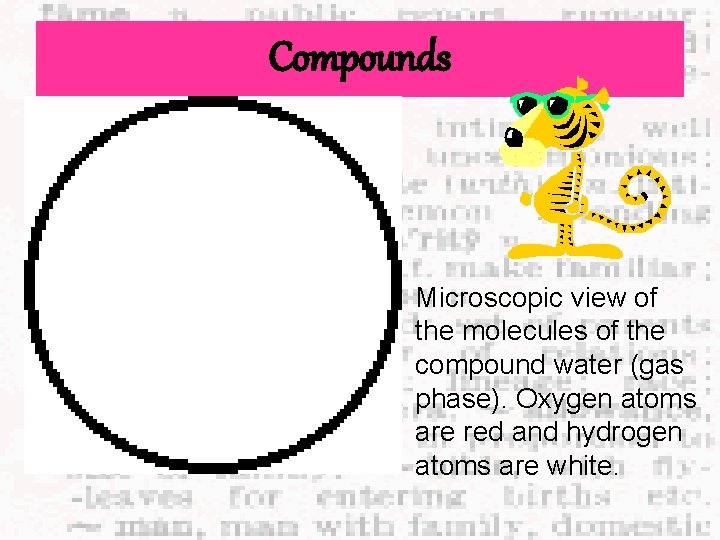
Compounds Microscopic view of the molecules of the compound water (gas phase). Oxygen atoms are red and hydrogen atoms are white.

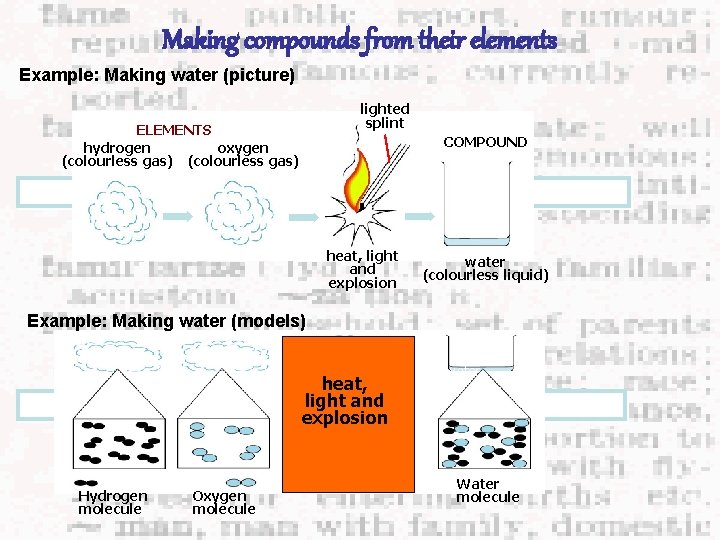
Making compounds from their elements Example: Making water (picture) lighted splint ELEMENTS hydrogen oxygen (colourless gas) COMPOUND heat, light and explosion water (colourless liquid) Example: Making water (models) mixture of hydrogen heat, and oxygen light and water explosion Hydrogen molecule Oxygen molecule Water molecule

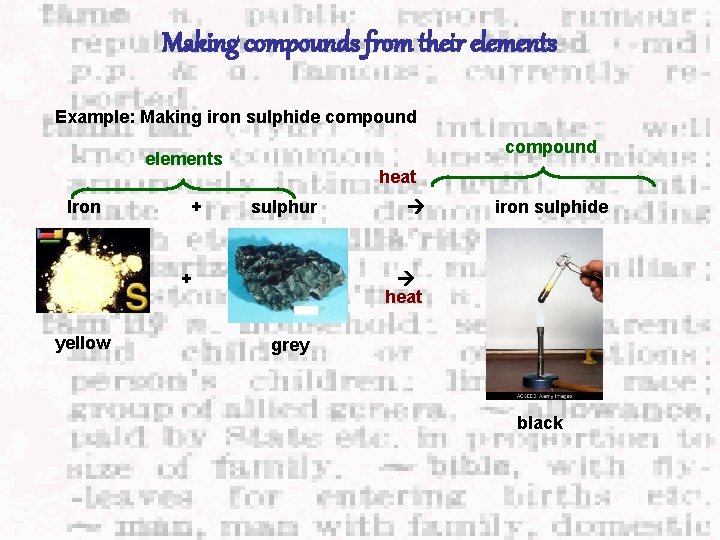
Making compounds from their elements Example: Making iron sulphide compound elements Iron + heat sulphur + yellow iron sulphide heat grey black

Summarizing • A compound is made up of two or more elements chemically joined together • A compound has a fixed composition • Every compound has a unique chemical formula • A compound has a completely different properties from its elements • A chemical reaction (decomposition or electrolysis) is needed to separate the elements in the compound

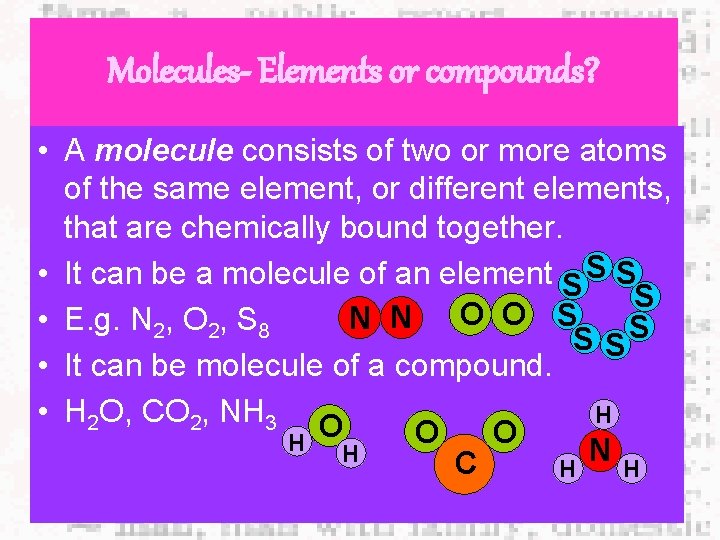
Molecules- Elements or compounds? • A molecule consists of two or more atoms of the same element, or different elements, that are chemically bound together. • It can be a molecule of an element S S N N O O S • E. g. N 2, O 2, S 8 S SS • It can be molecule of a compound. • H 2 O, CO 2, NH 3 O H O O H N H C H H

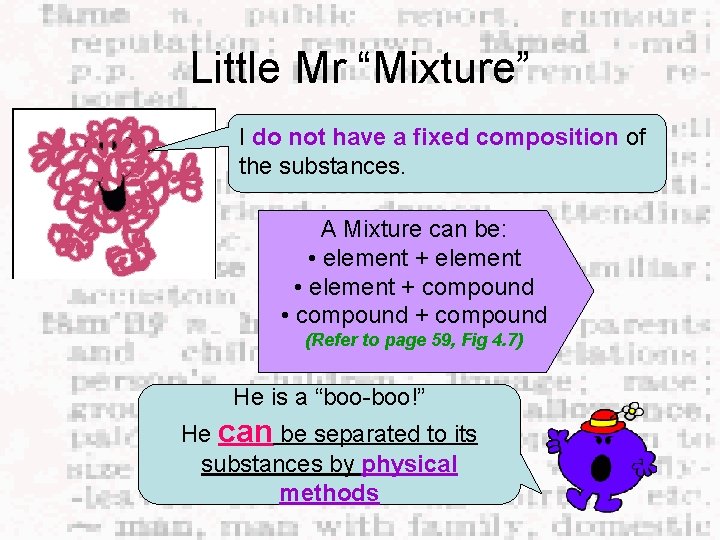
Introducing Little Mr. “Mixture” Burp!!!! I am messy! I am formed when two or more substances joined together physically (without chemical bonds) I have the same properties as all the substances I am his best friend! AIR

Mixtures Definition of a mixture: A mixture is not a pure substance as it contains a mixture of atoms of molecules which are not chemically combined together.

Mixtures • Note that a mixture: • consists of two or more different elements and/or compounds NOT chemically combined. • Can be homogeneous or non-homogeneous • can be separated into its components by physical means, and • often retains many of the properties of its components.

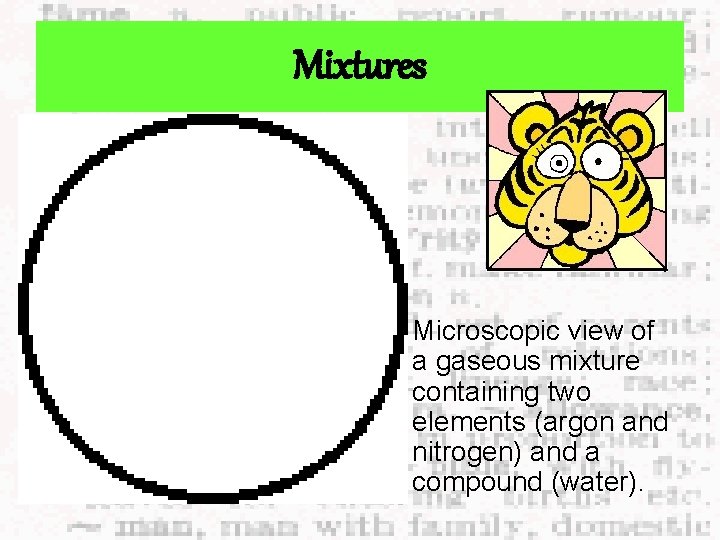
Mixtures Microscopic view of a gaseous mixture containing two elements (argon and nitrogen) and a compound (water).

Mixtures • Examples of mixtures include muddy water and air. Air is made up of gases such as nitrogen and oxygen mixed together.

Little Mr “Mixture” I do not have a fixed composition of the substances. A Mixture can be: • element + element • element + compound • compound + compound (Refer to page 59, Fig 4. 7) He is a “boo-boo!” He can be separated to its substances by physical methods

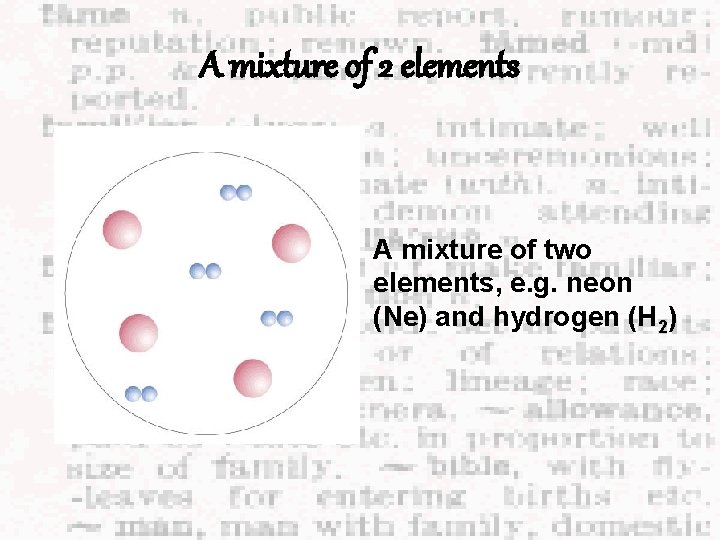
A mixture of 2 elements A mixture of two elements, e. g. neon (Ne) and hydrogen (H 2)

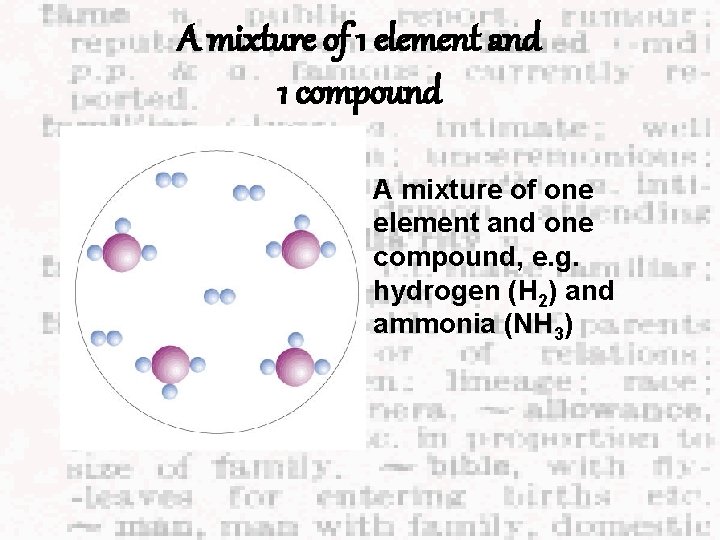
A mixture of 1 element and 1 compound A mixture of one element and one compound, e. g. hydrogen (H 2) and ammonia (NH 3)

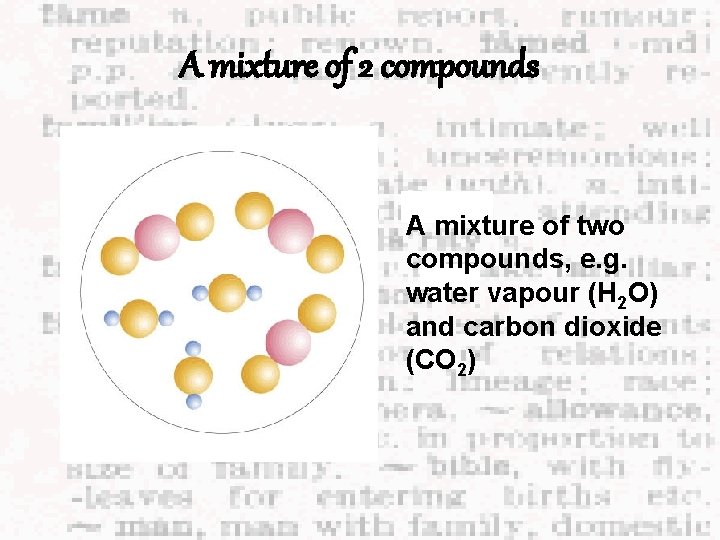
A mixture of 2 compounds A mixture of two compounds, e. g. water vapour (H 2 O) and carbon dioxide (CO 2)

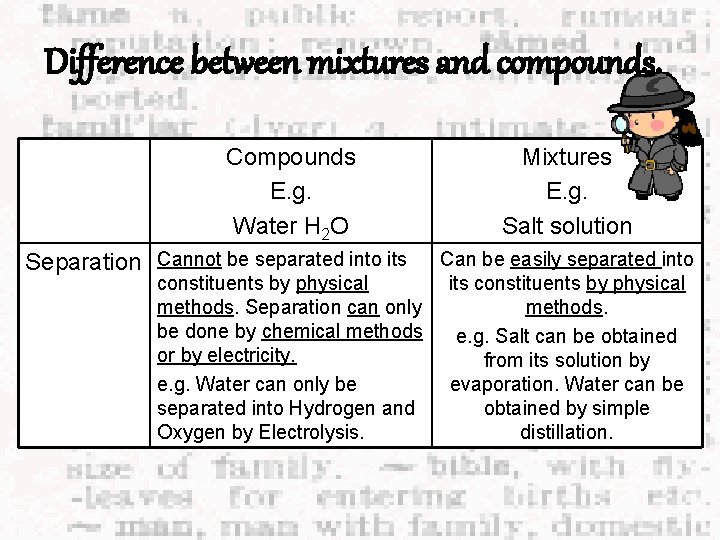
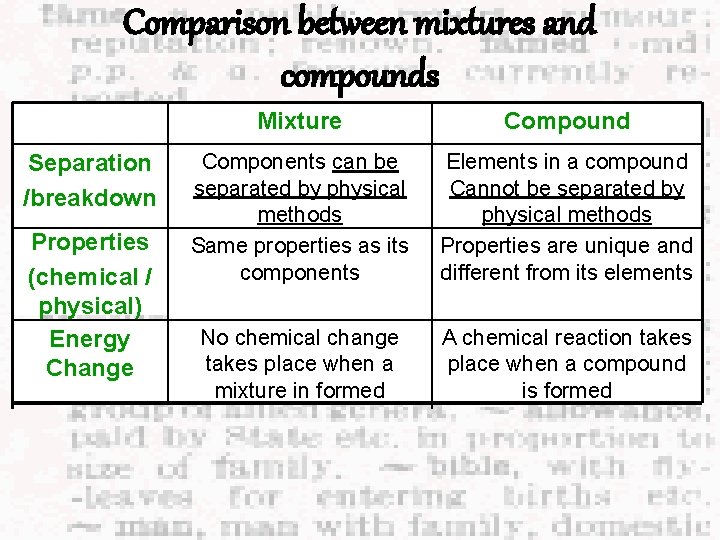
Difference between mixtures and compounds. Compounds E. g. Water H 2 O Separation Cannot be separated into its Mixtures E. g. Salt solution Can be easily separated into constituents by physical its constituents by physical methods. Separation can only methods. be done by chemical methods e. g. Salt can be obtained or by electricity. from its solution by e. g. Water can only be evaporation. Water can be separated into Hydrogen and obtained by simple Oxygen by Electrolysis. distillation.

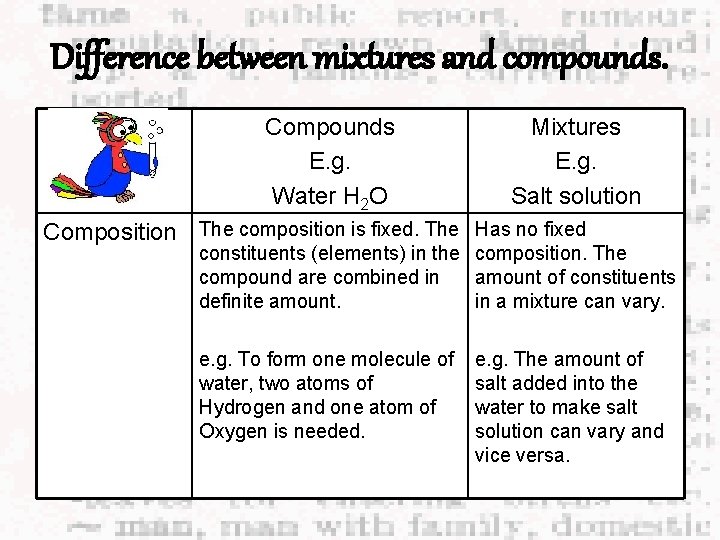
Difference between mixtures and compounds. Compounds E. g. Water H 2 O Mixtures E. g. Salt solution Composition The composition is fixed. The Has no fixed constituents (elements) in the composition. The compound are combined in amount of constituents definite amount. in a mixture can vary. e. g. To form one molecule of water, two atoms of Hydrogen and one atom of Oxygen is needed. e. g. The amount of salt added into the water to make salt solution can vary and vice versa.

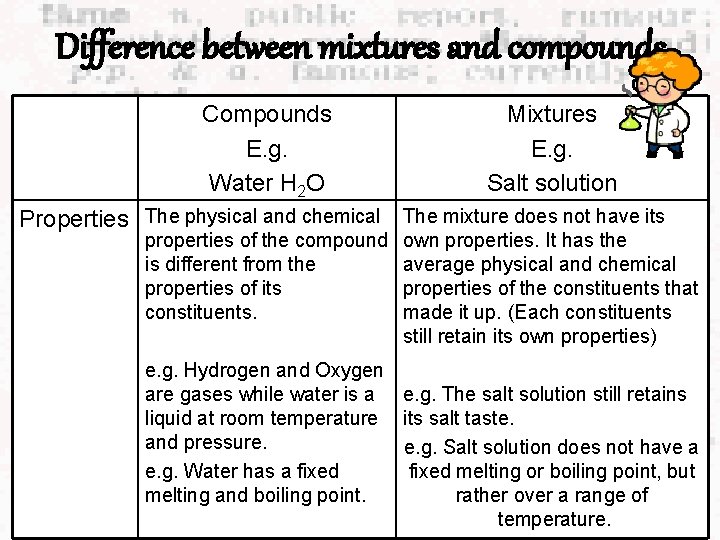
Difference between mixtures and compounds. Compounds E. g. Water H 2 O Mixtures E. g. Salt solution Properties The physical and chemical The mixture does not have its properties of the compound is different from the properties of its constituents. own properties. It has the average physical and chemical properties of the constituents that made it up. (Each constituents still retain its own properties) e. g. Hydrogen and Oxygen are gases while water is a e. g. The salt solution still retains liquid at room temperature its salt taste. and pressure. e. g. Salt solution does not have a e. g. Water has a fixed melting or boiling point, but melting and boiling point. rather over a range of temperature.

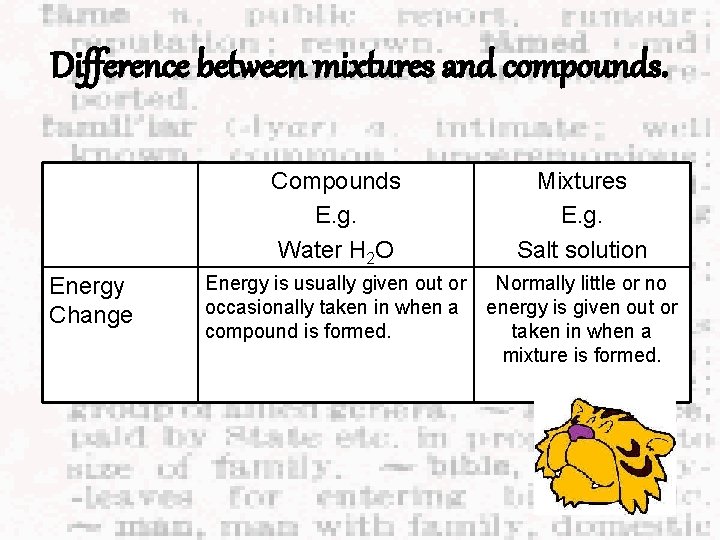
Difference between mixtures and compounds. Energy Change Compounds E. g. Water H 2 O Mixtures E. g. Salt solution Energy is usually given out or occasionally taken in when a compound is formed. Normally little or no energy is given out or taken in when a mixture is formed.

Differences between mixtures and compounds Laugh what? I know I am a little “bushy and hairy” than you… Mr Messy *giggles * He. E stupid… Little Miss Compound

Comparison between mixtures and compounds Separation /breakdown Properties (chemical / physical) Energy Change Mixture Compound Components can be separated by physical methods Same properties as its components Elements in a compound Cannot be separated by physical methods Properties are unique and different from its elements No chemical change takes place when a mixture in formed A chemical reaction takes place when a compound is formed

Challenge Time • Is mineral water an element, mixture or compound?

Challenge Time • Are YOU an element, mixture or compound?

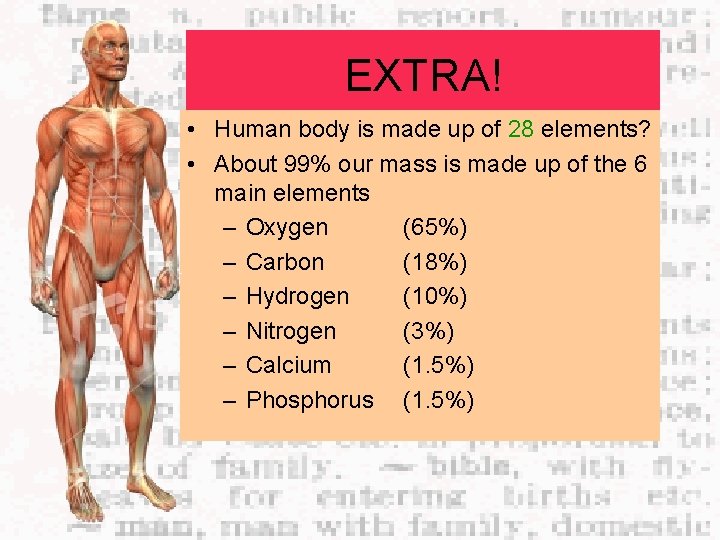
EXTRA! • Human body is made up of 28 elements? • About 99% our mass is made up of the 6 main elements – Oxygen (65%) – Carbon (18%) – Hydrogen (10%) – Nitrogen (3%) – Calcium (1. 5%) – Phosphorus (1. 5%)

The End……