CHAPTER 2 ELECTROLYTE SOLUTION 2 1 Strong and






![H+ Ac- + H 2 O HAc + OH- [HAc][OH-] K b = ────── H+ Ac- + H 2 O HAc + OH- [HAc][OH-] K b = ──────](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-22.jpg)




![Kw = [H 3 O+][OH-] Where Kw is the equilibrium constant for water (unitless) Kw = [H 3 O+][OH-] Where Kw is the equilibrium constant for water (unitless)](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-29.jpg)

![When: c/Ka ≥ 103, or α≤ 5%, c - [H+] ≈ c thus, Similarly, When: c/Ka ≥ 103, or α≤ 5%, c - [H+] ≈ c thus, Similarly,](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-33.jpg)
![● Mg(OH) 2 (s) Mg 2+ + 2 OH- Ksp = [M g 2+][OH-]2 ● Mg(OH) 2 (s) Mg 2+ + 2 OH- Ksp = [M g 2+][OH-]2](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-35.jpg)



- Slides: 45

CHAPTER 2 ELECTROLYTE SOLUTION 2 -1 Strong and Weak Electrolyte Solution 2 -2 Theory of Acid-base 2 -3 Acidity and Calculation of Solution 2 -4 Equilibrium Between Dissolution and Precipitation 1

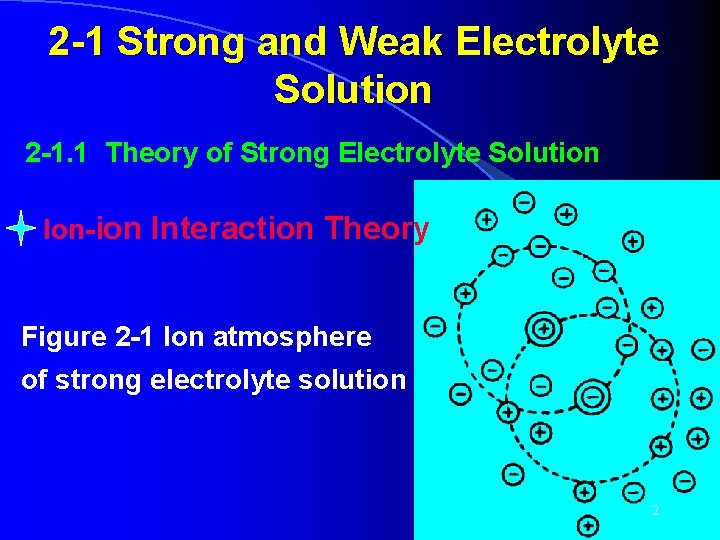
2 -1 Strong and Weak Electrolyte Solution 2 -1. 1 Theory of Strong Electrolyte Solution Ion-ion Interaction Theory Figure 2 -1 Ion atmosphere of strong electrolyte solution 2

Ion Activity and Activity Coefficient Activity(a): Ion concentration, which can play a real action in solution is ionic effective concentration, is called ion activity. actual concentration of ion (c) multiply a correction factor - activity coefficient ( f ). a = f ·c (2 -1) Generally, a<c, 0<f< 1 3

Activity coefficient are influenced by ion concentration the electric-charge number of ion has nothing to do with the nature of ion. 4

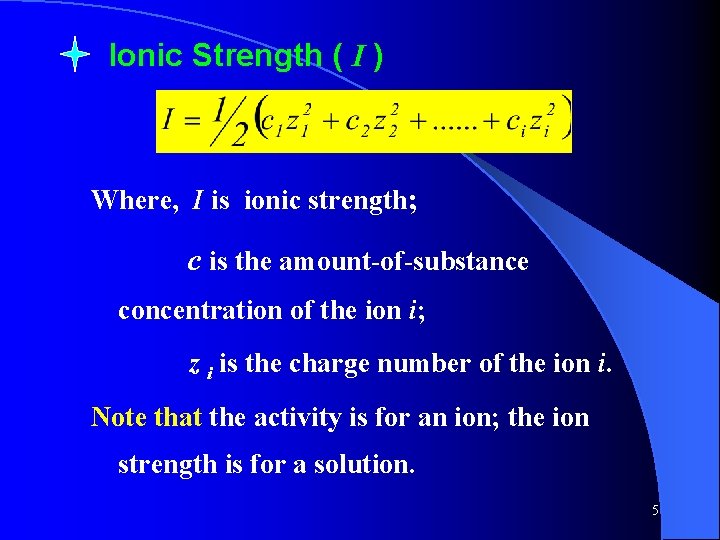
Ionic Strength ( I ) Where, I is ionic strength; c is the amount-of-substance concentration of the ion i; z i is the charge number of the ion i. Note that the activity is for an ion; the ion strength is for a solution. 5

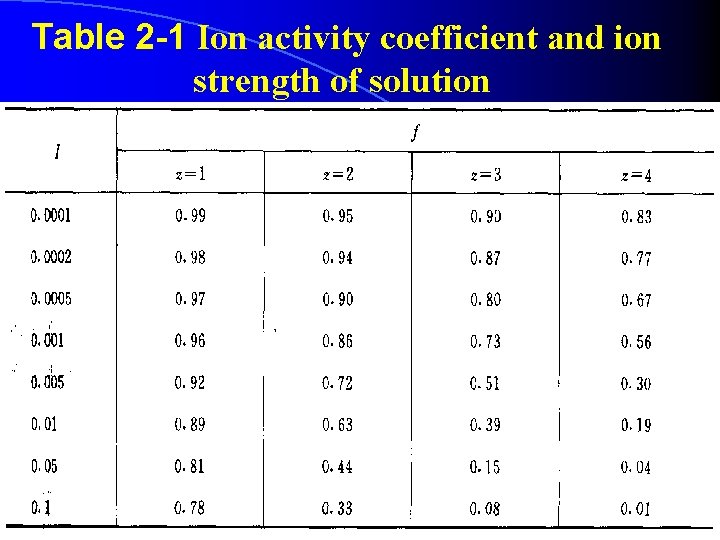
Table 2 -1 Ion activity coefficient and ion strength of solution 6

Example 2 -1 25 ml 0. 02 mol /L HCl mixed with 25 ml 0. 18 mol /L KCl, calculate activity of H+ ? Solution: (1) calculate the ion strength of the solution: I =( 0. 01× 12+0. 09× 12)/2 =0. 1 (2) look up the activity coefficient of ion has one charge: when I = 0. 1, Z =1, f = 0. 78 (3) calculate the activity of H+ (a. H+) c. H+ =0. 02/2 =0. 01 mol /L, f = 0. 78 So, a. H+ = f ·c = 0. 78× 0. 01 = 0. 0078 (mol /L) 7

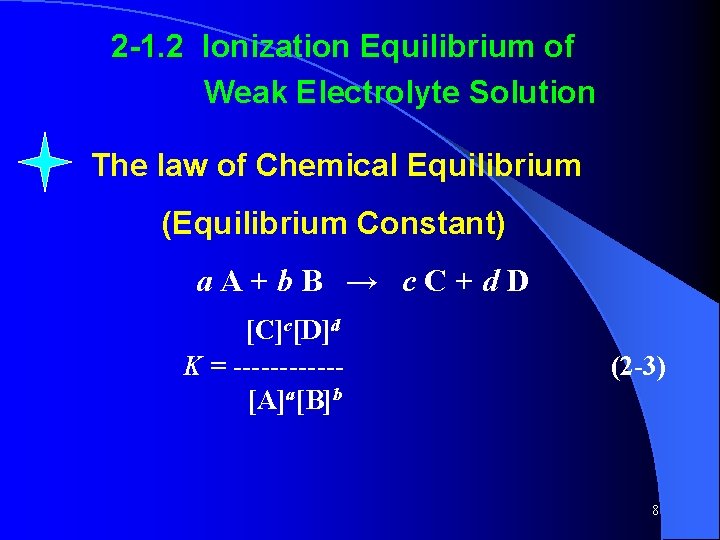
2 -1. 2 Ionization Equilibrium of Weak Electrolyte Solution The law of Chemical Equilibrium (Equilibrium Constant) a. A+b. B → c. C+d. D [C]c[D]d K = ------[A]a[B]b (2 -3) 8

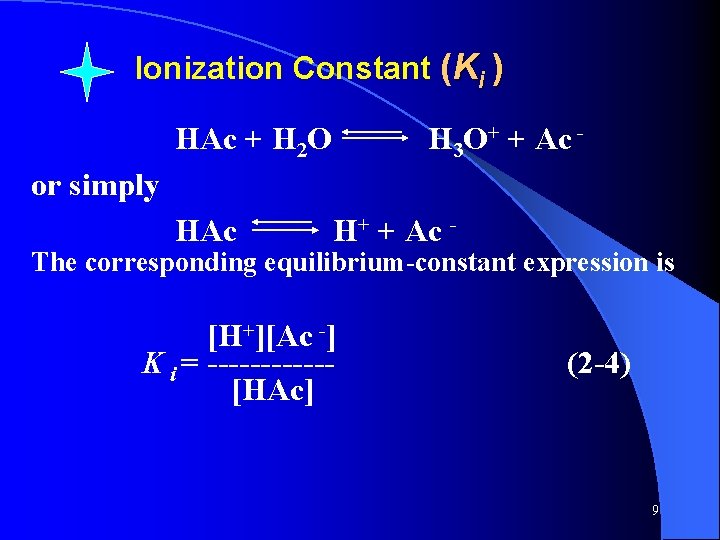
Ionization Constant (Ki ) HAc + H 2 O H 3 O+ + Ac - or simply HAc H+ + Ac - The corresponding equilibrium-constant expression is [H+][Ac -] K i = ------[HAc] (2 -4) 9

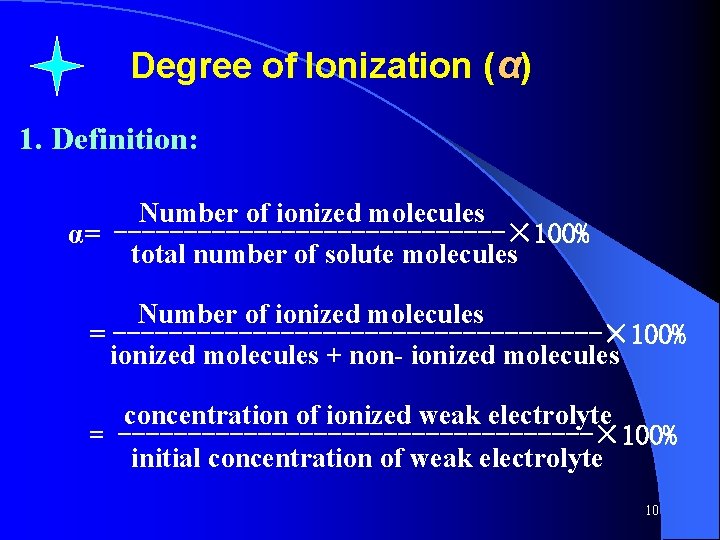
Degree of Ionization (α) 1. Definition: Number of ionized molecules α= --------------× 100% total number of solute molecules Number of ionized molecules = ------------------× 100% ionized molecules + non- ionized molecules concentration of ionized weak electrolyte = -----------------× 100% initial concentration of weak electrolyte 10

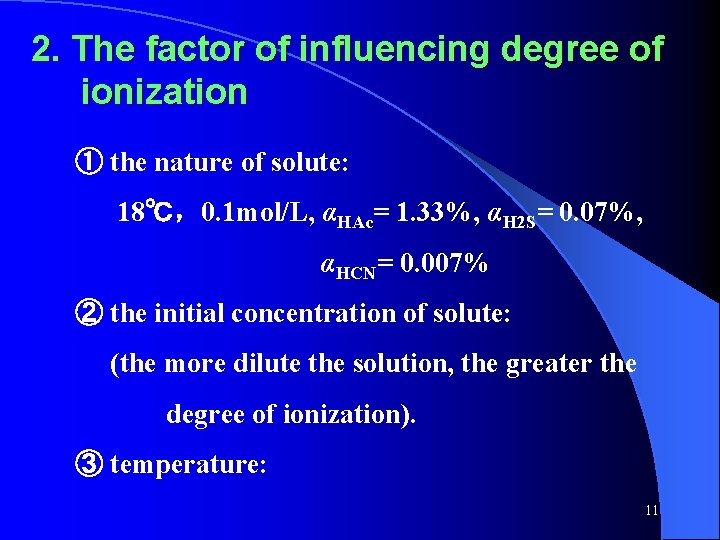
2. The factor of influencing degree of ionization ① the nature of solute: 18℃,0. 1 mol/L, αHAc= 1. 33%, αH 2 S= 0. 07%, αHCN= 0. 007% ② the initial concentration of solute: (the more dilute the solution, the greater the degree of ionization). ③ temperature: 11

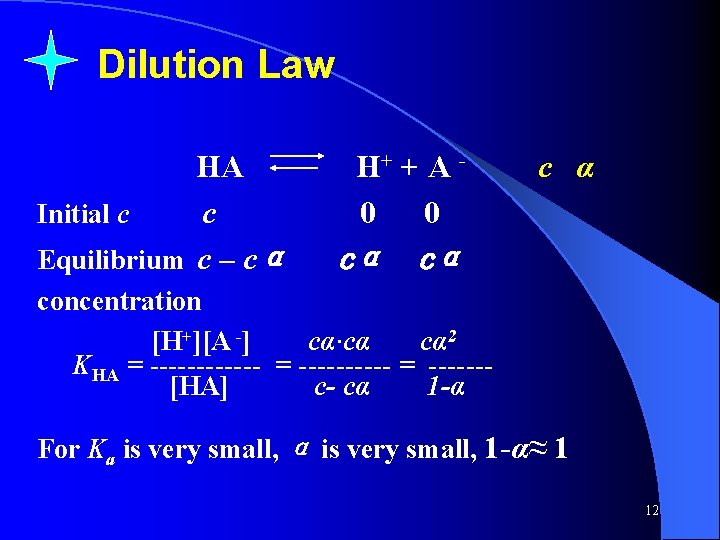
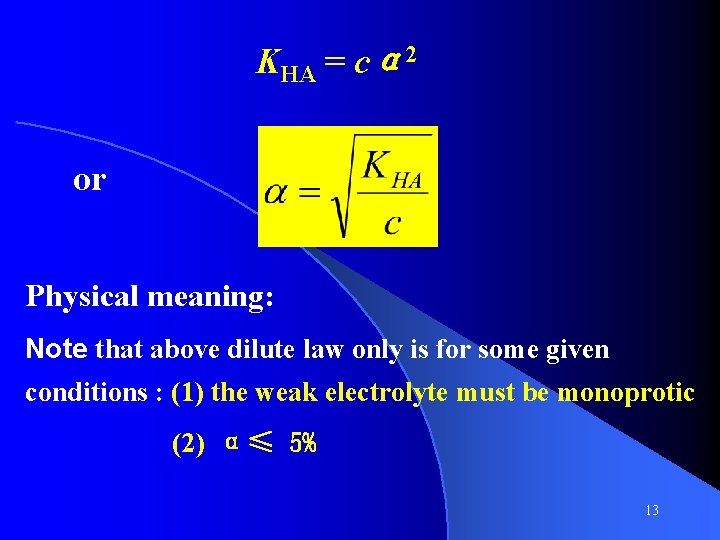
Dilution Law HA Initial c c Equilibrium c – cα H+ + A 0 0 c α cα cα concentration [H+][A -] cα·cα cα 2 KHA = ------ = ------[HA] c- cα 1 -α For Ka is very small, α is very small, 1 -α≈ 1 12

KHA = cα 2 or Physical meaning: Note that above dilute law only is for some given conditions : (1) the weak electrolyte must be monoprotic (2) α≤ 5% 13

The Common Ion Effect and Salt Effect 1. Common ion effect The ionization of a weak electrolyte is markedly decreased by the adding to the solution an ionic compound containing one of the ion of the weak electrolyte, this effect is called the common ion effect. For example, HAc Ac - + H+ Na. Ac → Ac – + Na+ shift the equilibrium from right to left, decreasing the [H+]. 14

2. Salt effect The ionization of a weak electrolyte is increased by adding to the solution an soluble strong electrolyte which not contains the common ion with the weak electrolyte. This effect is called salt effect. For example: 0. 1 mol/L [H Ac] α= 1. 33%, adding Na. Cl, [Na. Cl]= 0. 1 mol/L, α= 1. 68% 15

Example: There is a solution of c(HAc) =0. 1 mol/L, if we add Na. Ac, when c(Na. Ac)=0. 1 mol/L. Calculate the α of HAc. Solution: HAc H+ + Ac Initial c 0. 1 0 0 Equilibrium c 0. 1 -[H+ ] 0. 1+ [H+ ] ≈0. 1 [H+][Ac-] [H+]× 0. 1 Ka= ------- = [H+] = 1. 8× 10 -5 [H Ac] 0. 1 α= [H+]/[HAc] = 0. 018% << 1. 33% 16

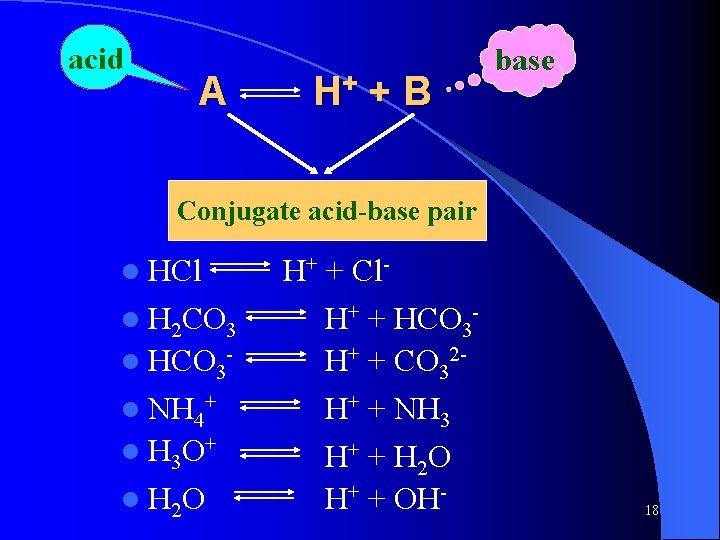
2 -2 Theory of Acid-base 2 -2. 2 Bronsted-Lowry Acids and Bases 1. Definition of acid and base Acid - is a substance capable of donating a proton. HCl, NH 4+, HSO 4 -, H 2 O Base - is a substance capable of accepting a proton. Cl-, NH 3, HSO 4 -, OH 17

acid A H+ + B base Conjugate acid-base pair l HCl l H 2 CO 3 l HCO 3 l NH 4+ l H 3 O+ l H 2 O H+ + Cl. H+ + HCO 3 H+ + CO 32 H+ + NH 3 H+ + H 2 O H+ + OH- 18

Conclusion: l Acid or base may be a molecule, atom, or ion. l Some molecules or ions are capable of donating a proton, and also accepting a proton, which named ampholyte. l There are no concepts of salt in acid-base proton theory. 19

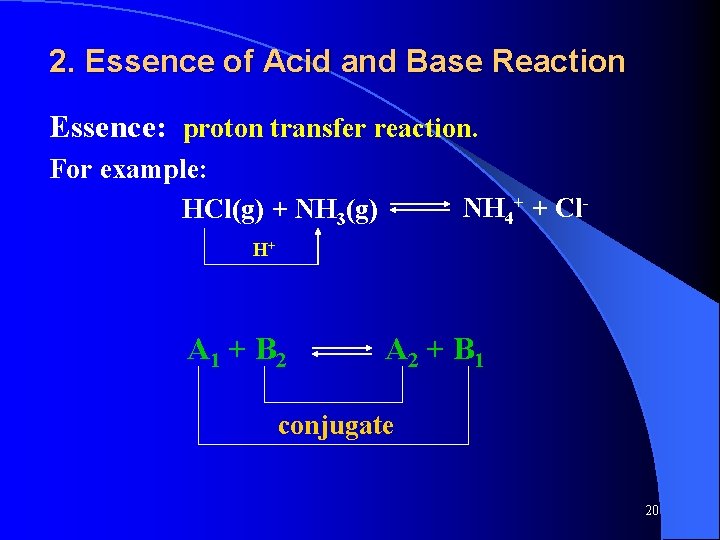
2. Essence of Acid and Base Reaction Essence: proton transfer reaction. For example: HCl(g) + NH 3(g) NH 4+ + Cl- H+ A 1 + B 2 A 2 + B 1 conjugate 20

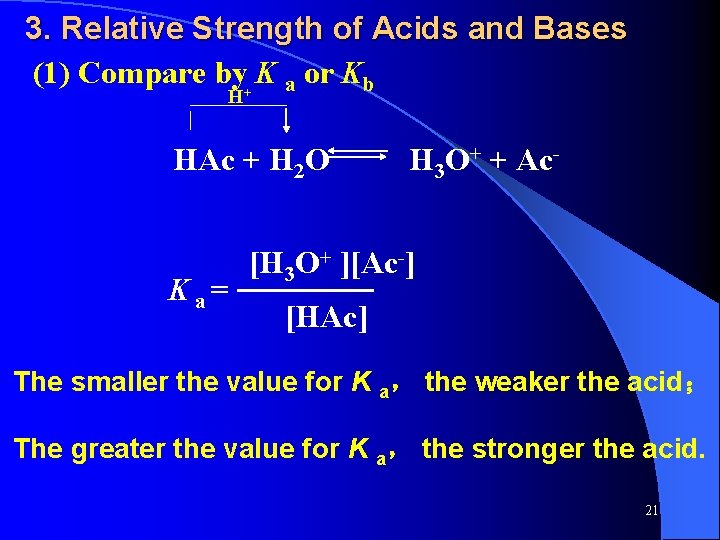
3. Relative Strength of Acids and Bases (1) Compare by+ K a or Kb H HAc + H 2 O H 3 O+ + Ac- [H 3 O+ ][Ac-] K a = ────── [HAc] The smaller the value for K a, the weaker the acid; The greater the value for K a, the stronger the acid. 21
![H Ac H 2 O HAc OH HAcOH K b H+ Ac- + H 2 O HAc + OH- [HAc][OH-] K b = ──────](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-22.jpg)
H+ Ac- + H 2 O HAc + OH- [HAc][OH-] K b = ────── [Ac -] The smaller the value for Kb, the weaker the base; The greater the value for Kb, the stronger the base. 22

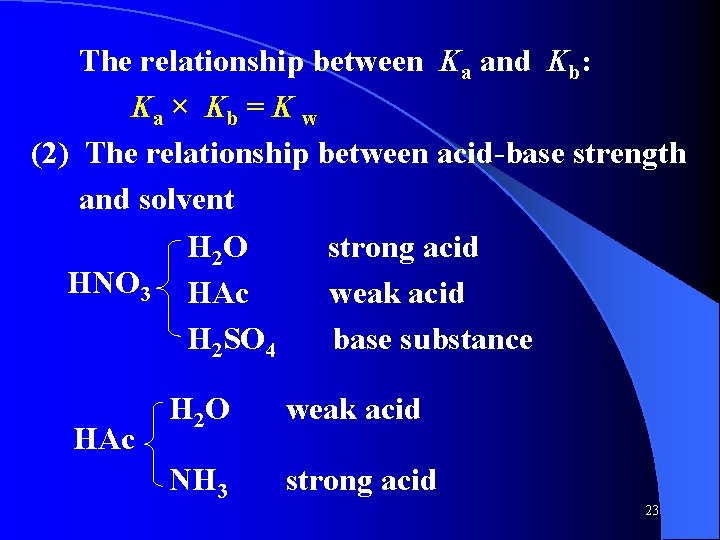
The relationship between Ka and Kb: Ka × Kb = K w (2) The relationship between acid-base strength and solvent H 2 O strong acid HNO 3 HAc weak acid H 2 SO 4 base substance HAc H 2 O weak acid NH 3 strong acid 23

4. The Leveling Effect and Differentiating Effect The leveling effect: The inability of a solvent to differentiate among the relative strengths of all acids stronger than the solvent’s conjugate acid is known as the leveling effect. Because the solvent is said to level the strengths of these acids, making them seen identical. leveling solvent: Strong acid such as HCl. O 4, HCl, HNO 3, H 2 SO 4 will appear to be of equal strength in aqueous solution. 24

• For strong acid or base Strong acids--hydrochloric acid (HCl), nitric acid (HNO 3), perchloric acid (HCl. O 4), and sulfuric acid (H 2 SO 4), for example- are all strong electrolytes. They may be assumed to be completely ionized in water. HCl(a q) + H 2 O(1) → H 3 O+(aq) + Cl - (aq) HNO 3(a q) + H 2 O(1) → H 3 O+(aq) + NO 3 -(aq) HCl. O 4(a q) + H 2 O(1) → H 3 O+(aq) + Cl. O 4 - (aq) H 2 SO 4(a q) + H 2 O(1) → H 3 O+(aq) + HSO 4 - (aq) we can not determine their strength, because H 3 O+ is the strongest acid that can exist in aqueous solution. 25

The differentiating effect: The ability of a solvent to differentiate among the relative strengths of all acids stronger than the solvent’s conjugate acid is known as the differentiating effect. If we use a more weakly basic solvent like acetic acid, acetic acid can function as a base by accepting a proton. Since acetic acid is a much weaker base than water, it is not as easily protonated. Thus there appreciable differences in the extent. 26

In acetic acid solvent, their relative strength increase as follows: HNO 3 <H 2 SO 4 <HCl< HCl. O 4 HCl(aq) + CH 3 COOH(l) CH 3 COOH 2+(aq) + Cl - (aq) HNO 3(aq) + CH 3 COOH (l) CH 3 COOH 2+ (aq) + NO 3 -(aq) HCl. O 4(aq)+ CH 3 COOH (l) CH 3 COOH 2+ (aq) + Cl. O 4 - (aq) H 2 SO 4(aq)+ CH 3 COOH (l) CH 3 COOH 2+ (aq) + HSO 4 - (aq) 27

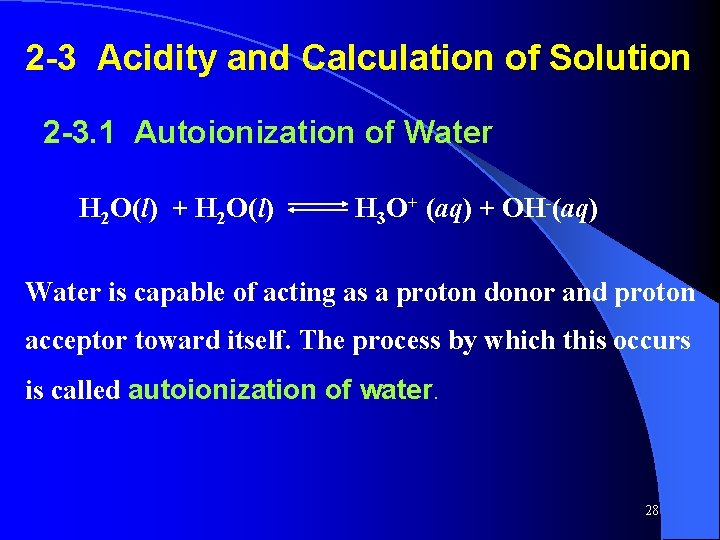
2 -3 Acidity and Calculation of Solution 2 -3. 1 Autoionization of Water H 2 O(l) + H 2 O(l) H 3 O+ (aq) + OH-(aq) Water is capable of acting as a proton donor and proton acceptor toward itself. The process by which this occurs is called autoionization of water. 28
![Kw H 3 OOH Where Kw is the equilibrium constant for water unitless Kw = [H 3 O+][OH-] Where Kw is the equilibrium constant for water (unitless)](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-29.jpg)
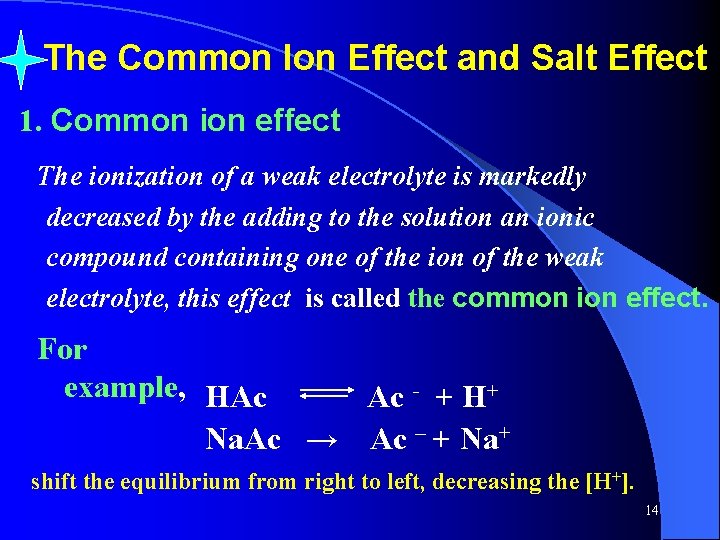
Kw = [H 3 O+][OH-] Where Kw is the equilibrium constant for water (unitless) , is called ion product of water or autoionization equilibrium constant. At 25℃, Kw = [H 3 O+][OH -] = 1. 0 × 10 -14 [H+] > [OH-] in acid solutions [H+] < [OH-] in basic solutions [H+] = [OH-] in neutral solutions 29

2 -3. 2 Acidity of solution p. H = - log a. H+ = -log [H 3 O+] p. OH = - log a. OH- = -log[OH-] For, [H+][OH-] = Kw= 1× 10 -14 So, p. H + p. OH =p. Kw= 14. 00 In neutral solutions, p. H= 7=p. OH, In acid solutions, p. H < 7 <p. OH In basic solutions, p. H > 7 >p. OH 30

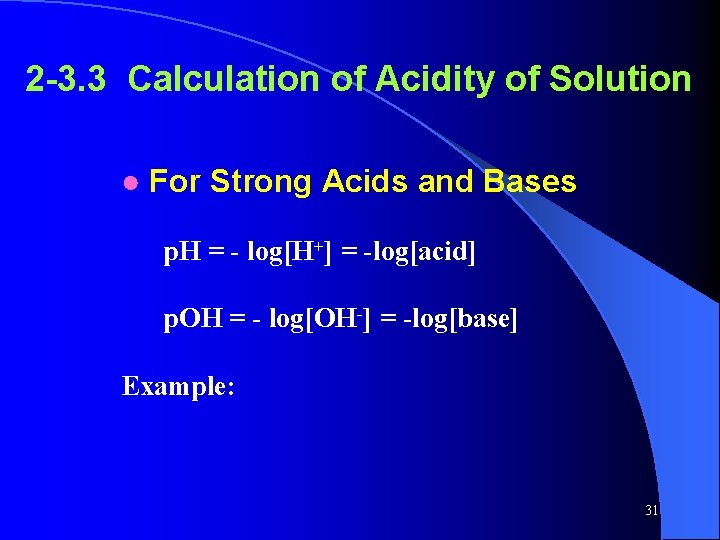
2 -3. 3 Calculation of Acidity of Solution ● For Strong Acids and Bases p. H = - log[H+] = -log[acid] p. OH = - log[OH-] = -log[base] Example: 31

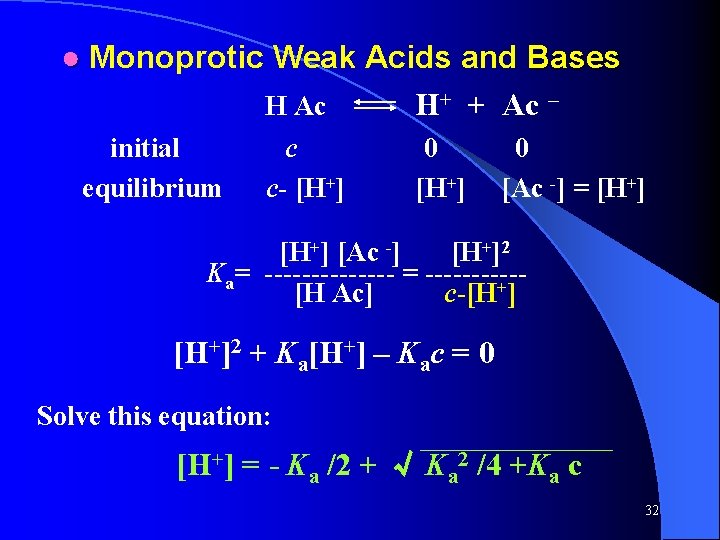
● Monoprotic Weak Acids and Bases H Ac H+ + Ac – initial equilibrium c c- [H+] 0 [Ac -] = [H+] [Ac -] [H+]2 Ka= ------- = -----[H Ac] c-[H+]2 + Ka[H+] – Kac = 0 Solve this equation: [H+] = - Ka /2 + √ Ka 2 /4 +Ka c 32
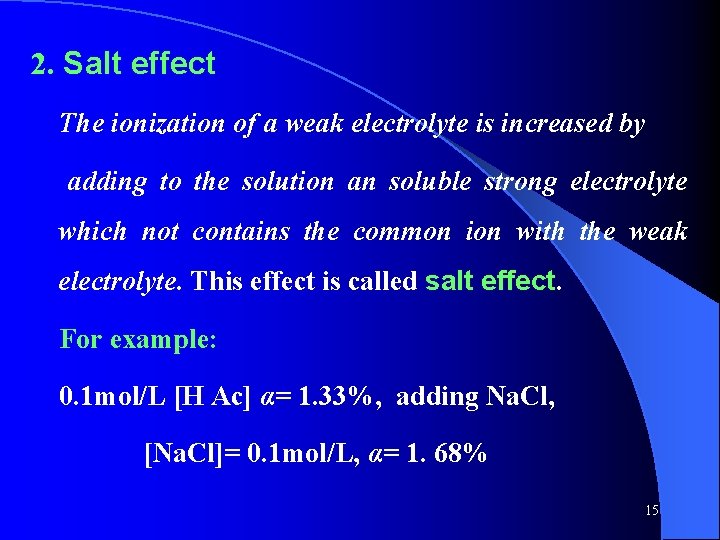
![When cKa 103 or α 5 c H c thus Similarly When: c/Ka ≥ 103, or α≤ 5%, c - [H+] ≈ c thus, Similarly,](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-33.jpg)
When: c/Ka ≥ 103, or α≤ 5%, c - [H+] ≈ c thus, Similarly, for weak base , there is an equation: Note that above equation is limited for some given condition: Example 4 -5: P 41 33

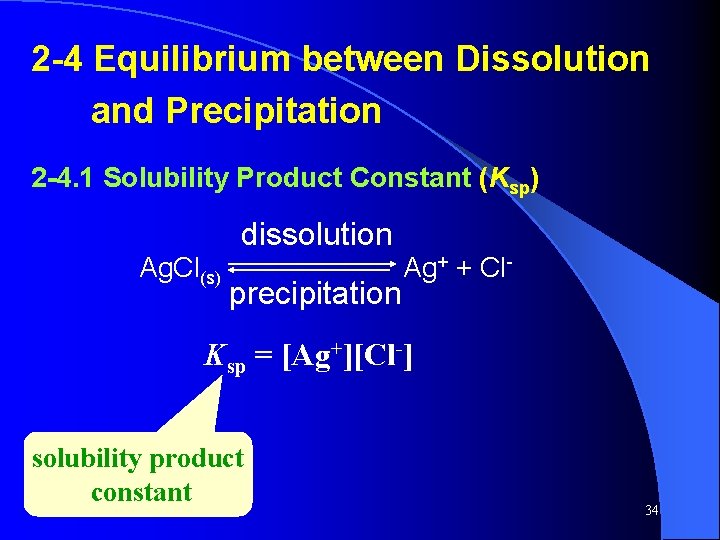
2 -4 Equilibrium between Dissolution and Precipitation 2 -4. 1 Solubility Product Constant (Ksp) dissolution Ag. Cl(s) precipitation Ag+ + Cl- Ksp = [Ag+][Cl-] solubility product constant 34
![MgOH 2 s Mg 2 2 OH Ksp M g 2OH2 ● Mg(OH) 2 (s) Mg 2+ + 2 OH- Ksp = [M g 2+][OH-]2](https://slidetodoc.com/presentation_image_h2/0f4d048a5a28375e8199e5dbdeec41b3/image-35.jpg)
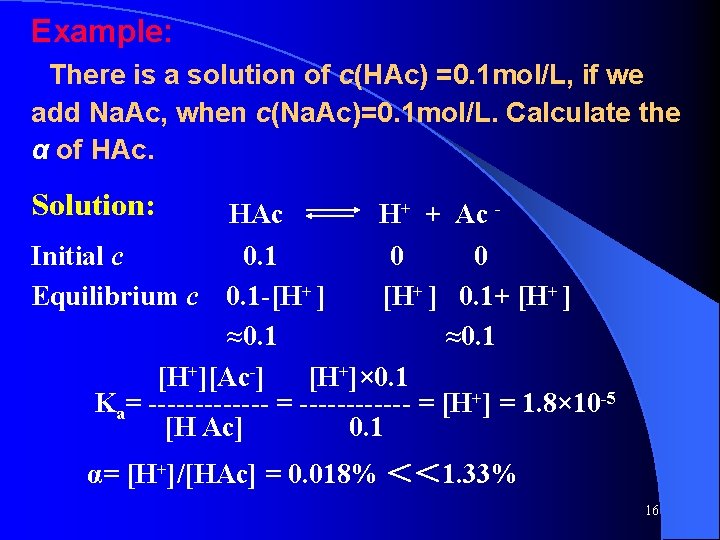
● Mg(OH) 2 (s) Mg 2+ + 2 OH- Ksp = [M g 2+][OH-]2 ● Ag 2 Cr. O 4 (s) 2 Ag+ + Cr. O 42 - Ksp = [A g+]2[Cr. O 42 -] ● Fe(OH) 3 (s) Fe 3+ + 3 OH- Ksp = [Fe 3+][OH-]3 ● Am. Bn(s) m. An+ + n. Bm- Ksp = [An+]m [Bm-]n 35

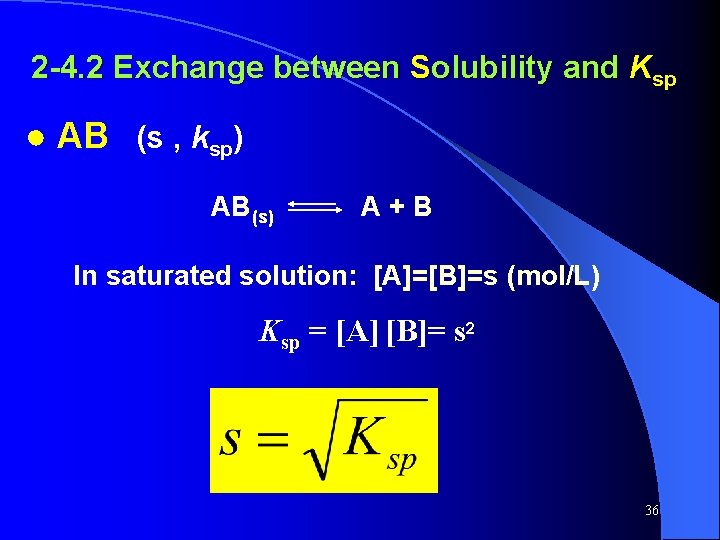
2 -4. 2 Exchange between Solubility and Ksp ● AB (s , ksp) AB(s) A+B In saturated solution: [A]=[B]=s (mol/L) Ksp = [A] [B]= s 2 36

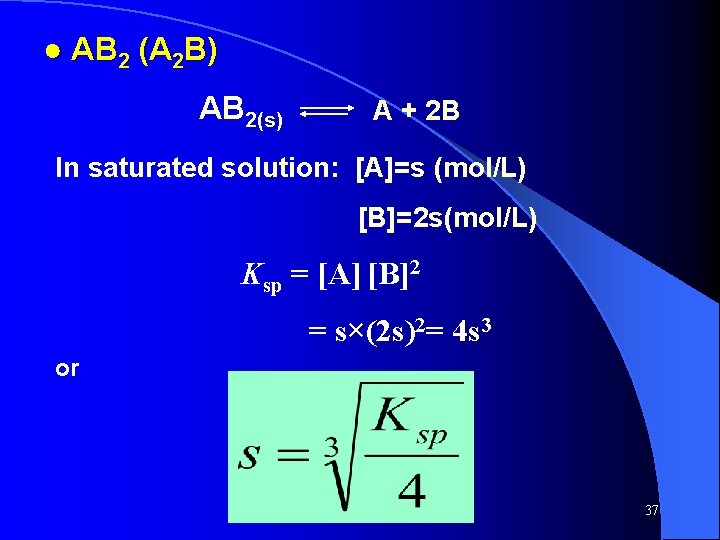
● AB 2 (A 2 B) AB 2(s) A + 2 B In saturated solution: [A]=s (mol/L) [B]=2 s(mol/L) Ksp = [A] [B]2 = s×(2 s)2= 4 s 3 or 37

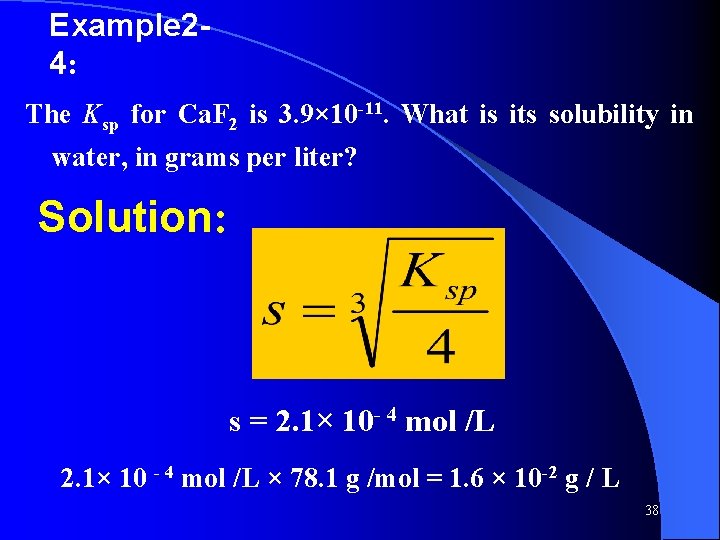
Example 24: The Ksp for Ca. F 2 is 3. 9× 10 -11. What is its solubility in water, in grams per liter? Solution: s = 2. 1× 10 - 4 mol /L 2. 1× 10 - 4 mol /L × 78. 1 g /mol = 1. 6 × 10 -2 g / L 38

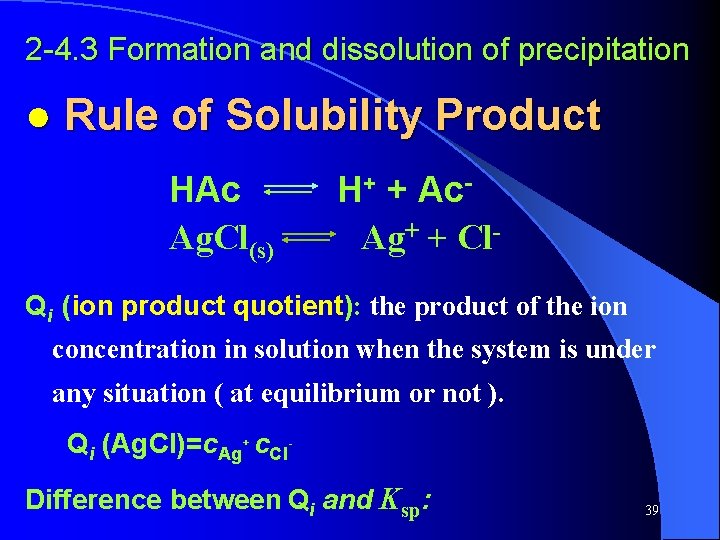
2 -4. 3 Formation and dissolution of precipitation ● Rule of Solubility Product HAc Ag. Cl(s) H+ + Ac. Ag+ + Cl- Qi (ion product quotient): the product of the ion concentration in solution when the system is under any situation ( at equilibrium or not ). Qi (Ag. Cl)=c. Ag+ c. Cl. Difference between Qi and Ksp: 39

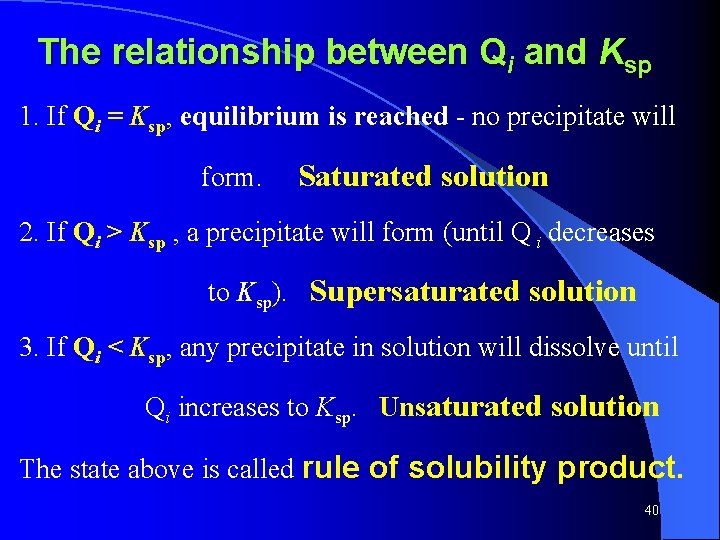
The relationship between Qi and Ksp 1. If Qi = Ksp, equilibrium is reached - no precipitate will form. Saturated solution 2. If Qi > Ksp , a precipitate will form (until Q i decreases to Ksp). Supersaturated solution 3. If Qi < Ksp, any precipitate in solution will dissolve until Qi increases to Ksp. Unsaturated solution The state above is called rule of solubility product. 40

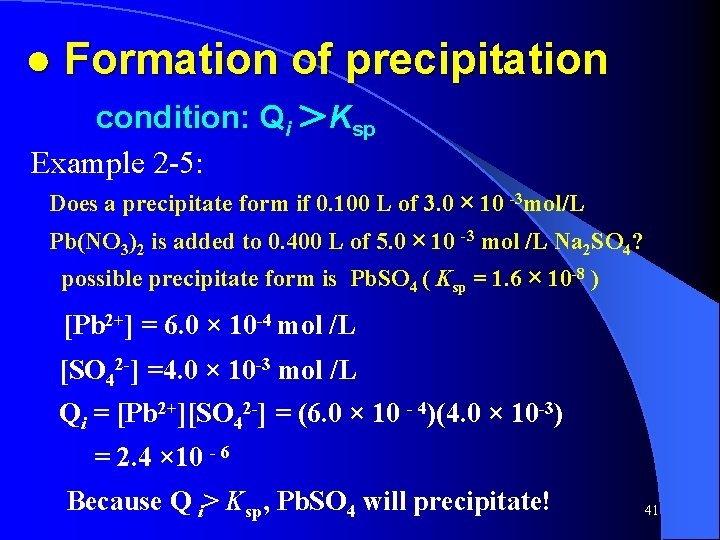
● Formation of precipitation condition: Qi >Ksp Example 2 -5: Does a precipitate form if 0. 100 L of 3. 0 × 10 -3 mol/L Pb(NO 3)2 is added to 0. 400 L of 5. 0 × 10 -3 mol /L Na 2 SO 4? possible precipitate form is Pb. SO 4 ( Ksp = 1. 6 × 10 -8 ) [Pb 2+] = 6. 0 × 10 -4 mol /L [SO 42 -] =4. 0 × 10 -3 mol /L Qi = [Pb 2+][SO 42 -] = (6. 0 × 10 - 4)(4. 0 × 10 -3) = 2. 4 × 10 - 6 Because Q i> Ksp, Pb. SO 4 will precipitate! 41

● Dissolution of precipitation condition: Qi < Ksp (ion product < solubility product) There are several methods to dissolve precipitation. 1. Forming weak electrolytes by adding some compounds make precipitation dissolve. 42

For example: Mg(OH)2 not only dissolve in acid, but also dissolve in NH 4 Cl solution. Mg(OH)2 + 2 HCl = Mg. Cl 2 + 2 H 2 O Mg 2+ + 2 OHMg(OH)2(s) + 2 HCl 2 Cl- + 2 H 2 O Because formed weak electrolyte H 2 O, [OH-] decrease, shift the equilibrium from left to right. 43

2. Forming coordination compounds by adding some agents make precipitation dissolve. For example, Ag. Cl precipitation dissolve in NH 3·H 2 O. Ag. Cl(s) + 2 NH 3 = [A g(NH 3)2 ]+ + Cl- Ag. Cl(s) Ag+ + Cl+ 2 NH 3 [A g(NH 3)2 ]+ 44

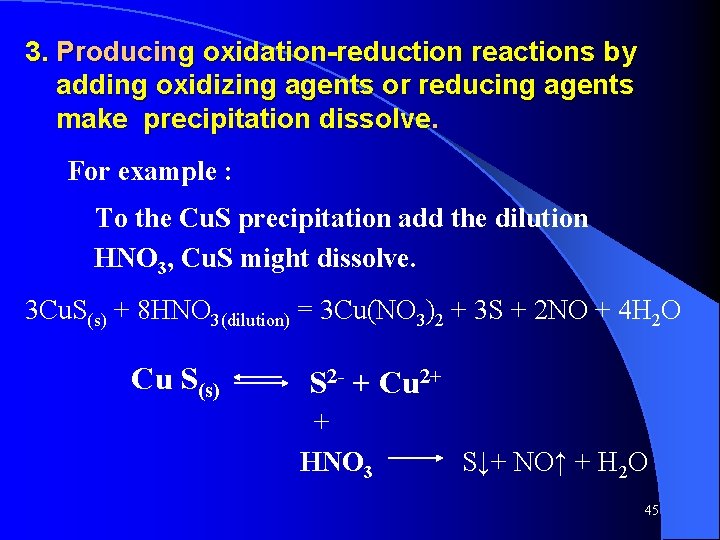
3. Producing oxidation-reduction reactions by adding oxidizing agents or reducing agents make precipitation dissolve. For example : To the Cu. S precipitation add the dilution HNO 3, Cu. S might dissolve. 3 Cu. S(s) + 8 HNO 3(dilution) = 3 Cu(NO 3)2 + 3 S + 2 NO + 4 H 2 O Cu S(s) S 2 - + Cu 2+ + HNO 3 S↓+ NO↑ + H 2 O 45