Chapter 2 Differential gene expression in Development Based

Chapter 2. Differential gene expression in Development Based on the basic assumtion, “Genomic equivalence”, scientist have asked “ how nuclear genes can direct development when these genes are exactly the same in every cell type? ” The answers are 1. Differentail gene expression 2. Selective nuclear RNA processing 3. Selective messenger RNA translation 4. Differential protein modification
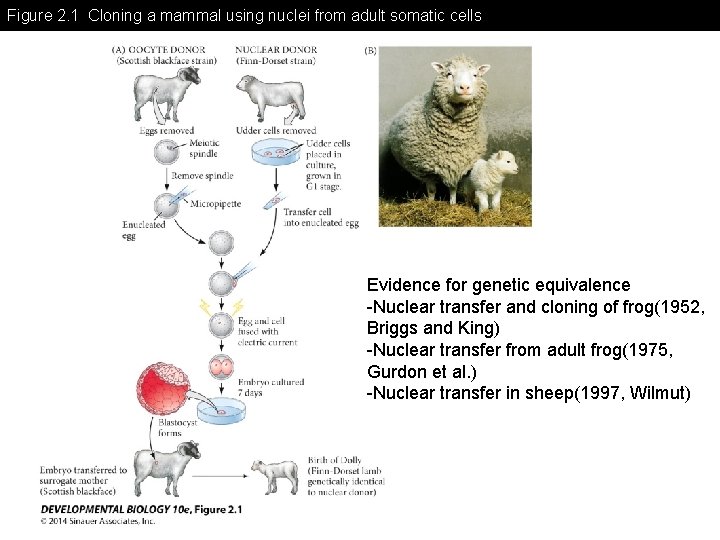
Figure 2. 1 Cloning a mammal using nuclei from adult somatic cells Evidence for genetic equivalence -Nuclear transfer and cloning of frog(1952, Briggs and King) -Nuclear transfer from adult frog(1975, Gurdon et al. ) -Nuclear transfer in sheep(1997, Wilmut)

Figure 2. 2 The kitten “CC” (From 9 th Edition) Resurrection is not possible!
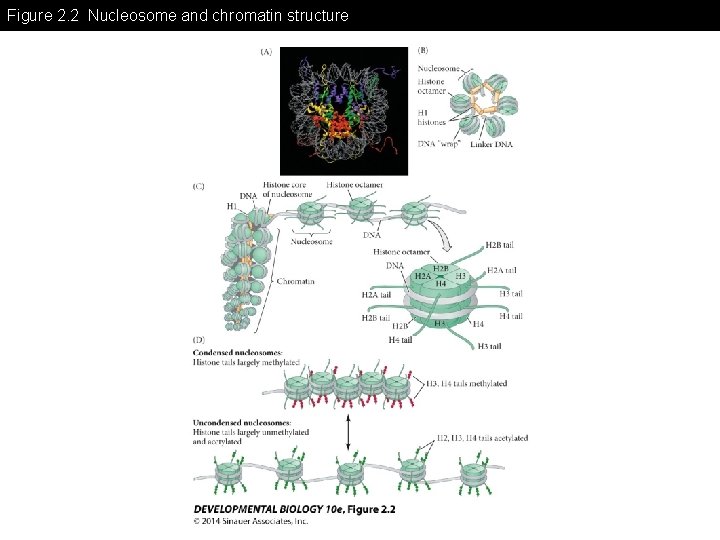
Figure 2. 2 Nucleosome and chromatin structure
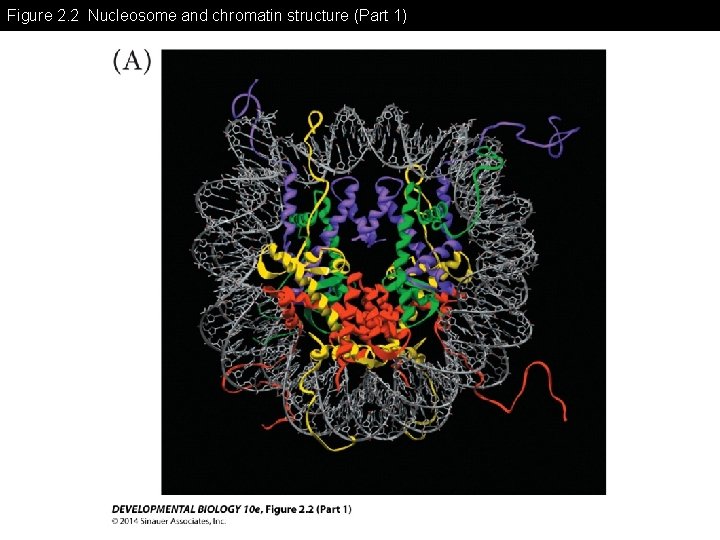
Figure 2. 2 Nucleosome and chromatin structure (Part 1)
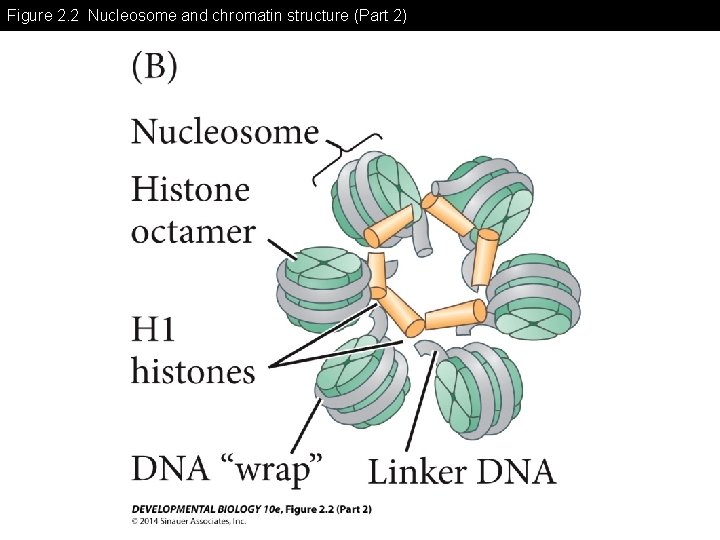
Figure 2. 2 Nucleosome and chromatin structure (Part 2)
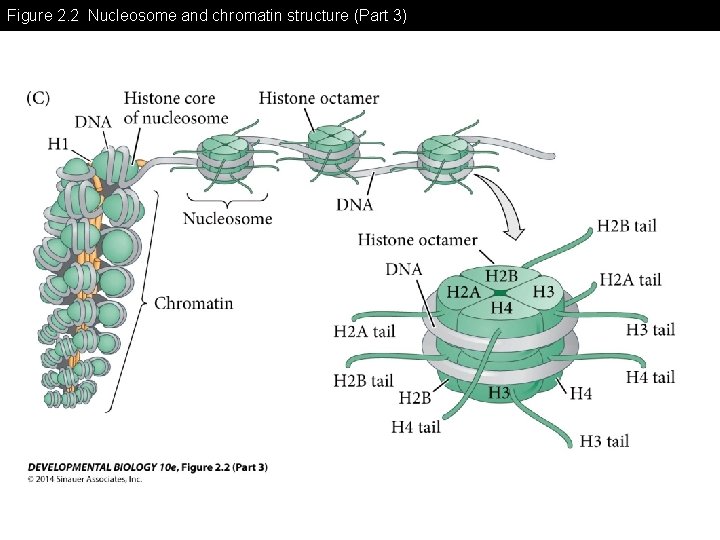
Figure 2. 2 Nucleosome and chromatin structure (Part 3)
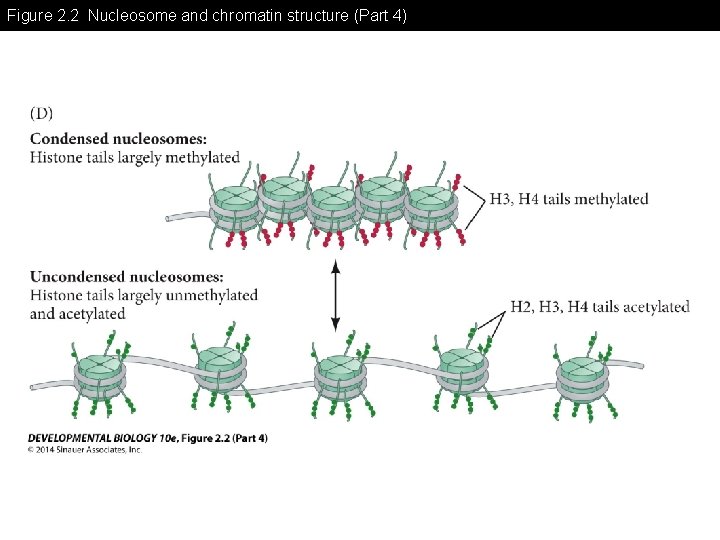
Figure 2. 2 Nucleosome and chromatin structure (Part 4)
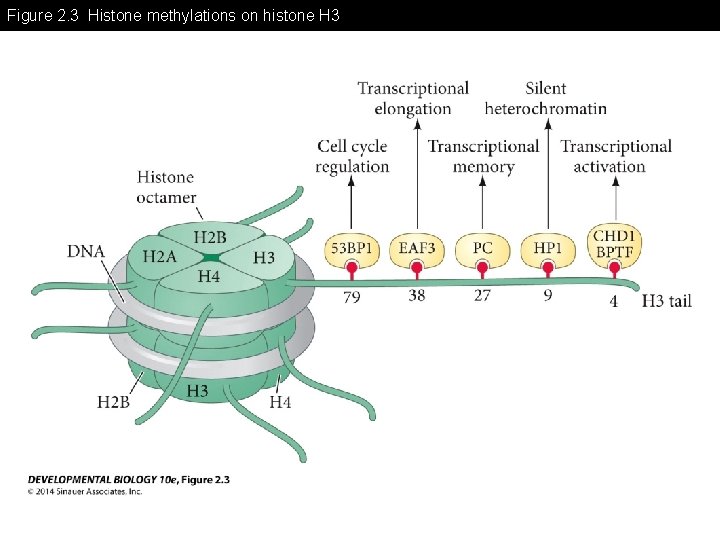
Figure 2. 3 Histone methylations on histone H 3
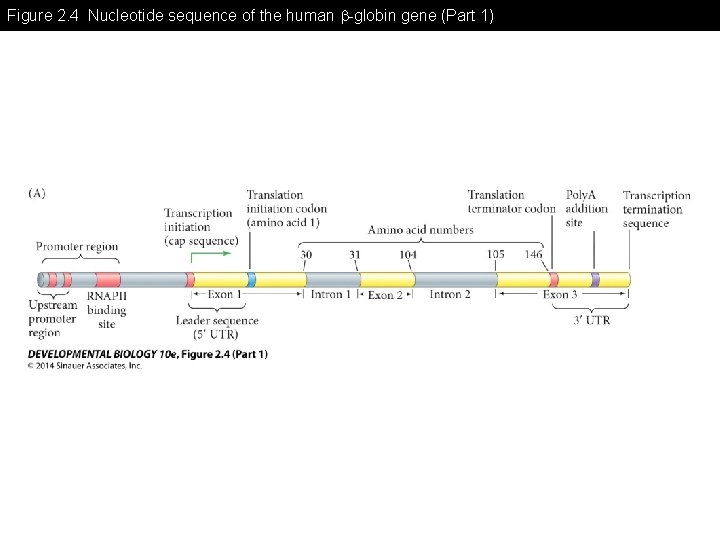
Figure 2. 4 Nucleotide sequence of the human -globin gene (Part 1)

Figure 2. 4 Nucleotide sequence of the human -globin gene (Part 2)
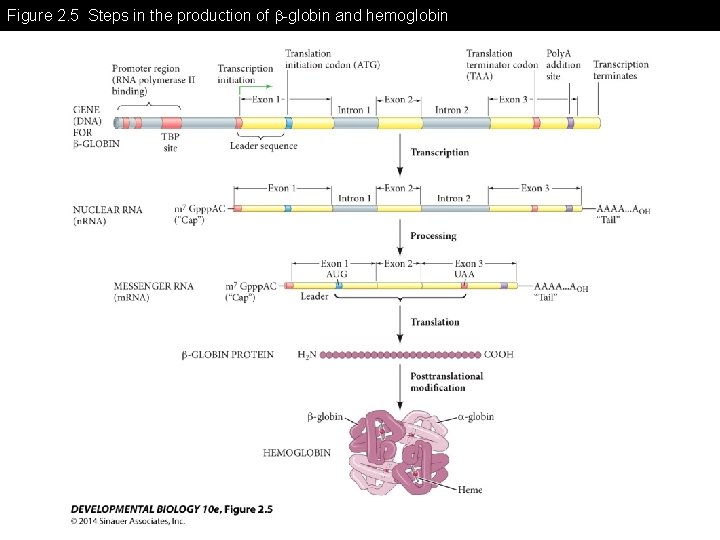
Figure 2. 5 Steps in the production of -globin and hemoglobin
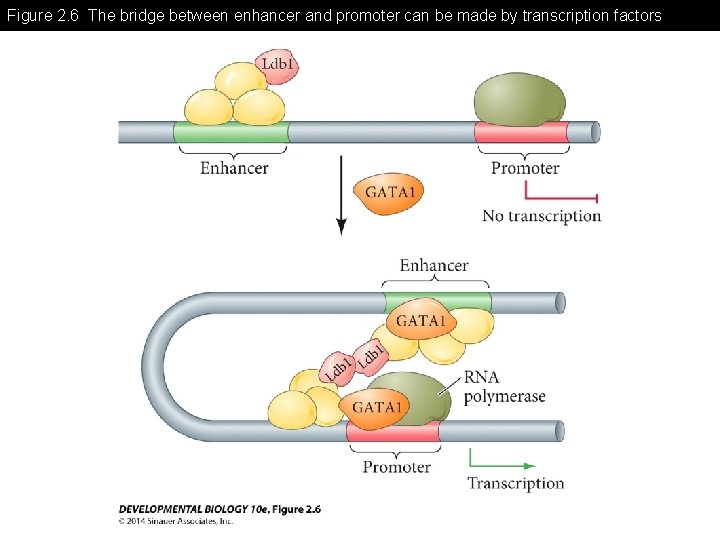
Figure 2. 6 The bridge between enhancer and promoter can be made by transcription factors
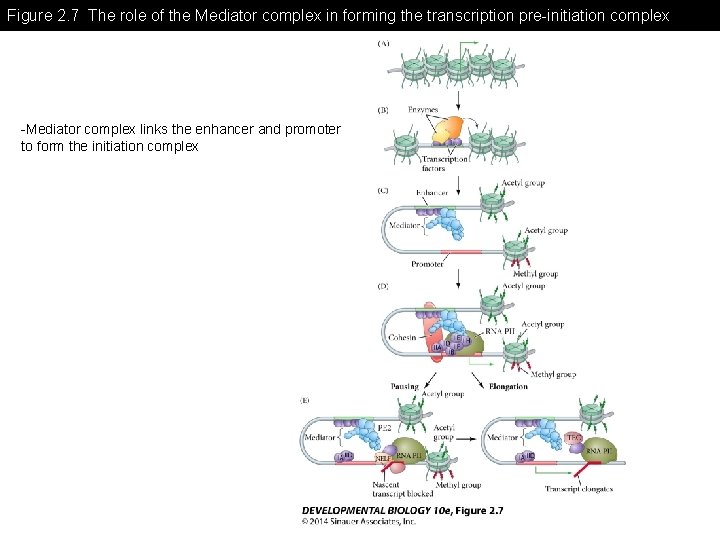
Figure 2. 7 The role of the Mediator complex in forming the transcription pre-initiation complex -Mediator complex links the enhancer and promoter to form the initiation complex
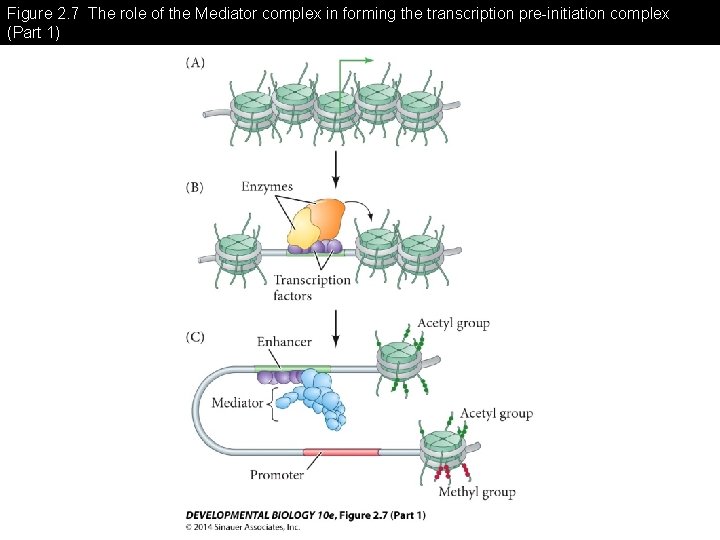
Figure 2. 7 The role of the Mediator complex in forming the transcription pre-initiation complex (Part 1)
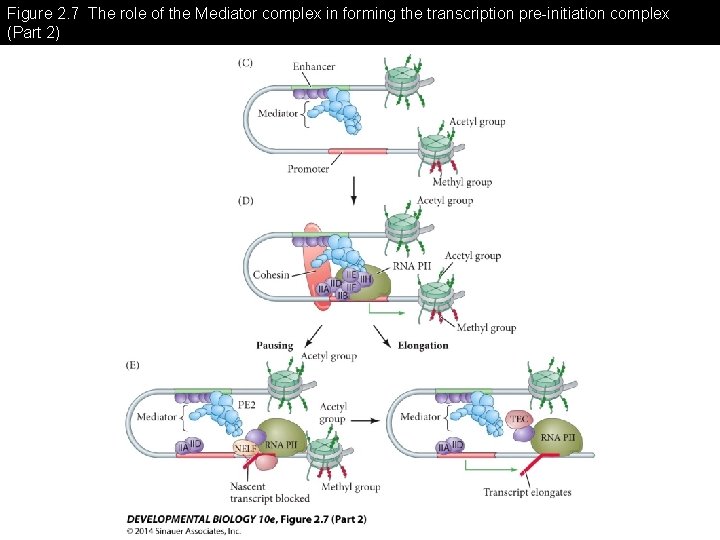
Figure 2. 7 The role of the Mediator complex in forming the transcription pre-initiation complex (Part 2)
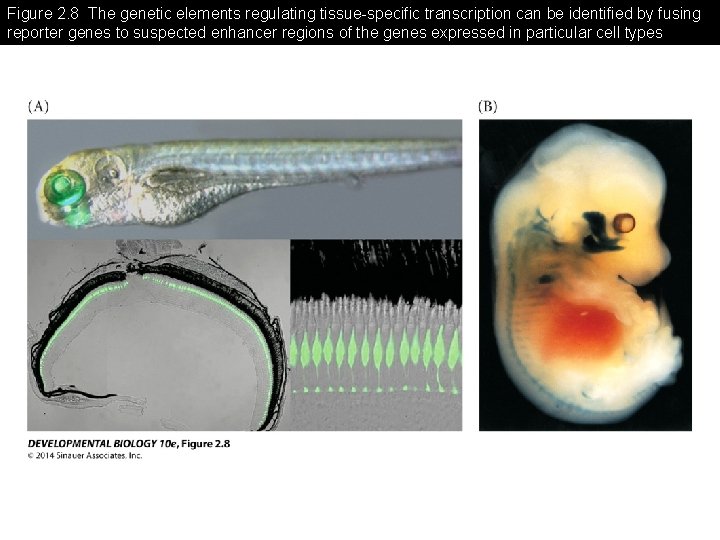
Figure 2. 8 The genetic elements regulating tissue-specific transcription can be identified by fusing reporter genes to suspected enhancer regions of the genes expressed in particular cell types
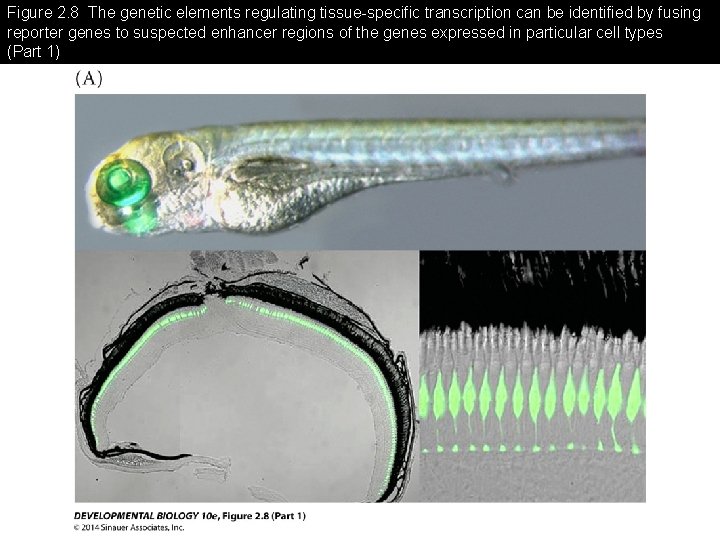
Figure 2. 8 The genetic elements regulating tissue-specific transcription can be identified by fusing reporter genes to suspected enhancer regions of the genes expressed in particular cell types (Part 1)
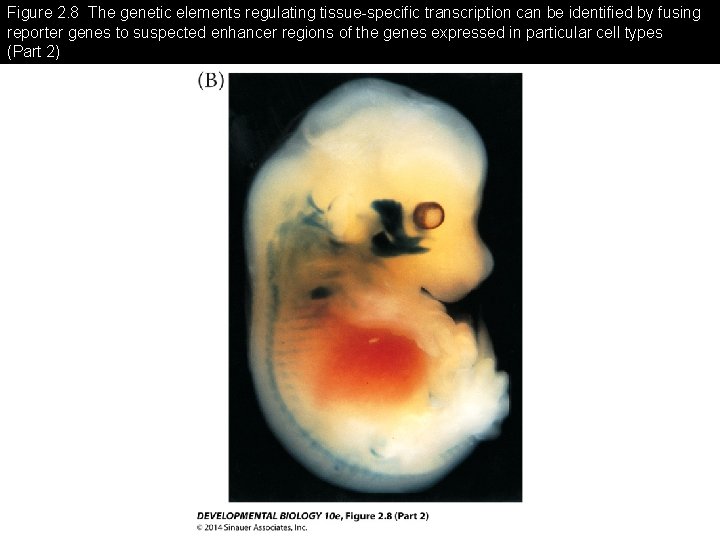
Figure 2. 8 The genetic elements regulating tissue-specific transcription can be identified by fusing reporter genes to suspected enhancer regions of the genes expressed in particular cell types (Part 2)
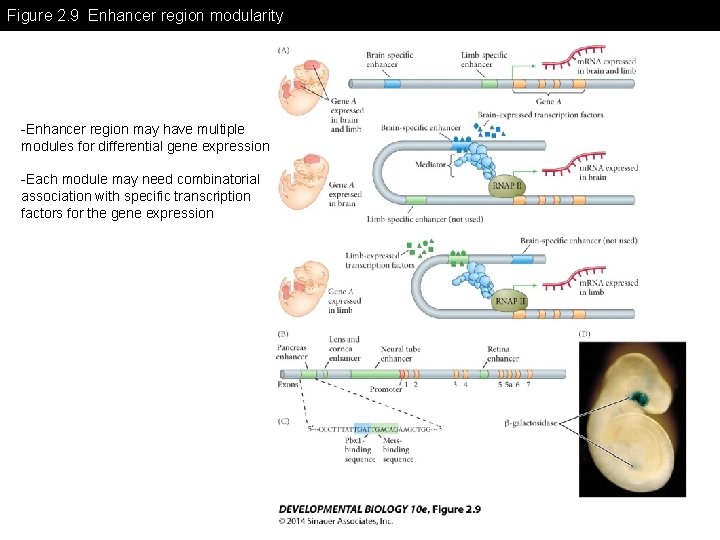
Figure 2. 9 Enhancer region modularity -Enhancer region may have multiple modules for differential gene expression -Each module may need combinatorial association with specific transcription factors for the gene expression
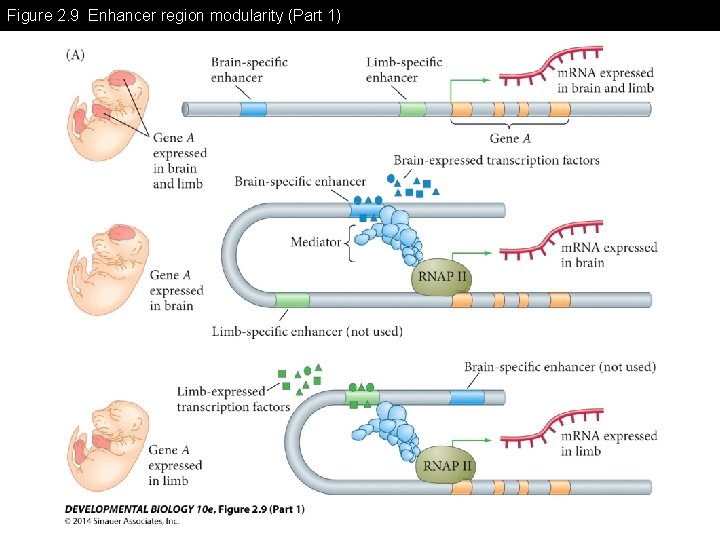
Figure 2. 9 Enhancer region modularity (Part 1)
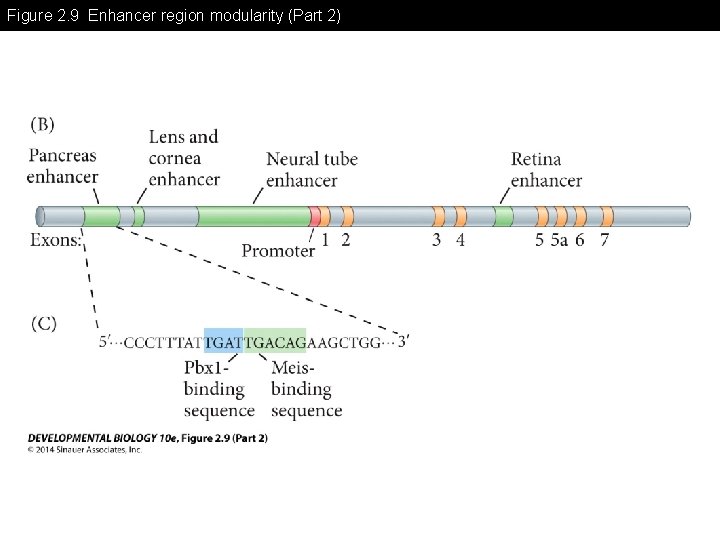
Figure 2. 9 Enhancer region modularity (Part 2)
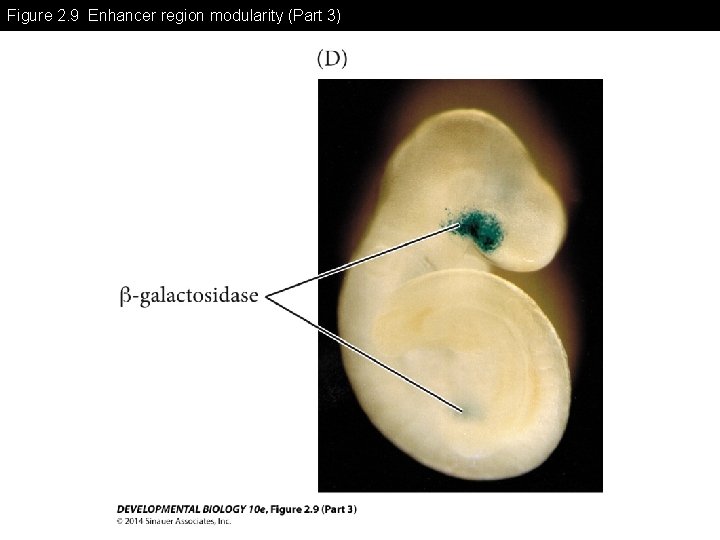
Figure 2. 9 Enhancer region modularity (Part 3)
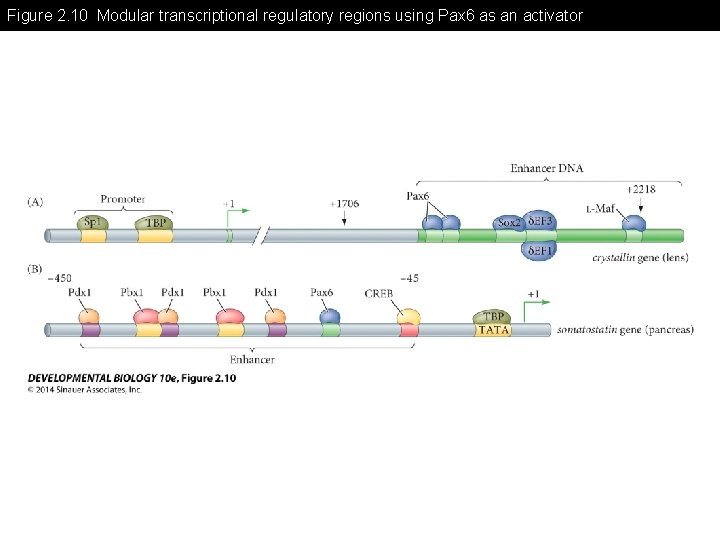
Figure 2. 10 Modular transcriptional regulatory regions using Pax 6 as an activator
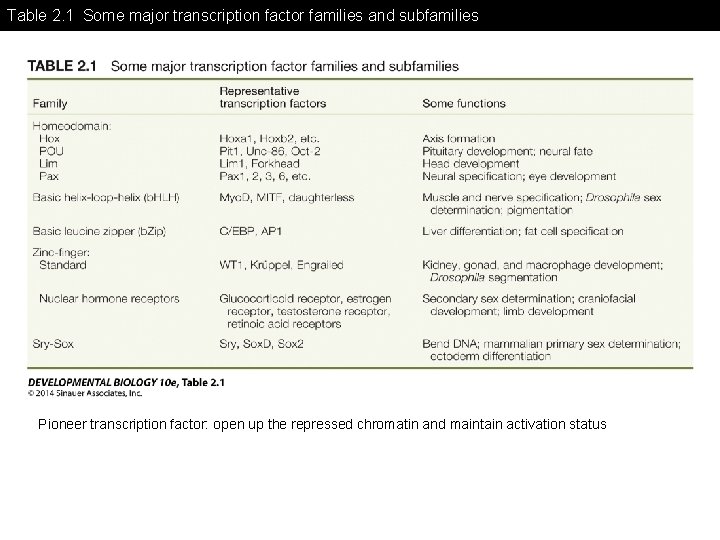
Table 2. 1 Some major transcription factor families and subfamilies Pioneer transcription factor: open up the repressed chromatin and maintain activation status
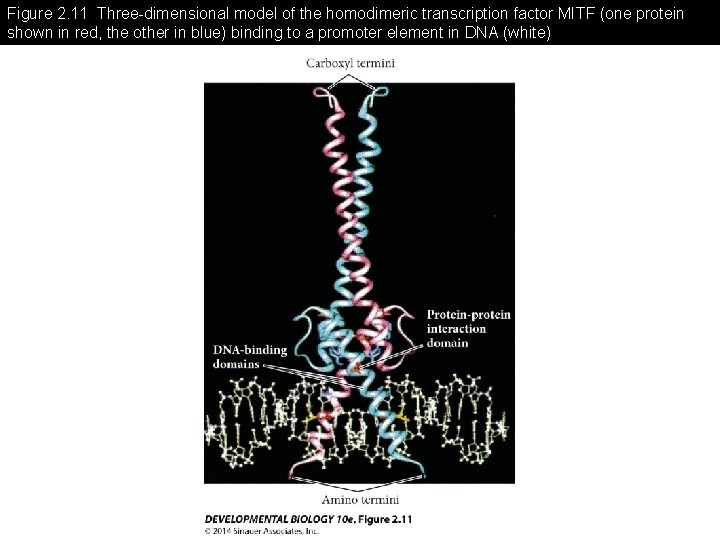
Figure 2. 11 Three-dimensional model of the homodimeric transcription factor MITF (one protein shown in red, the other in blue) binding to a promoter element in DNA (white)
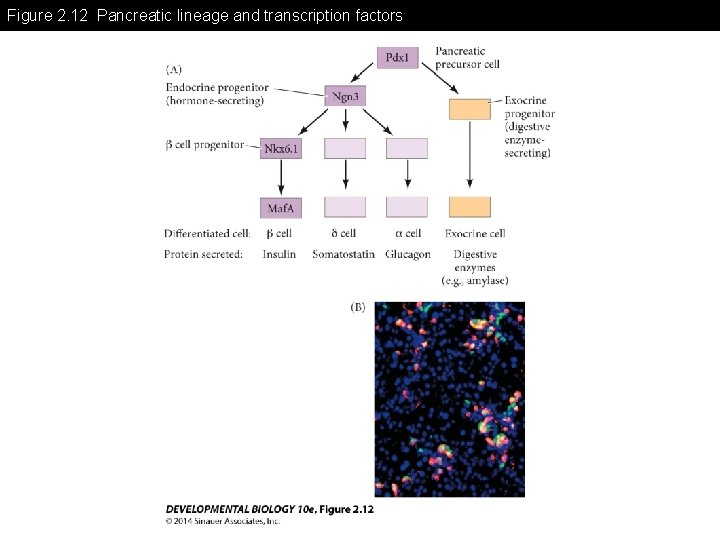
Figure 2. 12 Pancreatic lineage and transcription factors
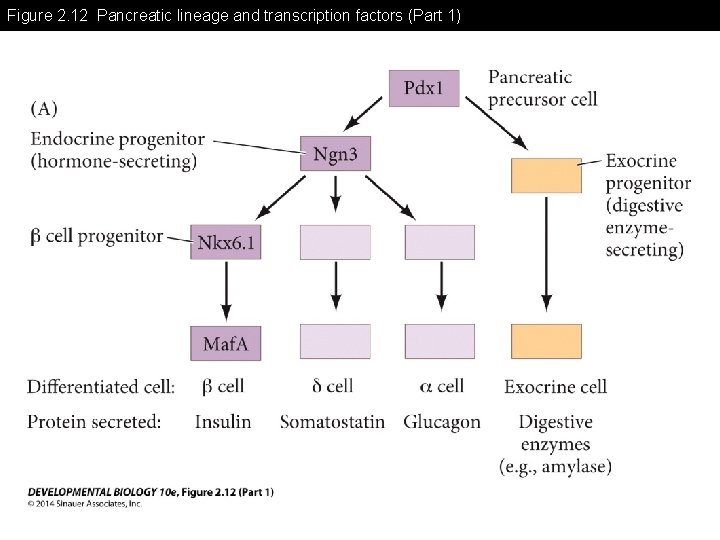
Figure 2. 12 Pancreatic lineage and transcription factors (Part 1)
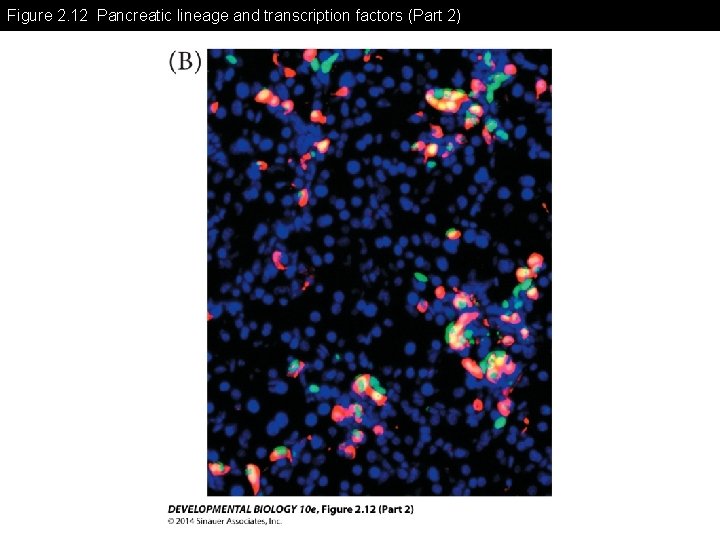
Figure 2. 12 Pancreatic lineage and transcription factors (Part 2)
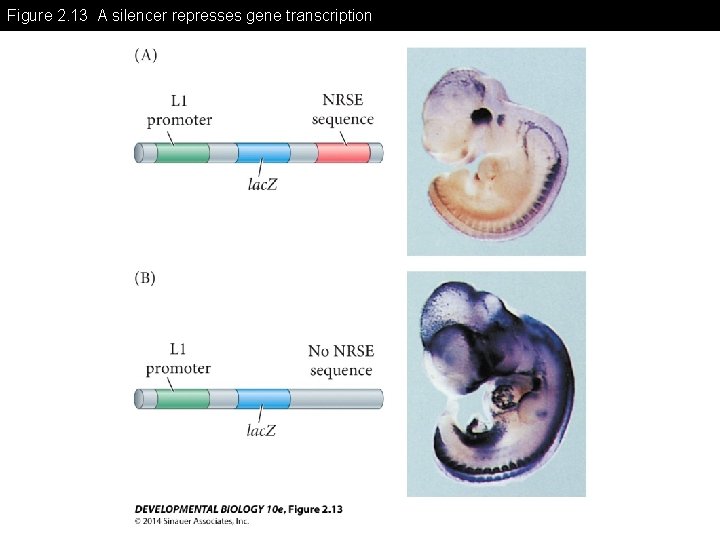
Figure 2. 13 A silencer represses gene transcription
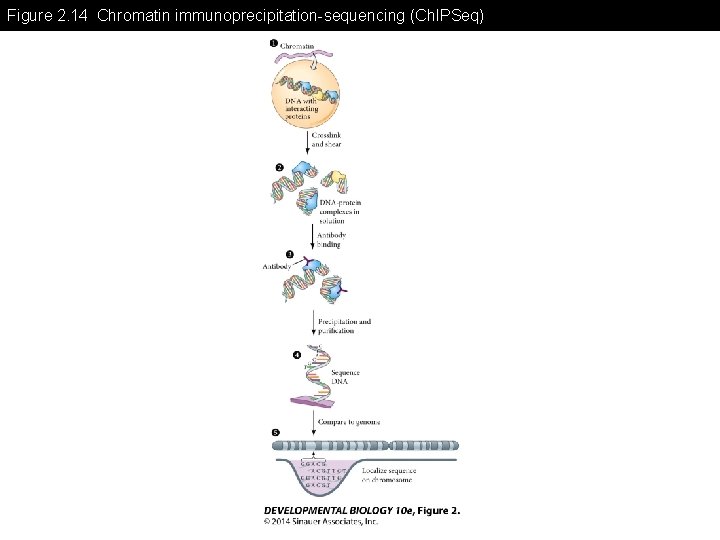
Figure 2. 14 Chromatin immunoprecipitation-sequencing (Ch. IPSeq)
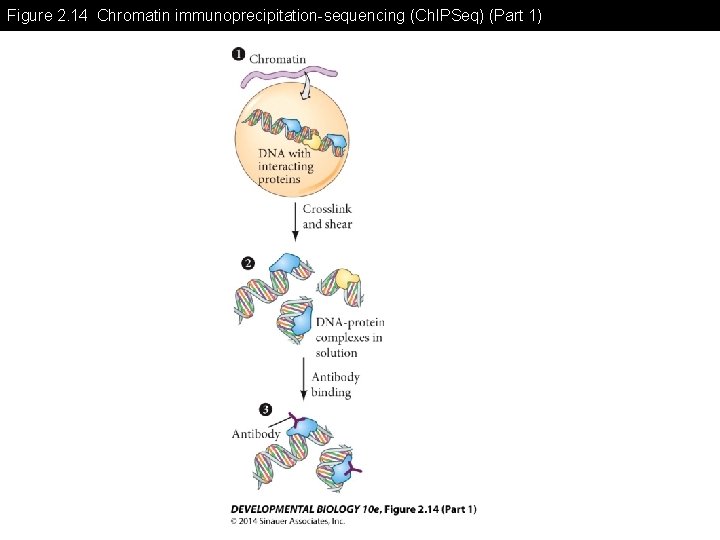
Figure 2. 14 Chromatin immunoprecipitation-sequencing (Ch. IPSeq) (Part 1)
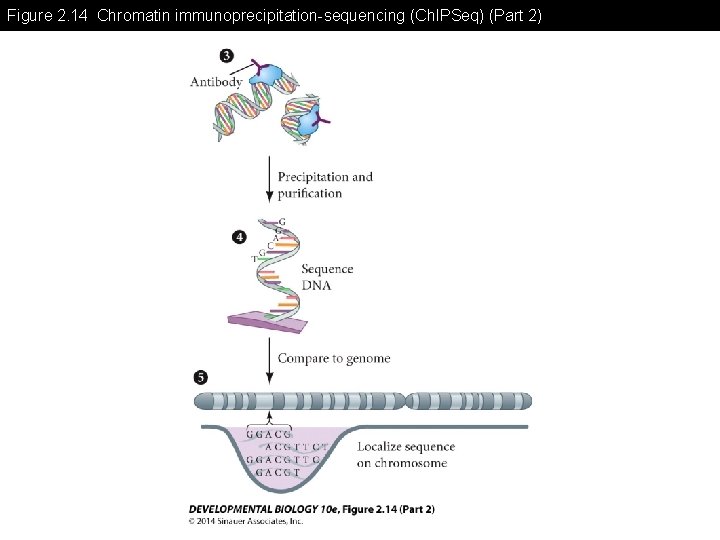
Figure 2. 14 Chromatin immunoprecipitation-sequencing (Ch. IPSeq) (Part 2)
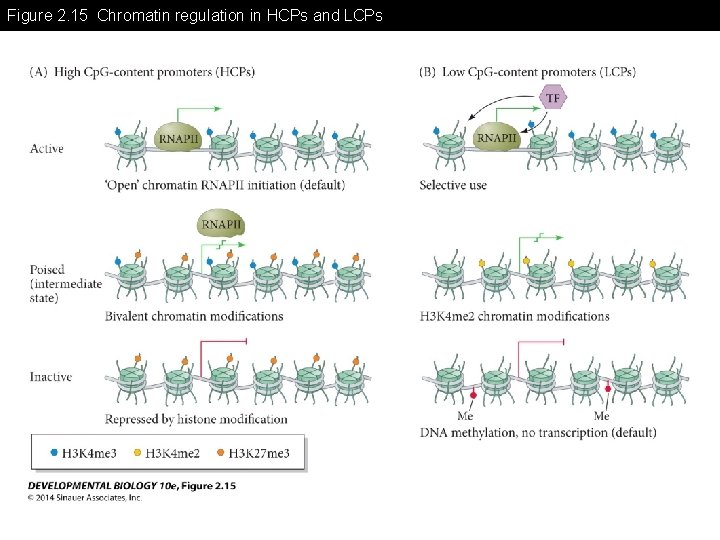
Figure 2. 15 Chromatin regulation in HCPs and LCPs
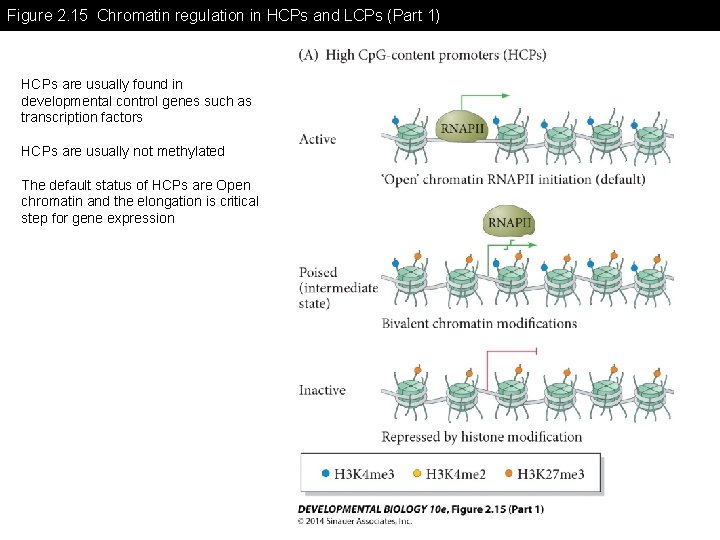
Figure 2. 15 Chromatin regulation in HCPs and LCPs (Part 1) HCPs are usually found in developmental control genes such as transcription factors HCPs are usually not methylated The default status of HCPs are Open chromatin and the elongation is critical step for gene expression
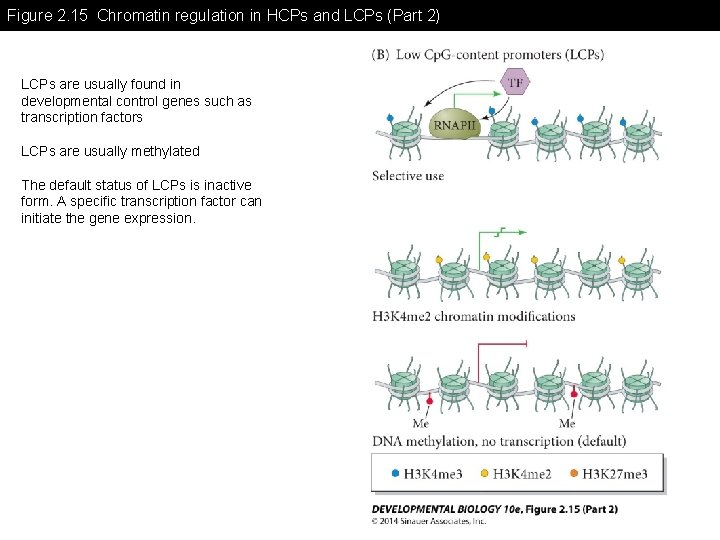
Figure 2. 15 Chromatin regulation in HCPs and LCPs (Part 2) LCPs are usually found in developmental control genes such as transcription factors LCPs are usually methylated The default status of LCPs is inactive form. A specific transcription factor can initiate the gene expression.
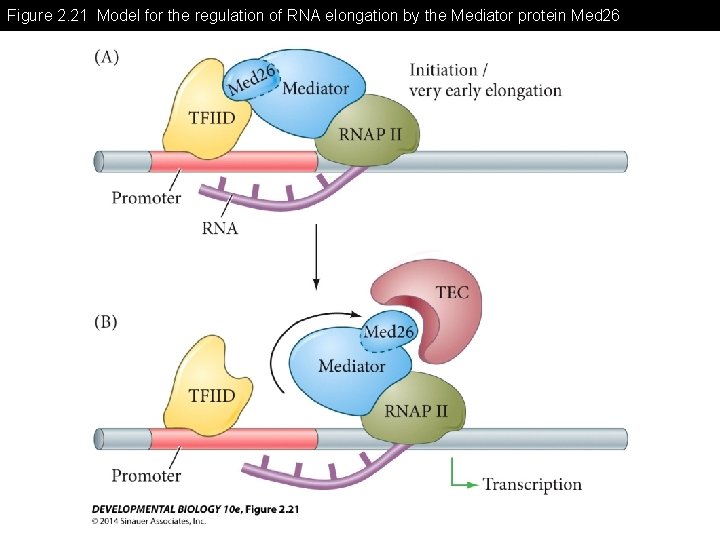
Figure 2. 21 Model for the regulation of RNA elongation by the Mediator protein Med 26
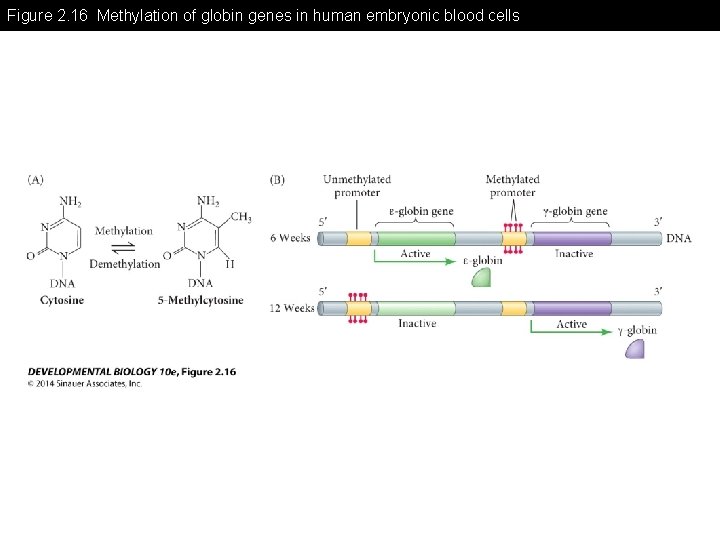
Figure 2. 16 Methylation of globin genes in human embryonic blood cells
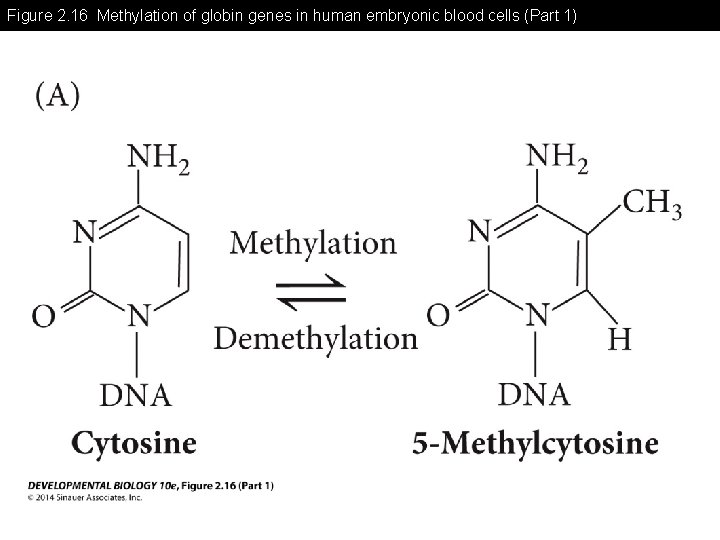
Figure 2. 16 Methylation of globin genes in human embryonic blood cells (Part 1)
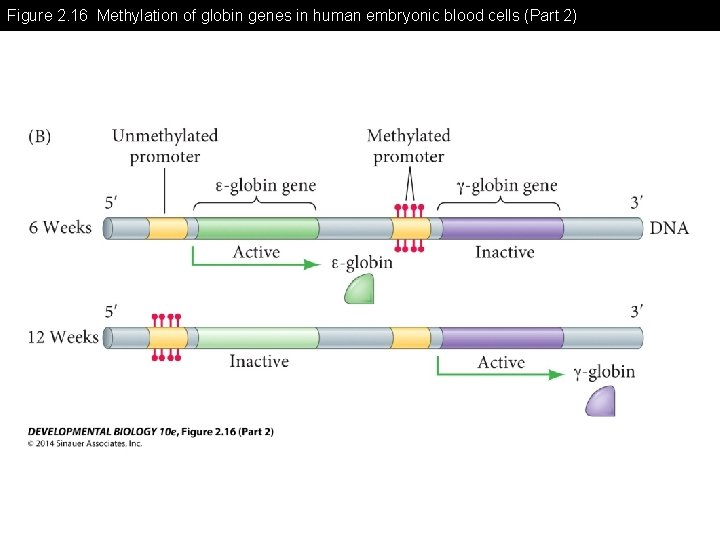
Figure 2. 16 Methylation of globin genes in human embryonic blood cells (Part 2)
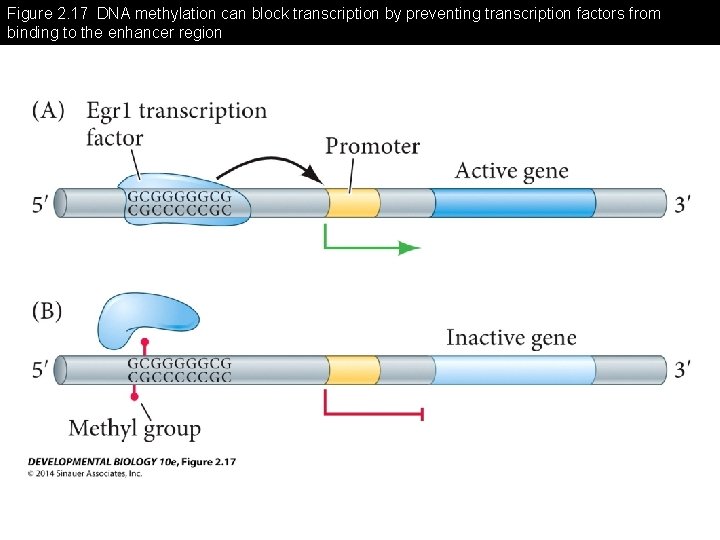
Figure 2. 17 DNA methylation can block transcription by preventing transcription factors from binding to the enhancer region
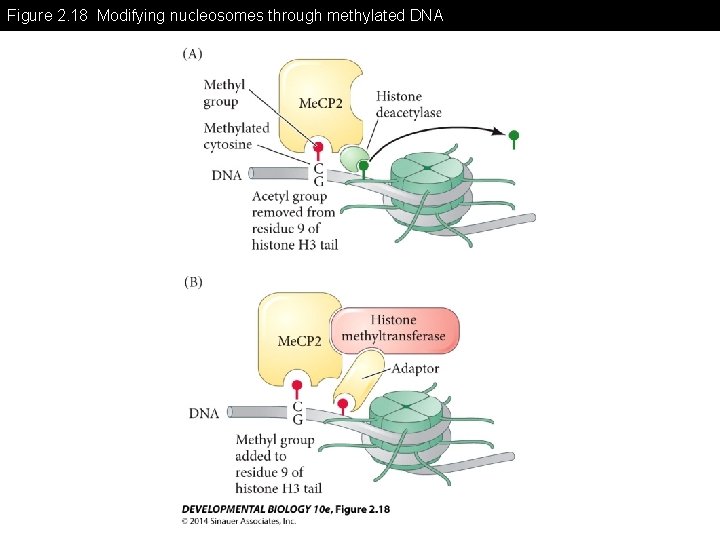
Figure 2. 18 Modifying nucleosomes through methylated DNA
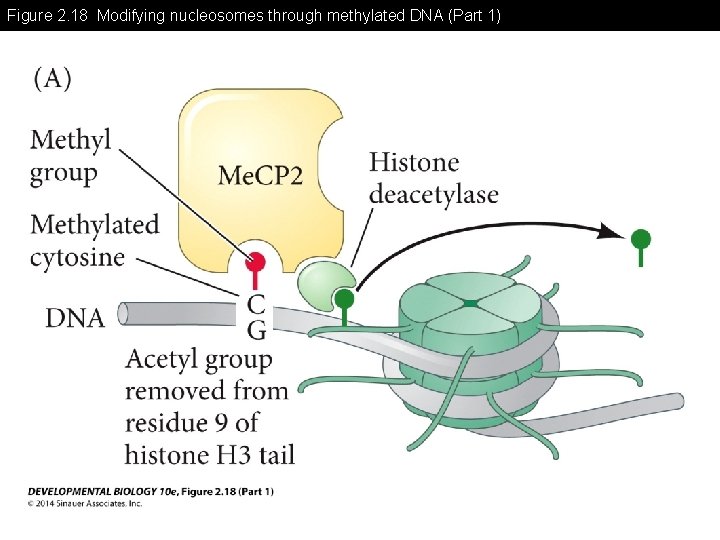
Figure 2. 18 Modifying nucleosomes through methylated DNA (Part 1)
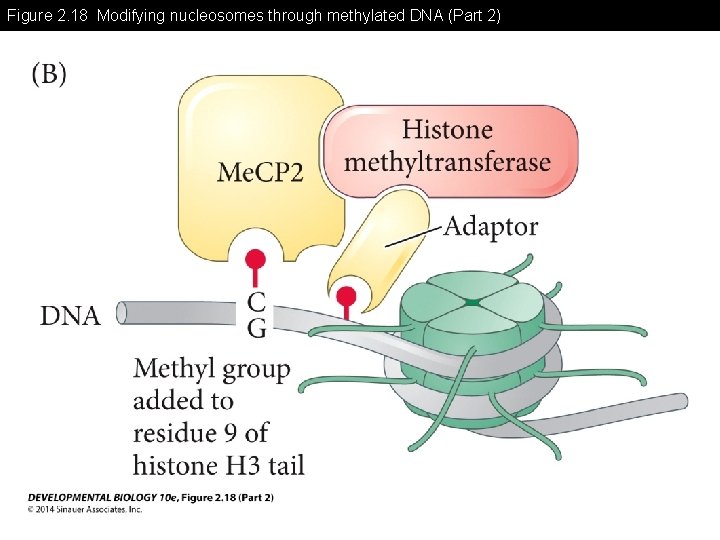
Figure 2. 18 Modifying nucleosomes through methylated DNA (Part 2)
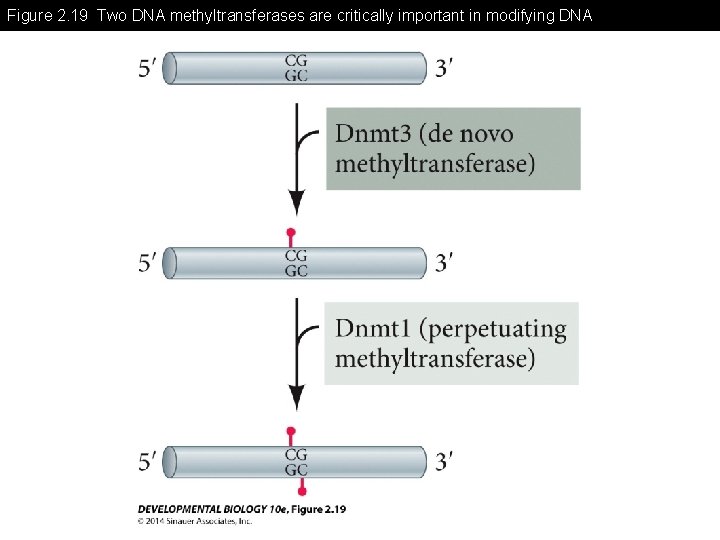
Figure 2. 19 Two DNA methyltransferases are critically important in modifying DNA
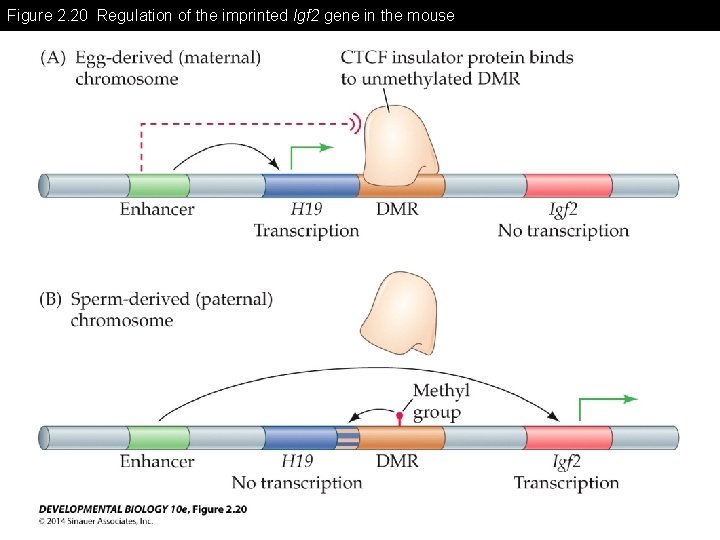
Figure 2. 20 Regulation of the imprinted Igf 2 gene in the mouse
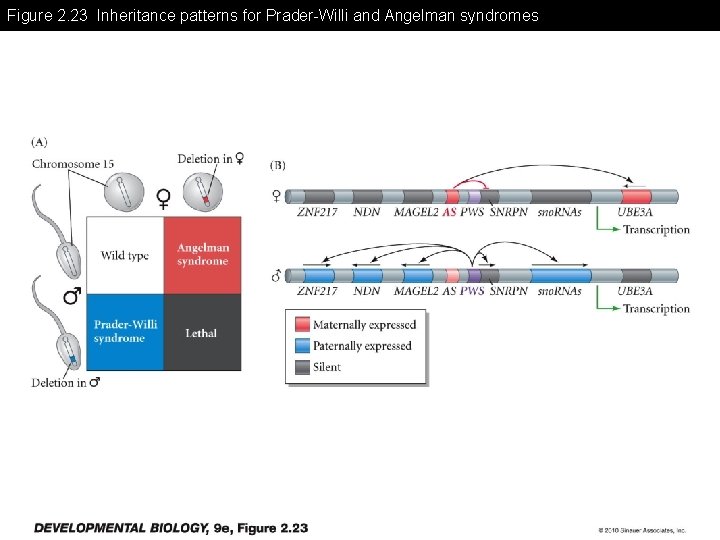
Figure 2. 23 Inheritance patterns for Prader-Willi and Angelman syndromes
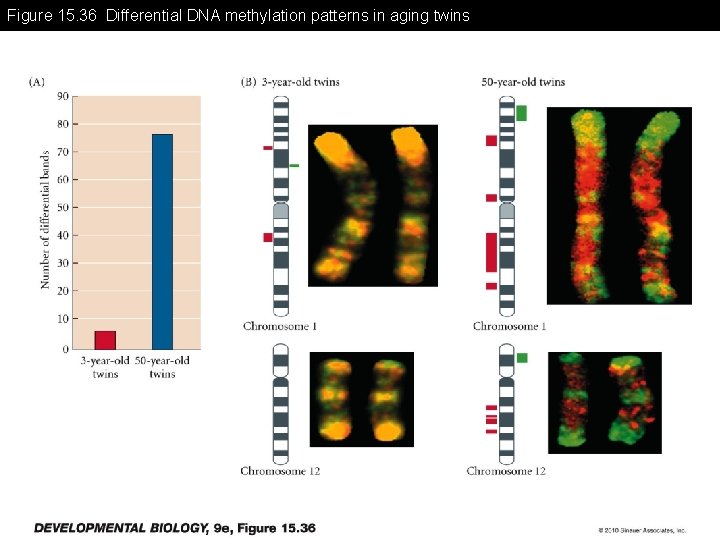
Figure 15. 36 Differential DNA methylation patterns in aging twins
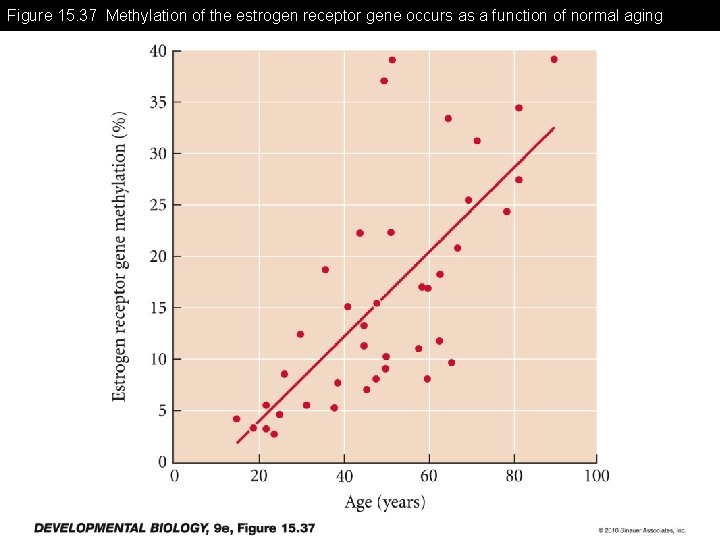
Figure 15. 37 Methylation of the estrogen receptor gene occurs as a function of normal aging
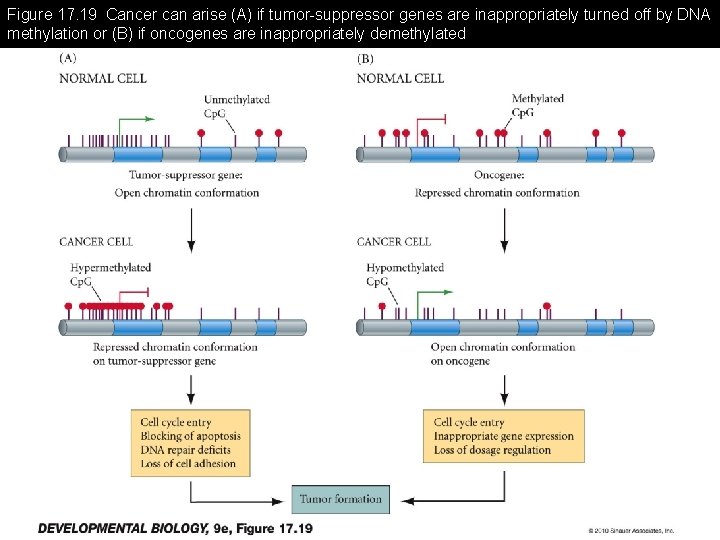
Figure 17. 19 Cancer can arise (A) if tumor-suppressor genes are inappropriately turned off by DNA methylation or (B) if oncogenes are inappropriately demethylated
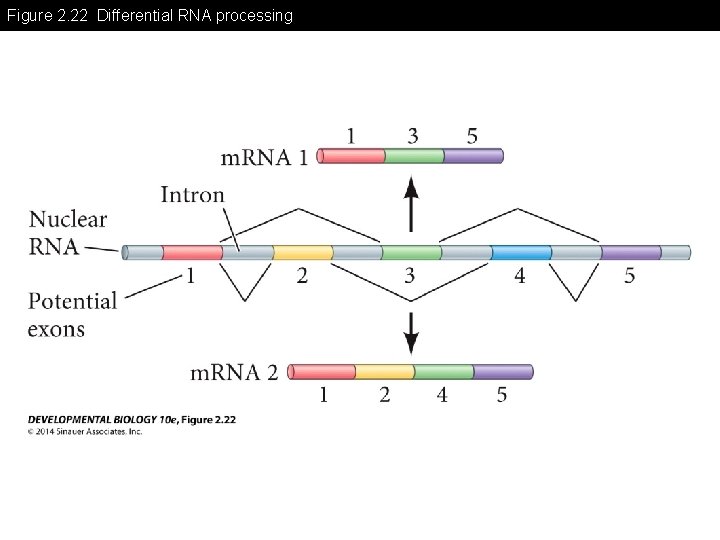
Figure 2. 22 Differential RNA processing

Figure 2. 23 The kitten “CC” (left) was the first household pet to be successfully cloned using somatic nuclear transfer from “Rainbow” (right), a female calico cat
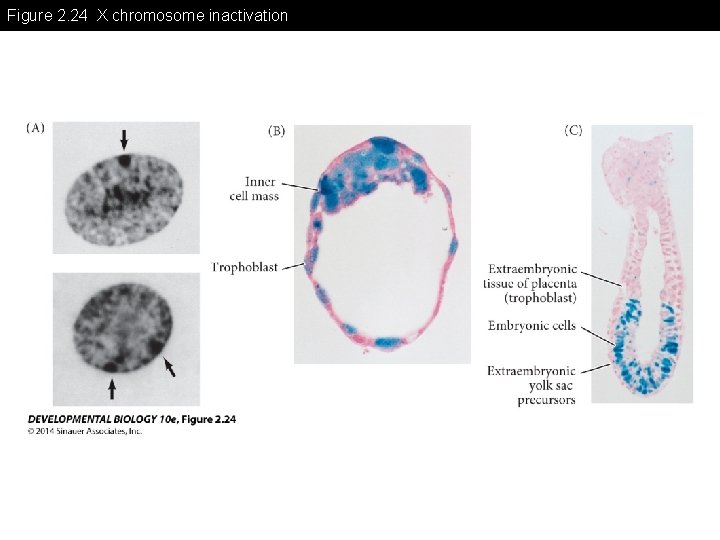
Figure 2. 24 X chromosome inactivation
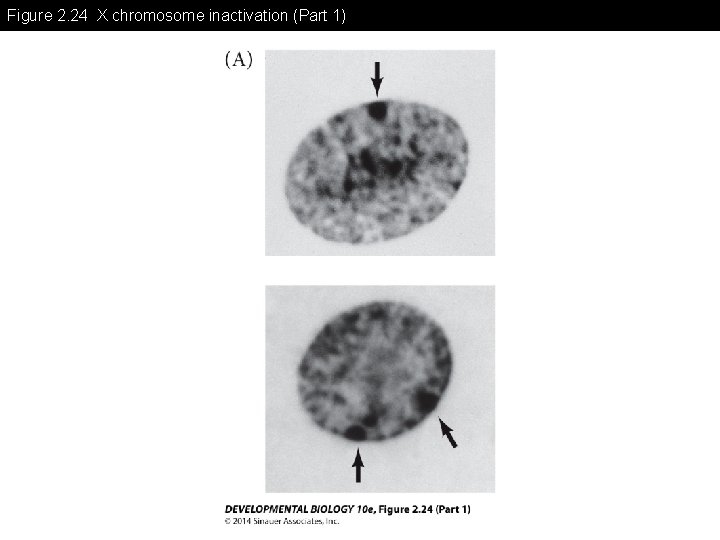
Figure 2. 24 X chromosome inactivation (Part 1)
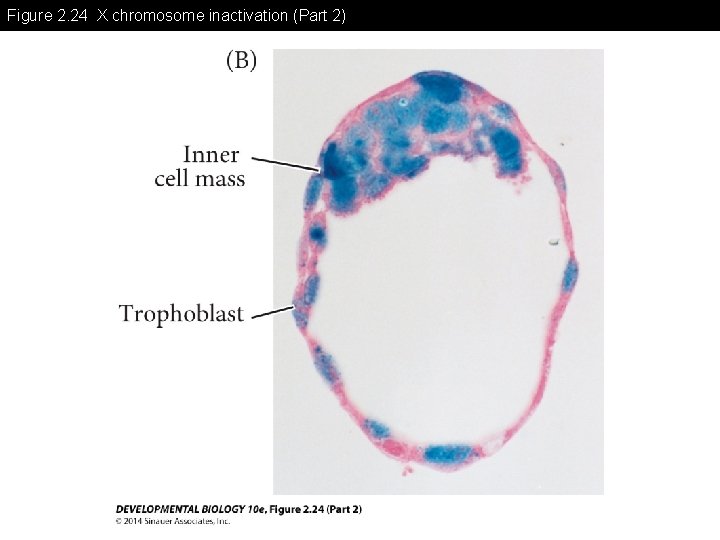
Figure 2. 24 X chromosome inactivation (Part 2)
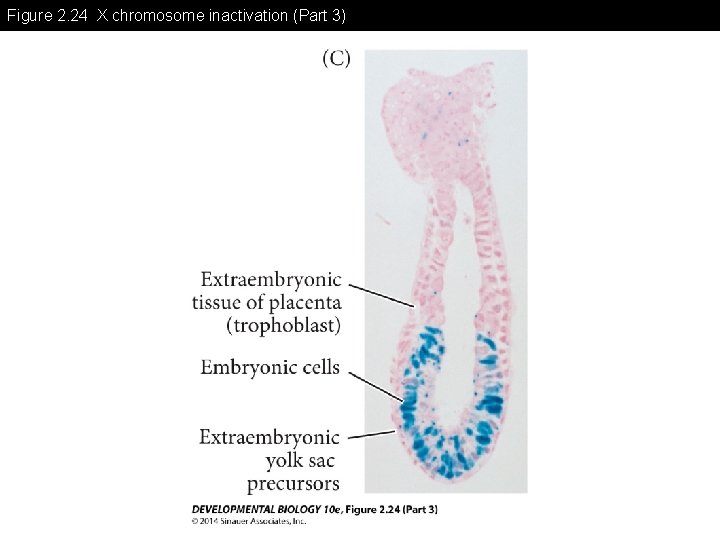
Figure 2. 24 X chromosome inactivation (Part 3)
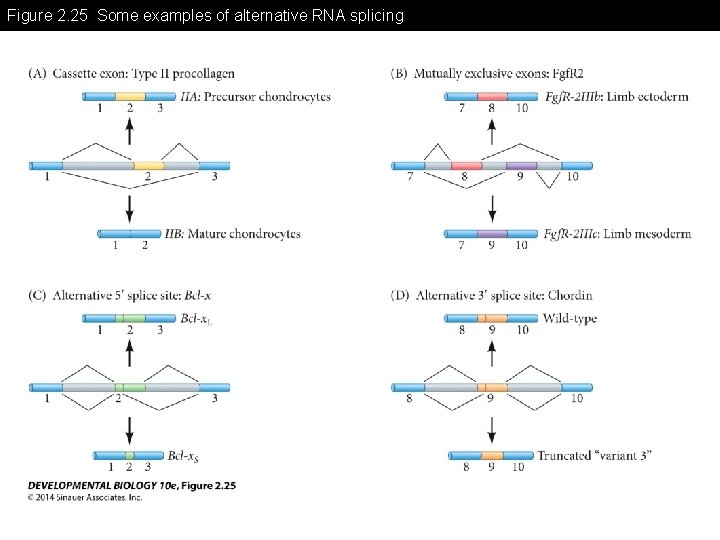
Figure 2. 25 Some examples of alternative RNA splicing
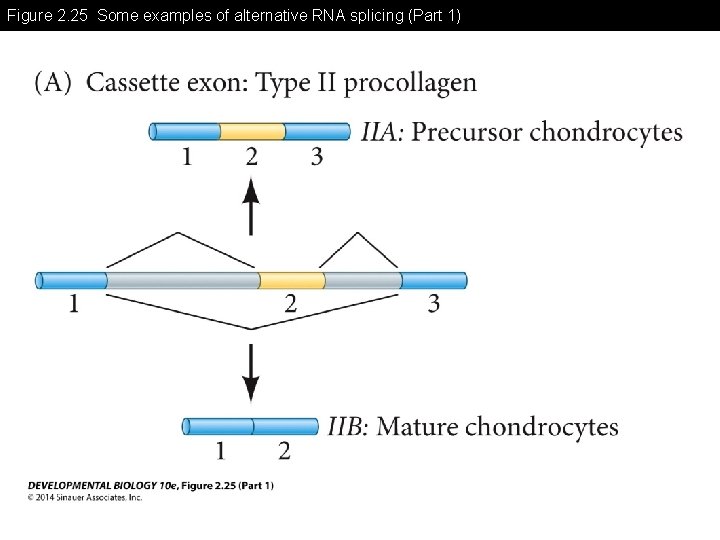
Figure 2. 25 Some examples of alternative RNA splicing (Part 1)
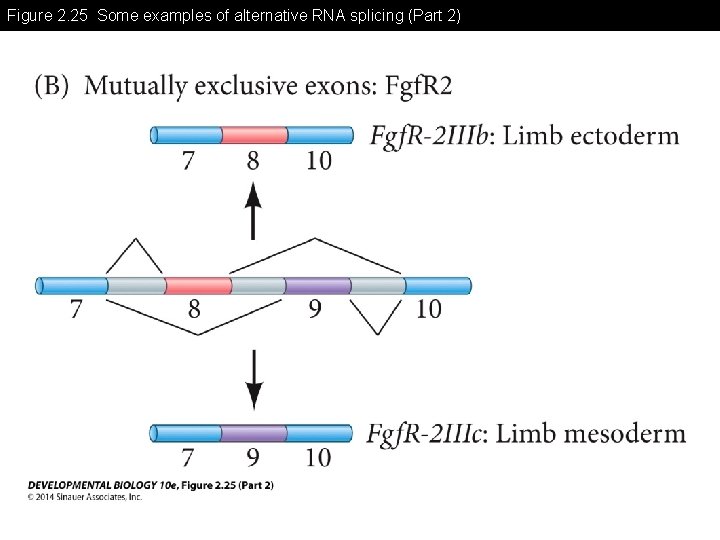
Figure 2. 25 Some examples of alternative RNA splicing (Part 2)
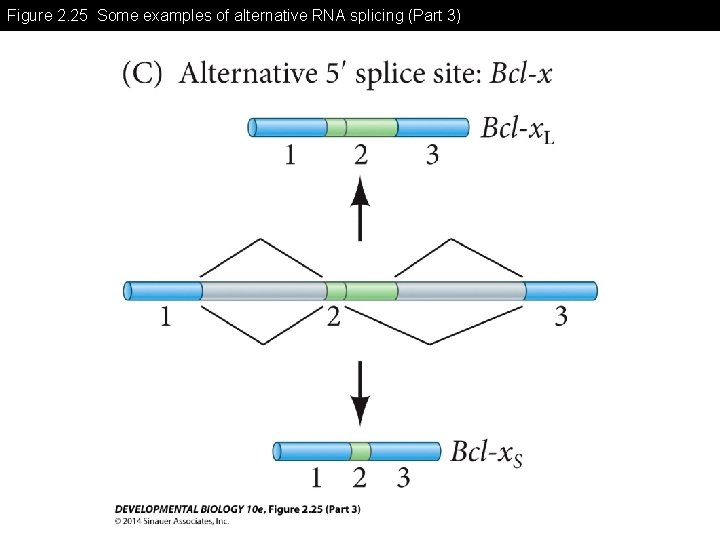
Figure 2. 25 Some examples of alternative RNA splicing (Part 3)
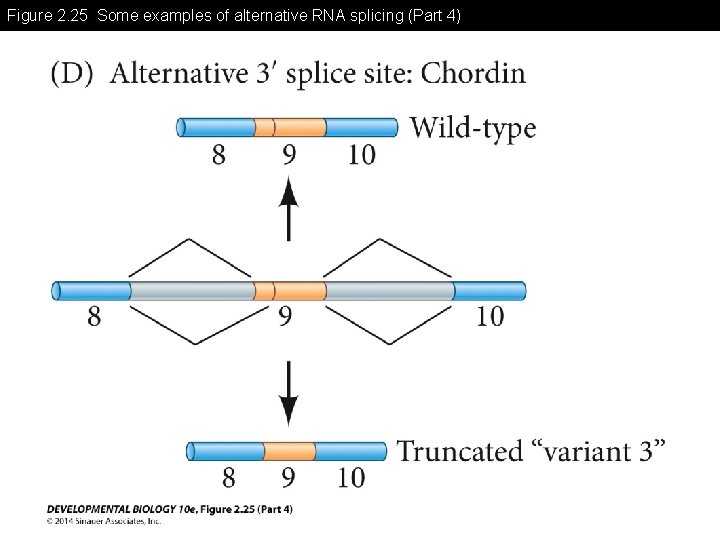
Figure 2. 25 Some examples of alternative RNA splicing (Part 4)
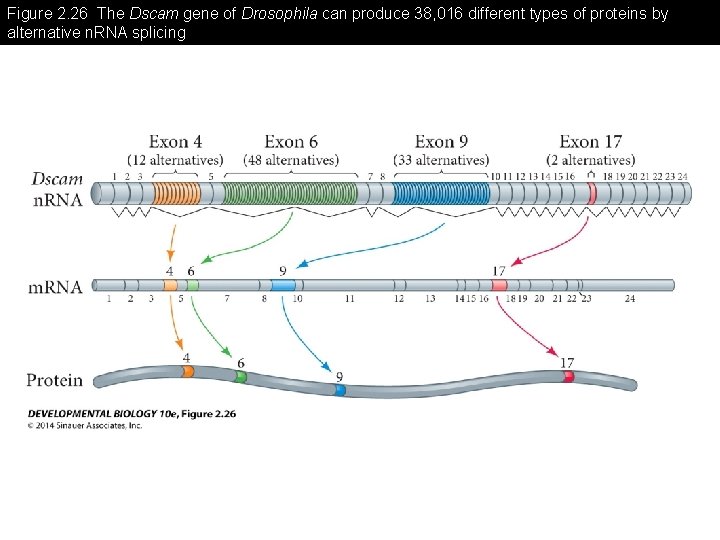
Figure 2. 26 The Dscam gene of Drosophila can produce 38, 016 different types of proteins by alternative n. RNA splicing
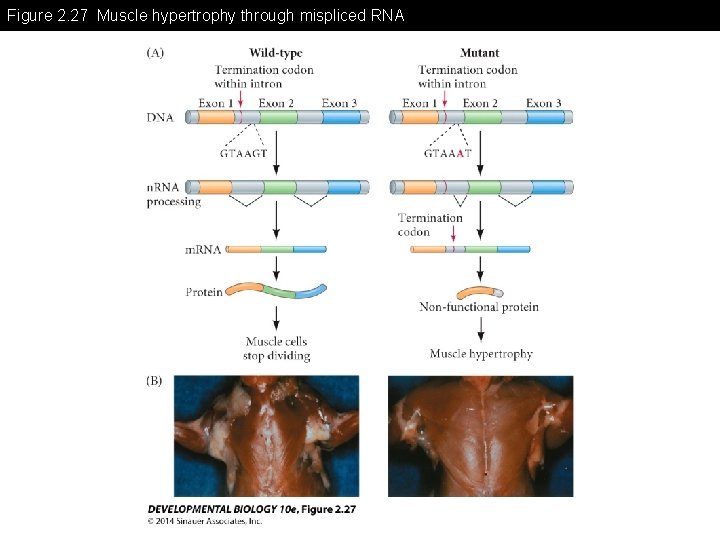
Figure 2. 27 Muscle hypertrophy through mispliced RNA
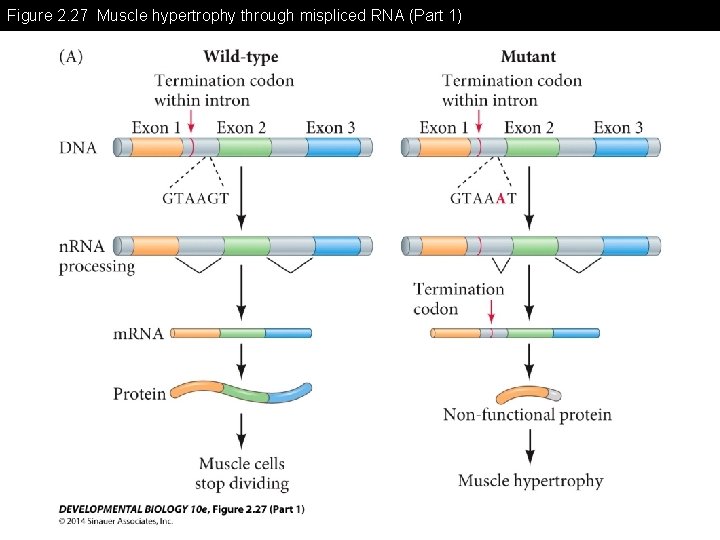
Figure 2. 27 Muscle hypertrophy through mispliced RNA (Part 1)
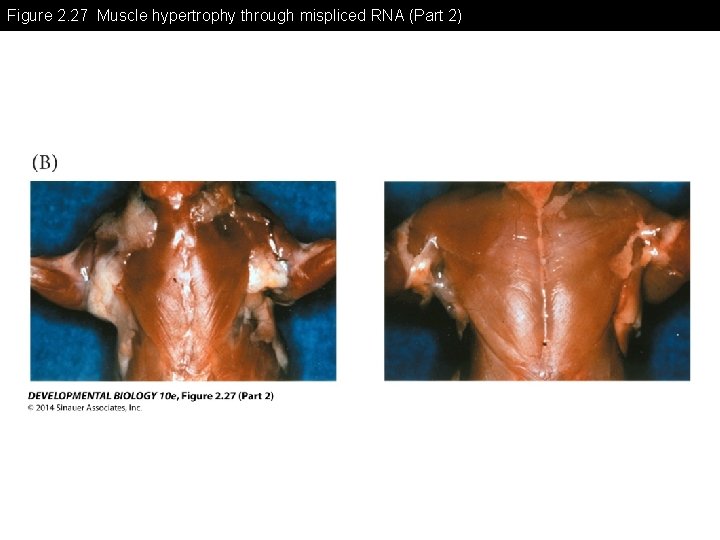
Figure 2. 27 Muscle hypertrophy through mispliced RNA (Part 2)
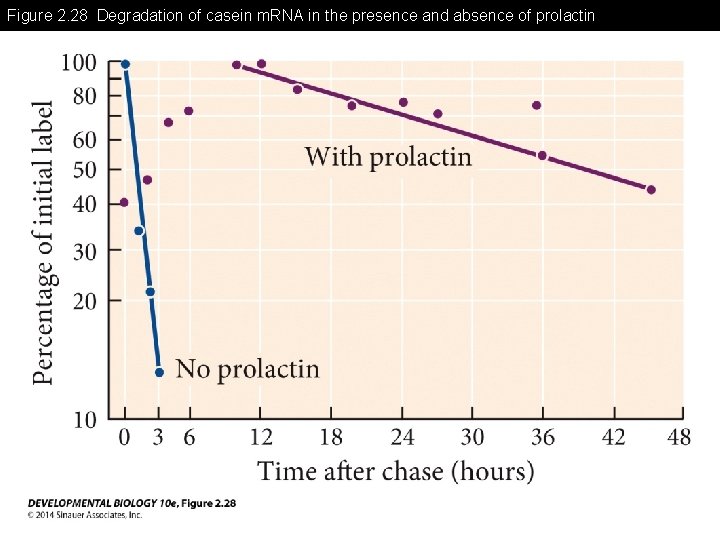
Figure 2. 28 Degradation of casein m. RNA in the presence and absence of prolactin
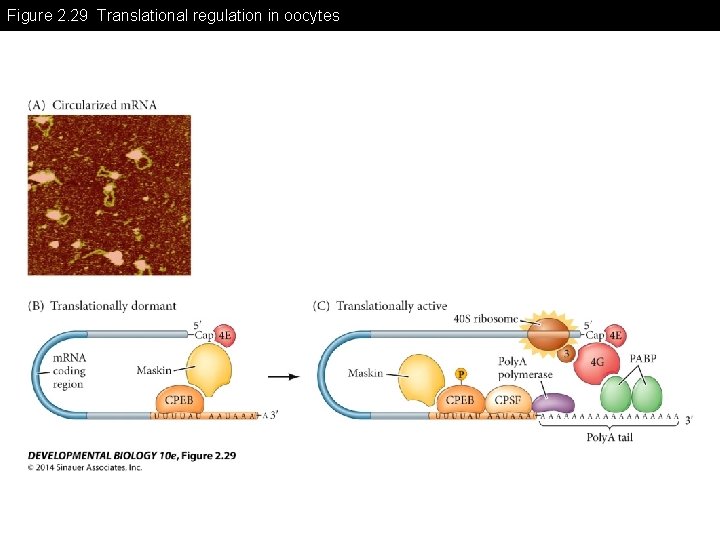
Figure 2. 29 Translational regulation in oocytes
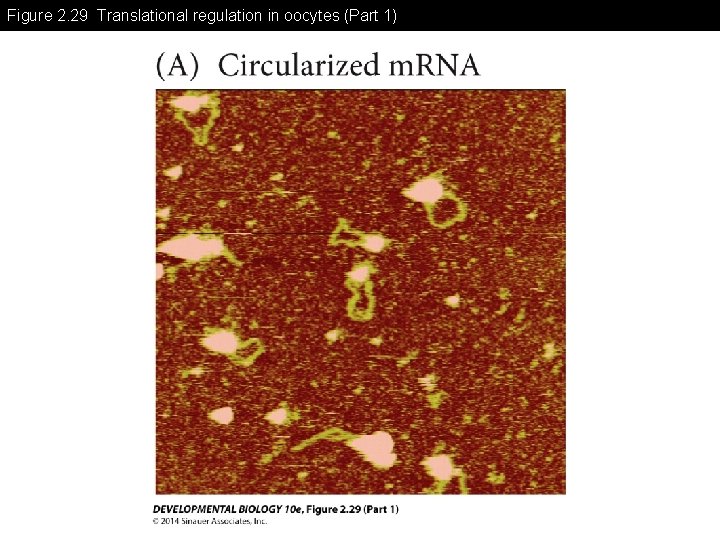
Figure 2. 29 Translational regulation in oocytes (Part 1)
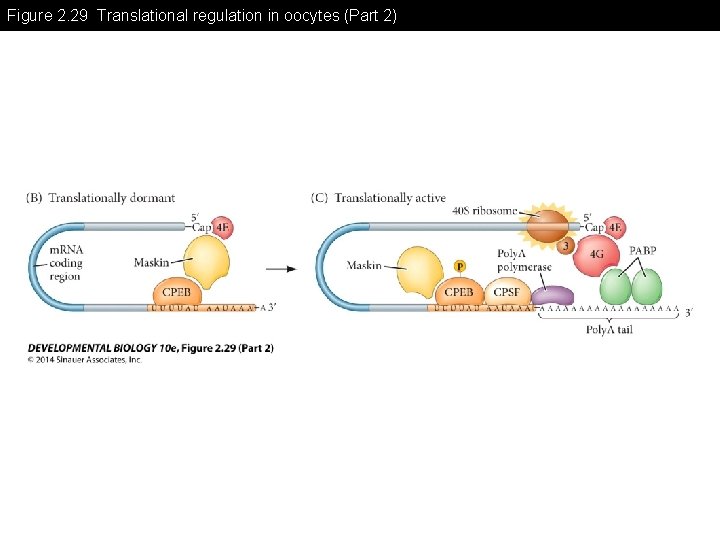
Figure 2. 29 Translational regulation in oocytes (Part 2)
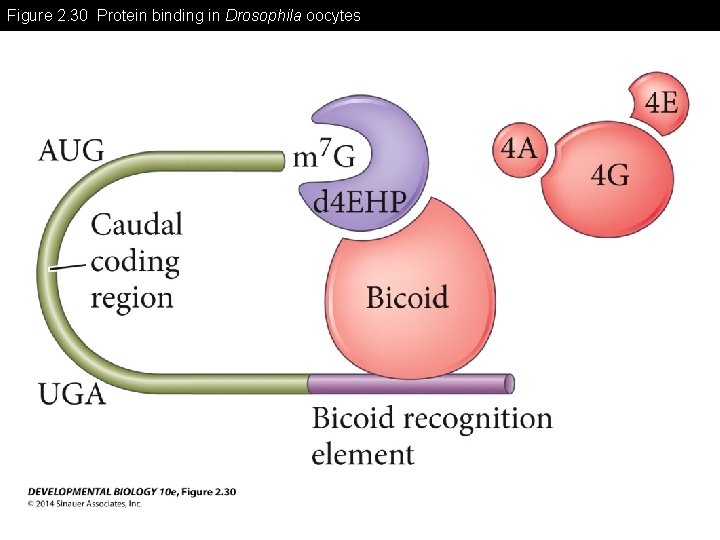
Figure 2. 30 Protein binding in Drosophila oocytes
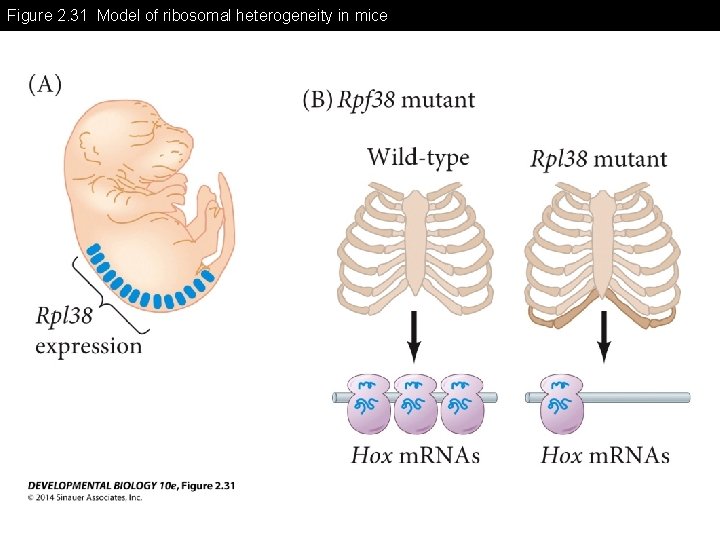
Figure 2. 31 Model of ribosomal heterogeneity in mice
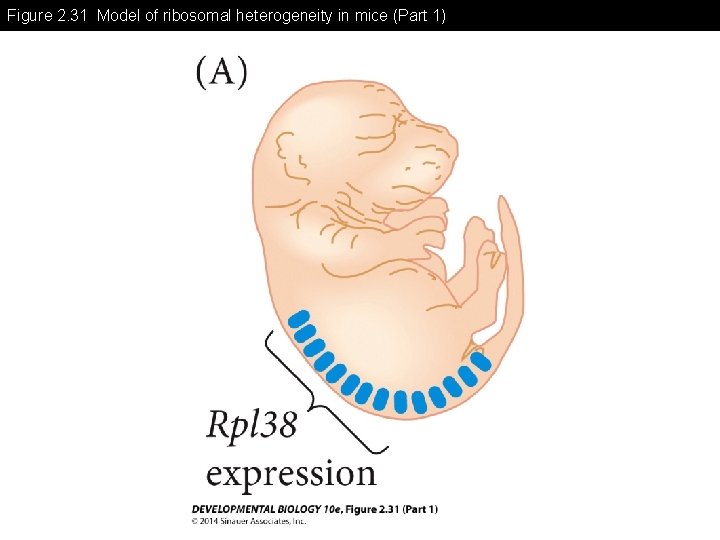
Figure 2. 31 Model of ribosomal heterogeneity in mice (Part 1)
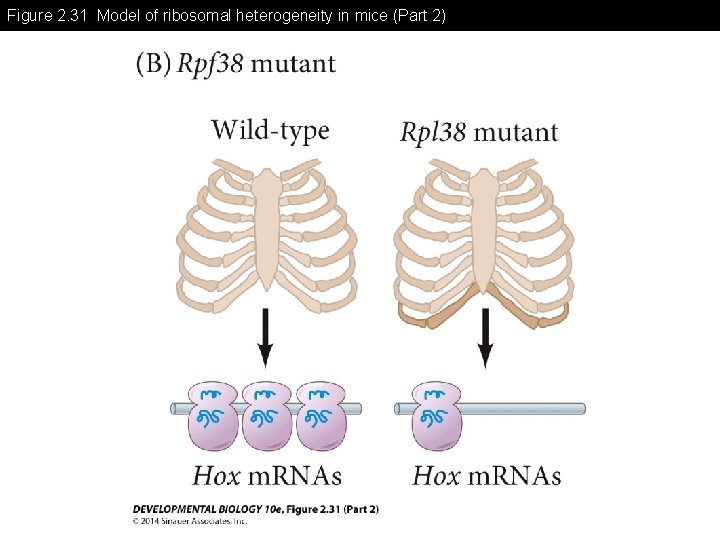
Figure 2. 31 Model of ribosomal heterogeneity in mice (Part 2)
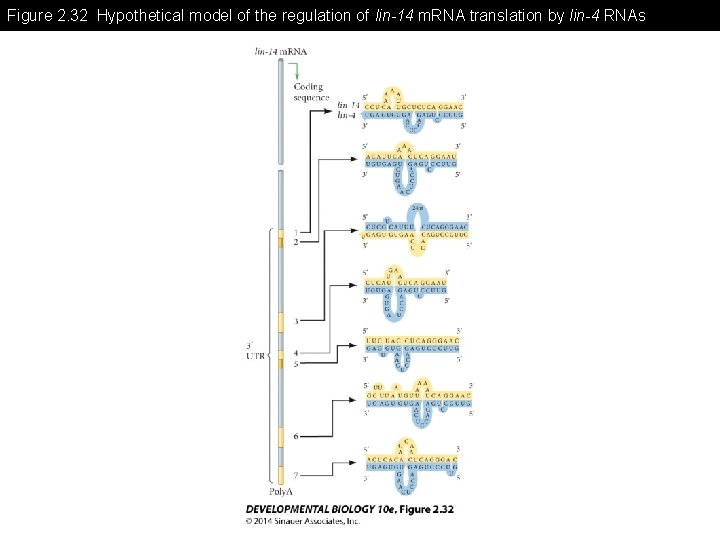
Figure 2. 32 Hypothetical model of the regulation of lin-14 m. RNA translation by lin-4 RNAs
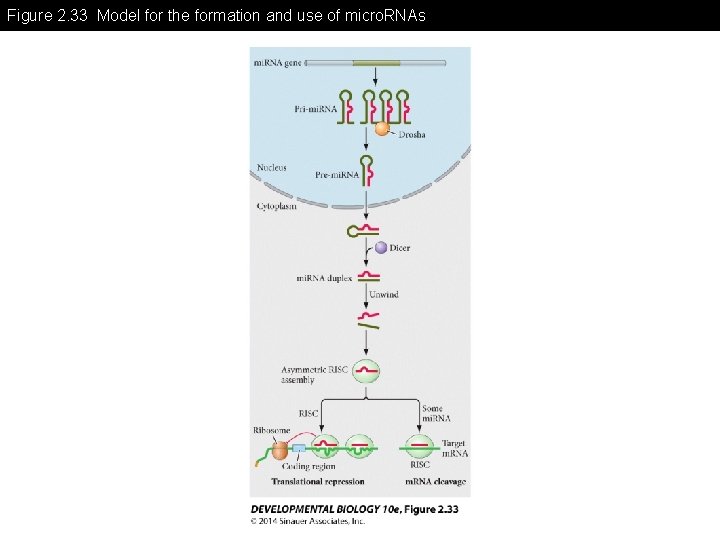
Figure 2. 33 Model for the formation and use of micro. RNAs
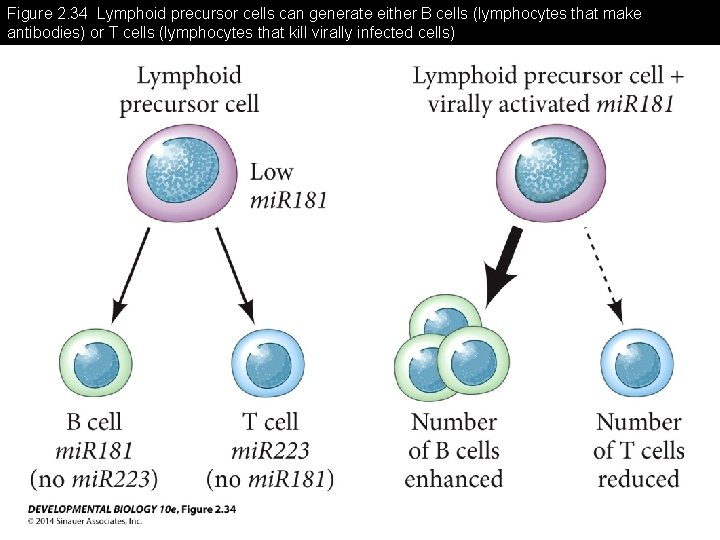
Figure 2. 34 Lymphoid precursor cells can generate either B cells (lymphocytes that make antibodies) or T cells (lymphocytes that kill virally infected cells)
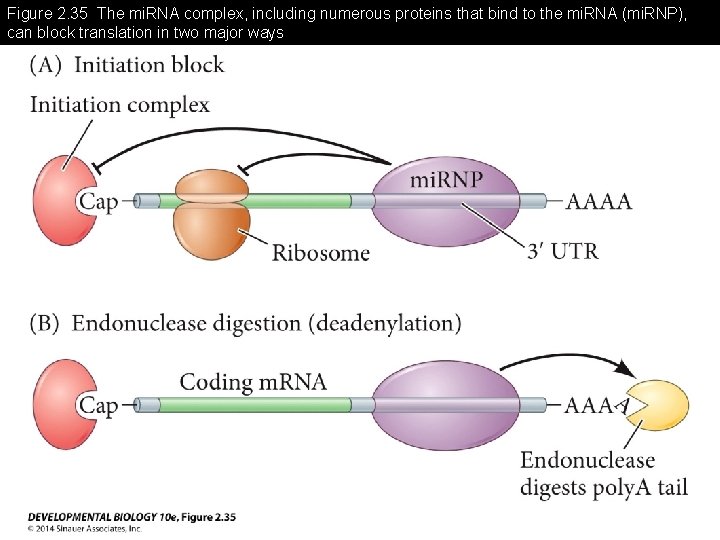
Figure 2. 35 The mi. RNA complex, including numerous proteins that bind to the mi. RNA (mi. RNP), can block translation in two major ways
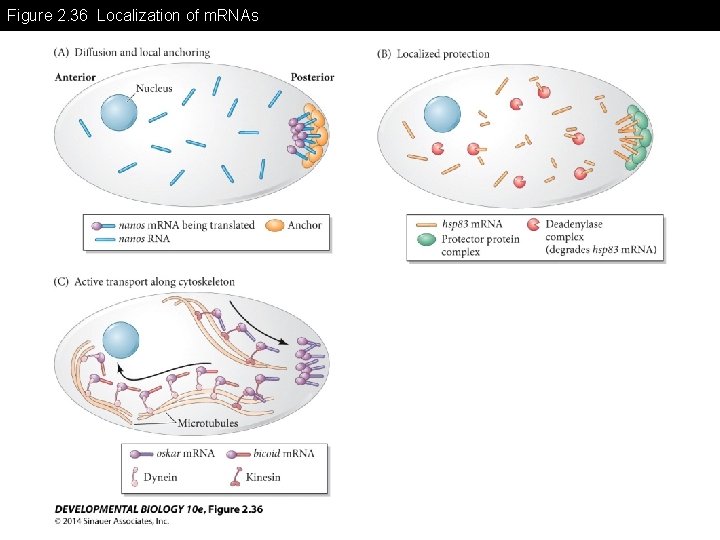
Figure 2. 36 Localization of m. RNAs
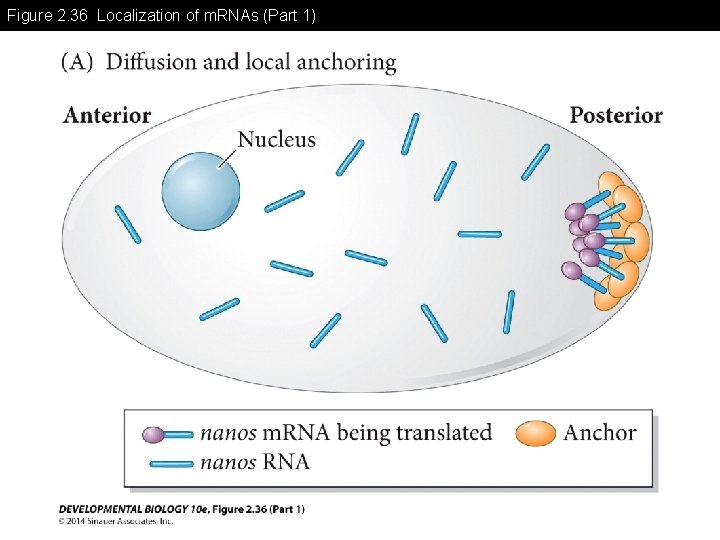
Figure 2. 36 Localization of m. RNAs (Part 1)
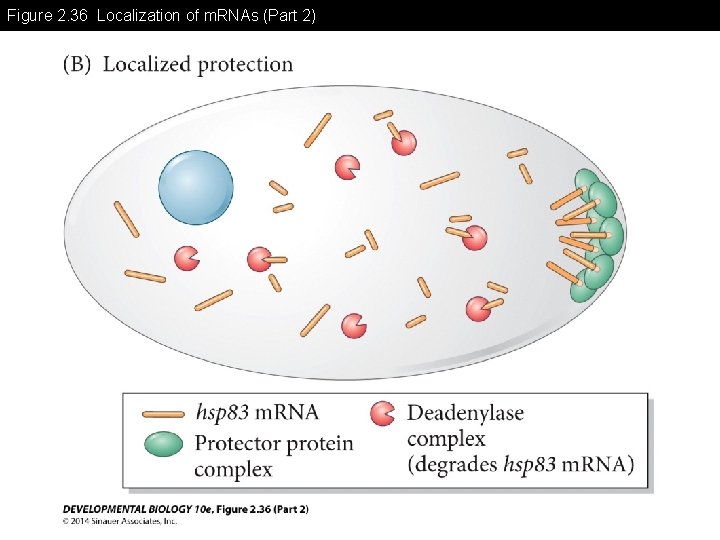
Figure 2. 36 Localization of m. RNAs (Part 2)
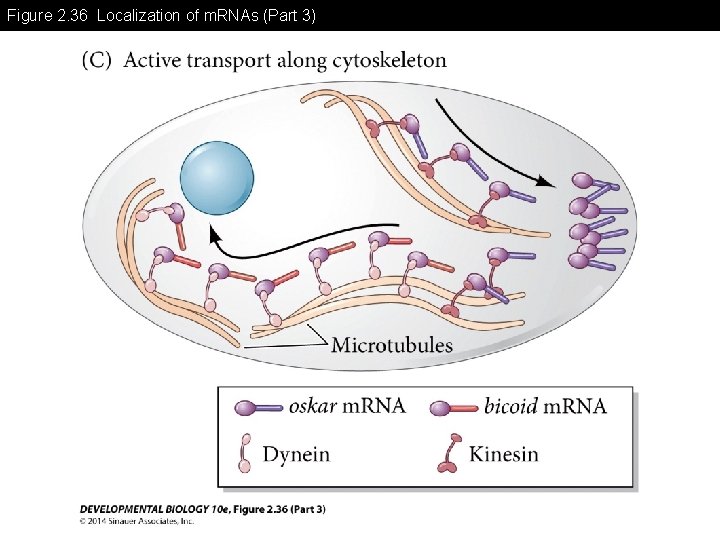
Figure 2. 36 Localization of m. RNAs (Part 3)
- Slides: 81