Chapter 2 Data Analysis WarmUp 1 Match the






















- Slides: 35

Chapter 2 - Data Analysis Warm-Up! • 1. – – Match the unit on the left with the value on the right. a. Milli 1. 1000 x b. Kilo 2. 0. 10 x c. Centi 3. 0. 001 x d. Deci 4. 0. 01 x • 2. – – – Name the metric unit most appropriate for measuring: A. Mass of a penny d. Volume of Pepsi B. Distance to Tahoe e. Your mass C. Length of your shoe f. Temperature of your coffee • 3. a) You have a gold ring with a volume of 0. 75 cm 3. Given that the density of gold is 19. 31 g/cm 3, what is the mass of that gold? – b) If gold is worth $900 per ounce, how much is your ring worth? (0. 040 ounce/gram) 9. 04

Chapter 2 - Data Analysis Test Your Metric Knowledge 1. A gram is about the weight of: (a) _____ an apple (b) _____ a dime (c) _____ a pineapple 2. A meter is about the height of: (a) _____ a door (b) _____ a kitchen counter, or a doorknob (c) _____ the seat of a chair 3. Water freezes and boils at: (a) _____ 32 °C and 212 °C (b) _____ 100 °C and 200 °C (c) _____ 0 °C and 100 °C 4. A coffee cup holds about (a) _____ 2 milliliters (m. L) (b) _____ 20 m. L (c) _____ 250 m. L 5. A newborn baby weighs about: (a) _____ 3 kilograms (kg) (b) _____ 30 kg 9. 04 (c) _____ 300 kg 6. The height of a tall man is about: (a) _____ 20 centimeters (cm) (b) _____ 200 cm (c) _____ 2000 cm 7. Normal body temperature is: (a) _____ 25 °C (degrees Celsius) (b) _____ 37 °C (c) _____ 45 °C 8. A liter of milk is: (a) _____ larger than a quart (b) _____ smaller than a quart (c) _____ the same size as a quart 9. A liter of water weighs: (a) _____ 1000 grams (g) (b) _____ 20 g (c) _____ 100 g 10. The thickness of a dime is about: (a) _____ 0. 1 millimeters (mm) (b) _____ 1 mm (c) _____ 5 mm

Chapter 2 - Data Analysis Answers: 1. b 2. b 3. c 4. c 5. a 6. b 7. b 8. a 9. a 10. b 9. 04

Chapter 2 - Data Analysis • Why is measurement important? 9. 04

Chapter 2 - Data Analysis I. Units of Measurement 2. 1 (pages 25 – 30) A. Why the Metric System? National Metric Week: Oct. 9 - 15, 2011 (10 th month and the week containing the 10 th day) 9. 04

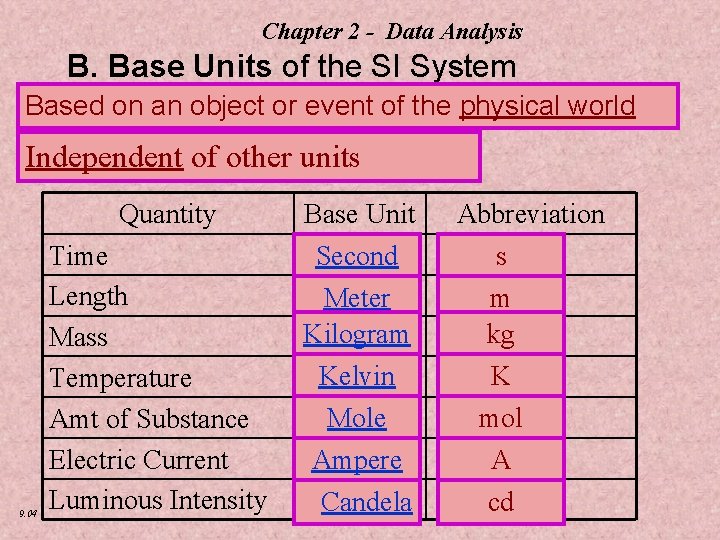
Chapter 2 - Data Analysis B. Base Units of the SI System Based on an object or event of the physical world Independent of other units Quantity 9. 04 Time Length Mass Temperature Amt of Substance Electric Current Luminous Intensity Base Unit Second Meter Kilogram Kelvin Mole Ampere Candela Abbreviation s m kg K mol A cd

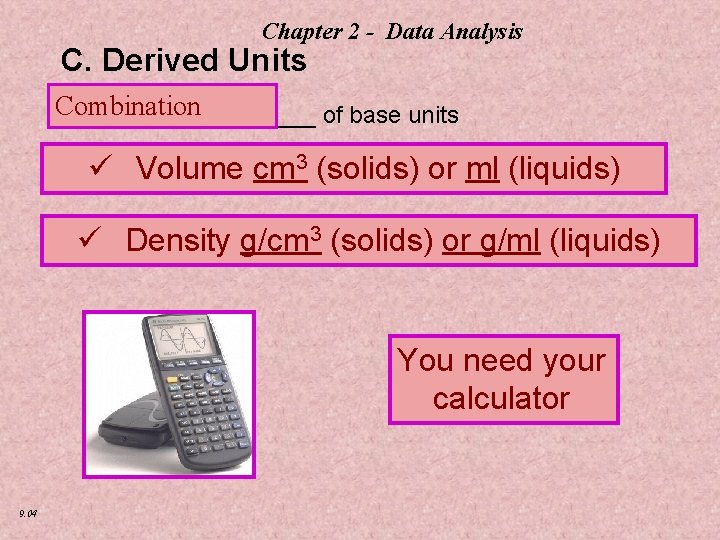
Chapter 2 - Data Analysis C. Derived Units Combination __________ of base units ü Volume cm 3 (solids) or ml (liquids) ü Density g/cm 3 (solids) or g/ml (liquids) You need your calculator 9. 04

Chapter 2 - Data Analysis Density Challenge! • Goal: Determine the largest amt of sand that can be added to a film canister so that it can still float in a container of water. What to do: – Obtain a film canister and ruler. – Calculate total mass needed for floating (water has a density of 1 g/cm 3) – Add sand to container to get to obtain desired mass – Bring film canister w/ sand to Ms. Buchanan for testing – If it floats (without tipping over) – you get 10 lab pts! • You can buy a hint for 2 pts. 9. 04 – If it sinks or tips, you get 5 pts. – Highest mass that floats – 5 xc points! – Second highest mass that floats – 3 xc points!

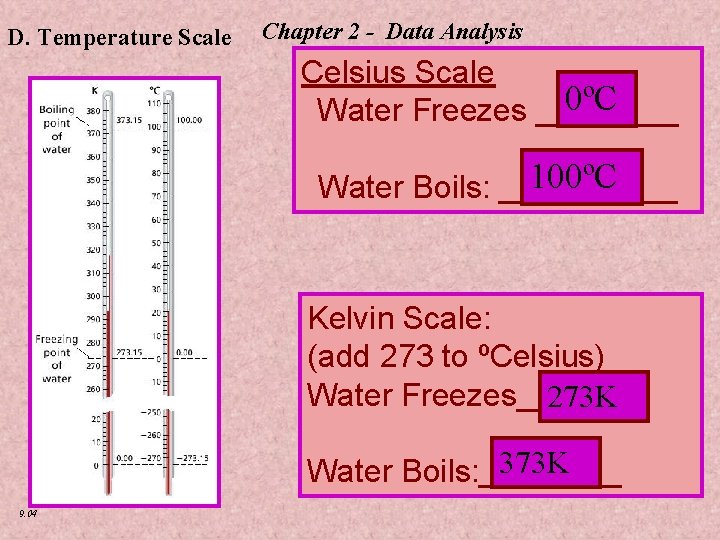
D. Temperature Scale Chapter 2 - Data Analysis Celsius Scale 0ºC Water Freezes ____ 100ºC Water Boils: _____ Kelvin Scale: (add 273 to ºCelsius) Water Freezes_______ 273 K 373 K Water Boils: ____ 9. 04

Chapter 2 - Data Analysis II. Scientific Notation & Dimensional Analysis A. Scientific Notation 1. Handling numbers: üin a gram of Hydrogen there are 602, 214, 000, 000 atoms üdistance between particles in a salt crystal is 0. 000 002 814 cm üadd 0. 000 000 036 + 0. 000 000 046 = ? Easier to use scientific notation 9. 04 M x 10 n M = between 1 & 10 n = integer (1, 2, 3. . . )

Chapter 2 - Data Analysis Examples: (from above) 1) 6. 02214 x 1023 2) 2. 814 x 10 -9 3) 3. 6 x 10 -11 + 4. 6 x 10 -14 = ? 3. 6046 x 10 -11 9. 04

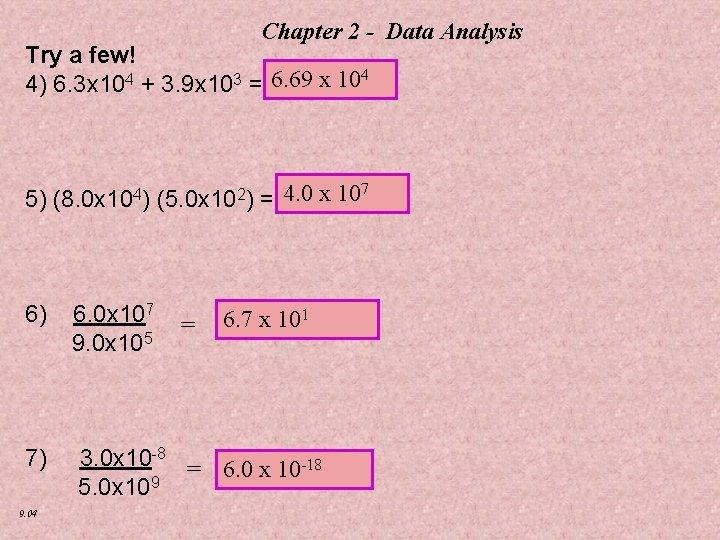
Chapter 2 - Data Analysis Try a few! 4 4) 6. 3 x 104 + 3. 9 x 103 =? 6. 69 x 10 7 5) (8. 0 x 104) (5. 0 x 102) =? 4. 0 x 10 6) 6. 0 x 107 = 6. 7 x 101 9. 0 x 105 7) 3. 0 x 10 -8 = 6. 0 x 10 -18 5. 0 x 109 9. 04

Chapter 2 - Data Analysis Estimate how far the winning jump was in feet. 9. 04

7. 15 m X 100 cm 1 m X Chapter 2 - Data Analysis 1 inch 1 ft 2. 54 cm X 12 in J. Faklaris = = 23. 5 feet 9. 04

Chapter 2 - Data Analysis D. Dimensional Analysis (aka Factor label) p. 34 -35 Activity Directions: • Table groups, take the cards out of your envelope. • Find the card showing the island people. How many people live on the island? • Now find a card with a person and a house. • How many houses are on the island? • How many dogs are on the island? • How many cats are on the island? • How many pine trees are on the island? 9. 04

Chapter 2 - Data Analysis 2. Examples – without the cards! a. How many meters in a one hundred yard dash? 1 inch = 2. 54 cm 100 yds X 3 ft 1 yd 9. 04 X 12 in 1 ft X 2. 54 cm 1 in X 1 m 100 cm 91. 4 m = ? m

Chapter 2 - Data Analysis b. How many kg in a 4. 00 ounce Mc. Donald's hamburger? 1 kg = 1000 g 16 ounces = 1 pound = 454 grams 0. 114 kg 9. 04

Chapter 2 - Data Analysis c. If Shaq is 7'2" tall how many millimeters tall is he? 1 inch = 2. 54 cm 2184 mm 9. 04

Chapter 2 - Data Analysis d. Convert 8 wags to warps. 1 wag = 12 zooms 1000 warps = 1 bam 32, 000 warps 9. 04 3 zoom = 1 bam

Chapter 2 - Data Analysis e. A computer switches 60 times in a microsecond, how many times does it switch in a minute? 1, 000 microsecond = 1 sec 3. 6 x 109 switches in a minute 9. 04

Chapter 2 - Data Analysis f. How many milliliters in a 12 fl oz can of soda? 1000 ml = 1 L, 1 L = 1. 06 quarts, 4 quarts = 1 gal, 1 gal = 128 fluid oz. 354 m. L 9. 04

Chapter 2 - Data Analysis Warm-Up – Dimensional Analysis • Calculate the time required for a student aide to earn $567 at $9. 00 per hour. – Answer – 63. 0 hours • How many square feet are in 6. 60 square yards? – Answer: 59. 4 ft 2 • Change 15 mph to feet per second. – Answer = 22 feet per second 9. 04

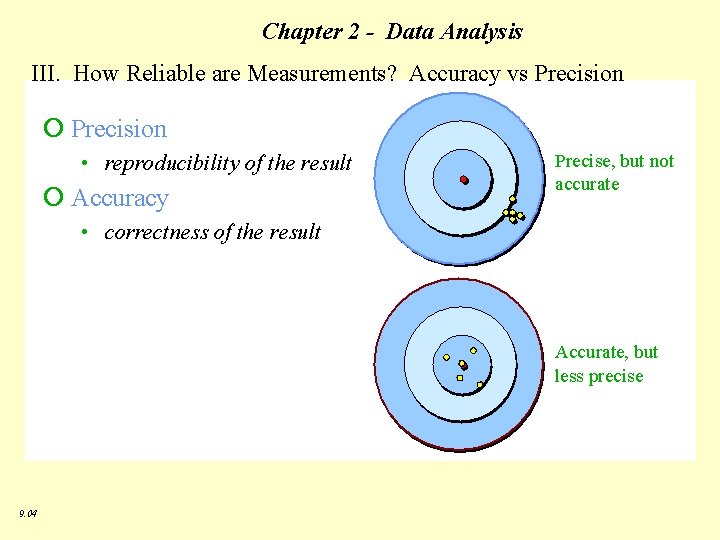
Chapter 2 - Data Analysis III. How Reliable are Measurements? Accuracy vs Precision 9. 04

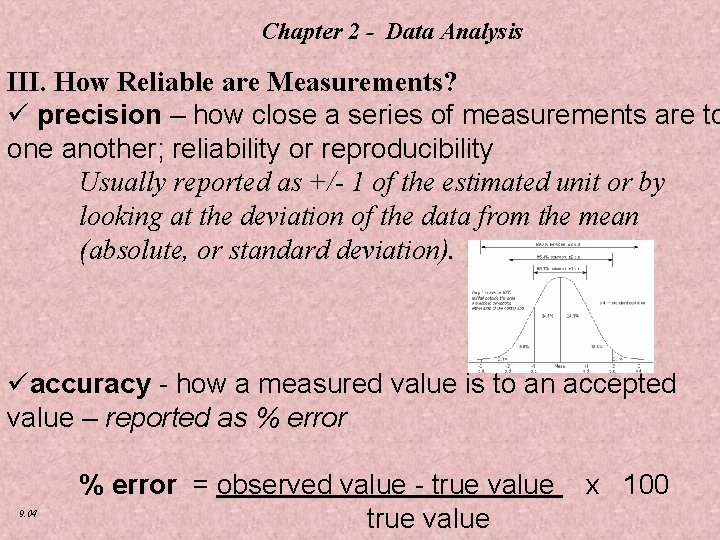
Chapter 2 - Data Analysis III. How Reliable are Measurements? ü precision – how close a series of measurements are to one another; reliability or reproducibility Usually reported as +/- 1 of the estimated unit or by looking at the deviation of the data from the mean (absolute, or standard deviation). üaccuracy - how a measured value is to an accepted value – reported as % error 9. 04 % error = observed value - true value x 100 true value

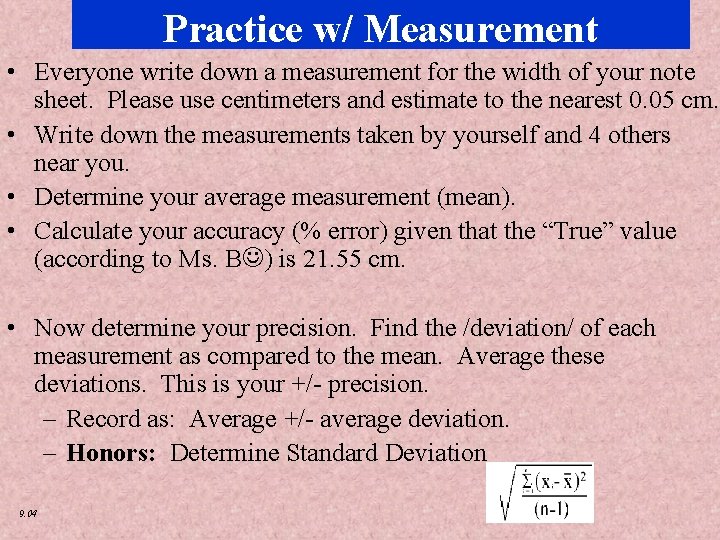
Chapter - Data Analysis Practice w/2 Measurement • Everyone write down a measurement for the width of your note sheet. Please use centimeters and estimate to the nearest 0. 05 cm. • Write down the measurements taken by yourself and 4 others near you. • Determine your average measurement (mean). • Calculate your accuracy (% error) given that the “True” value (according to Ms. B ) is 21. 55 cm. • Now determine your precision. Find the /deviation/ of each measurement as compared to the mean. Average these deviations. This is your +/- precision. – Record as: Average +/- average deviation. – Honors: Determine Standard Deviation 9. 04

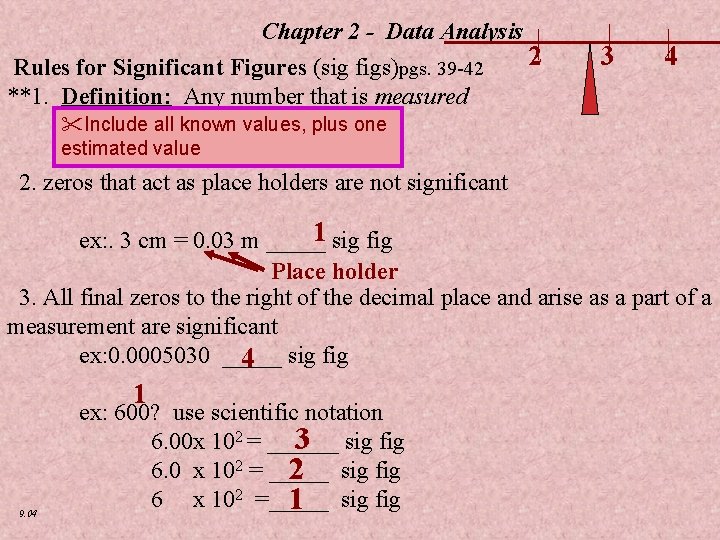
Chapter 2 - Data Analysis Rules for Significant Figures (sig figs)pgs. 39 -42 **1. Definition: Any number that is measured Include all known values, plus one 2 3 4 estimated value 2. zeros that act as place holders are not significant 1 ex: . 3 cm = 0. 03 m _____ sig fig Place holder 3. All final zeros to the right of the decimal place and arise as a part of a measurement are significant ex: 0. 0005030 _____ sig fig 4 1 9. 04 ex: 600? use scientific notation 3 6. 00 x 102 = ______ sig fig 6. 0 x 102 = _____ sig fig 2 6 x 102 =_____ sig fig 1

Chapter 2 - Data Analysis 4. Non-zero measurements are always significant 5. Zeros between non-zero numbers are always significant 9. 04

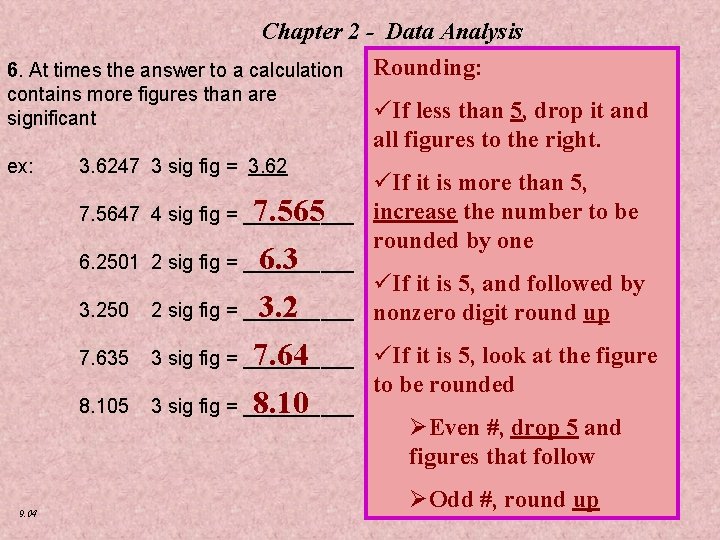
Chapter 2 - Data Analysis 6. At times the answer to a calculation contains more figures than are significant ex: 3. 6247 3 sig fig = 3. 62 7. 5647 4 sig fig = _____ 6. 2501 2 sig fig = _____ 3. 250 2 sig fig = _____ 7. 635 3 sig fig = _____ 8. 105 3 sig fig = _____ 7. 565 6. 3 3. 2 7. 64 8. 10 9. 04 Rounding: üIf less than 5, drop it and all figures to the right. üIf it is more than 5, increase the number to be rounded by one üIf it is 5, and followed by nonzero digit round up üIf it is 5, look at the figure to be rounded ØEven #, drop 5 and figures that follow ØOdd #, round up

Chapter 2 - Data Analysis 7. The result of an addition or subtraction should be reported to the same number of decimal places as that of the term with the least number of decimal places. ex: 1611. 032 5. 6 + 32. 4524 1649. 0844? 9. 04 =1649. 1

Chapter 2 - Data Analysis 8. The answer to a multiplication or division problem is rounded off to the same number of sig fig as is possessed by the term having the fewest significant figures used in the calculation. ex: 152. 06 x 0. 24 = 36. 4944? = 36 9. 04

Chapter 2 - Data Analysis Based on the data given which day received the most rain? How might this data be better organized? 9. 04

Chapter 2 - Data Analysis The End 9. 04

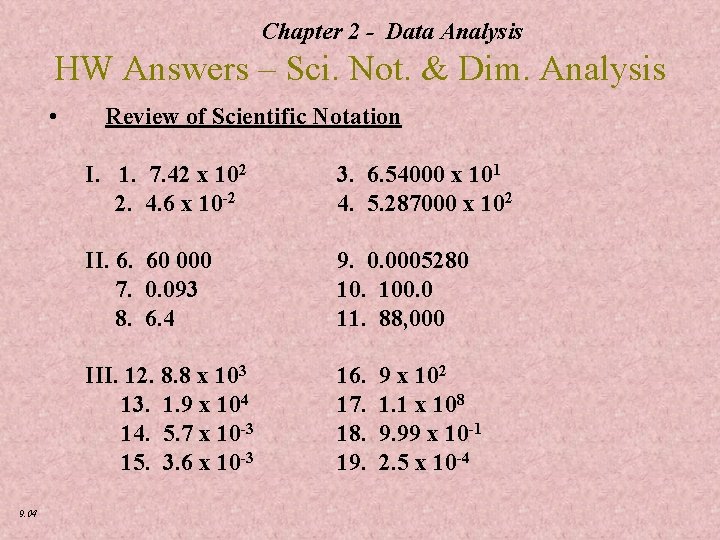
Chapter 2 - Data Analysis HW Answers – Sci. Not. & Dim. Analysis • 9. 04 Review of Scientific Notation I. 1. 7. 42 x 102 2. 4. 6 x 10 -2 3. 6. 54000 x 101 4. 5. 287000 x 102 II. 6. 60 000 7. 0. 093 8. 6. 4 9. 0. 0005280 10. 100. 0 11. 88, 000 III. 12. 8. 8 x 103 13. 1. 9 x 104 14. 5. 7 x 10 -3 15. 3. 6 x 10 -3 16. 17. 18. 19. 9 x 102 1. 1 x 108 9. 99 x 10 -1 2. 5 x 10 -4

Chapter 2 - Data Analysis HW: Dimensional Analysis Practice Problems • • • 9. 04 1. 4, 741 km 2. 7. 265 L 3. 0. 93 mi 4. 0. 918 g 5. 2. 40 m. L 6. 8 servings 7. 27. 8 m/s 8. 7. 99 g/m. L 9. 6. 25 x 106 kg 10 – 0. 0964 mm

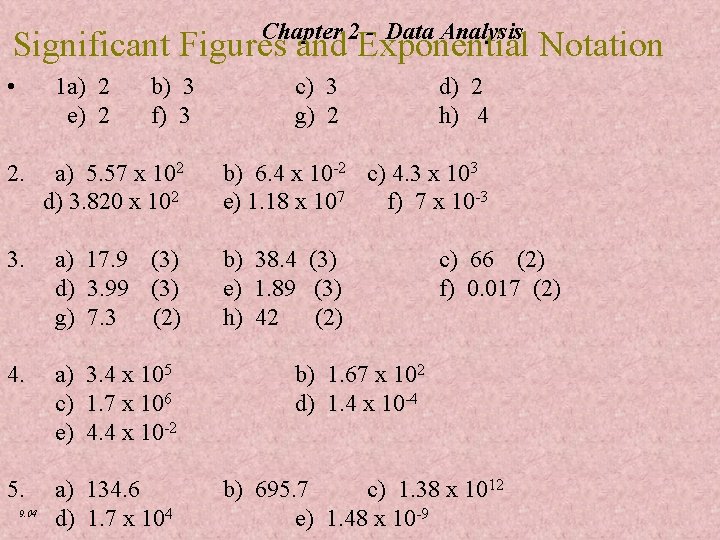
Chapter 2 - Data Analysis Significant Figures and Exponential Notation • 1 a) 2 e) 2 b) 3 f) 3 c) 3 g) 2 d) 2 h) 4 2. a) 5. 57 x 102 b) 6. 4 x 10 -2 c) 4. 3 x 103 d) 3. 820 x 102 e) 1. 18 x 107 f) 7 x 10 -3 3. a) 17. 9 (3) d) 3. 99 (3) g) 7. 3 (2) 4. a) 3. 4 x 105 c) 1. 7 x 106 e) 4. 4 x 10 -2 b) 1. 67 x 102 d) 1. 4 x 10 -4 5. a) 134. 6 d) 1. 7 x 104 b) 695. 7 c) 1. 38 x 1012 e) 1. 48 x 10 -9 9. 04 b) 38. 4 (3) e) 1. 89 (3) h) 42 (2) c) 66 (2) f) 0. 017 (2)