CHAPTER 2 CHEMISTRY OF LIFE Level of organisation












- Slides: 32

CHAPTER 2 CHEMISTRY OF LIFE

Level of organisation § ATOM § MOLECULE § ELEMENT § COMPOUND § SOLUTION Water

§ Only 4 of the 90 elements make up more than 96% of the mass of the human body. They are: Carbon (C) Hydrogen (H) Oxygen (O) Nitrogen (N)

Mixture and Solutions § When elements combine to form a compound, the elements no longer have their original properties. § A mixture is a combination of substance in which the individual components retain their properties. Ex: Sand sugar

§ A solution is mixture in which one or more substances (solute) are distributed evenly in another substance (solvent). Ex: Kool-aid § *The concentration of solute is important to organisms § A suspension is a mixture of water and nondissolved materials

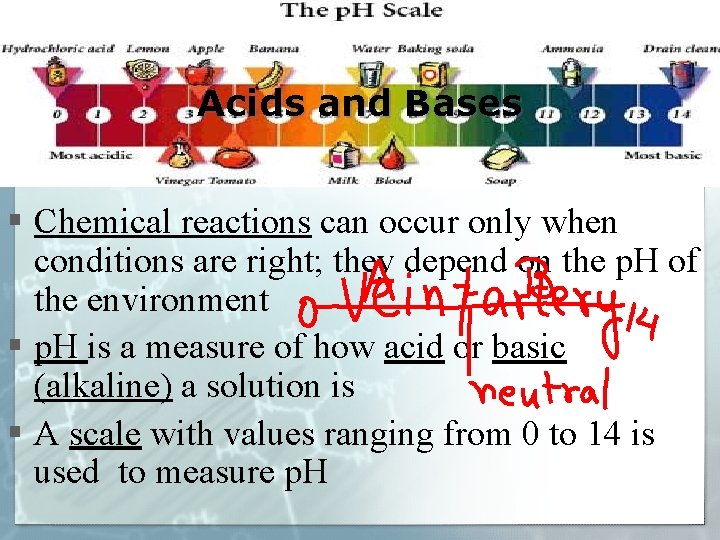
Acids and Bases § Chemical reactions can occur only when conditions are right; they depend on the p. H of the environment § p. H is a measure of how acid or basic (alkaline) a solution is § A scale with values ranging from 0 to 14 is used to measure p. H

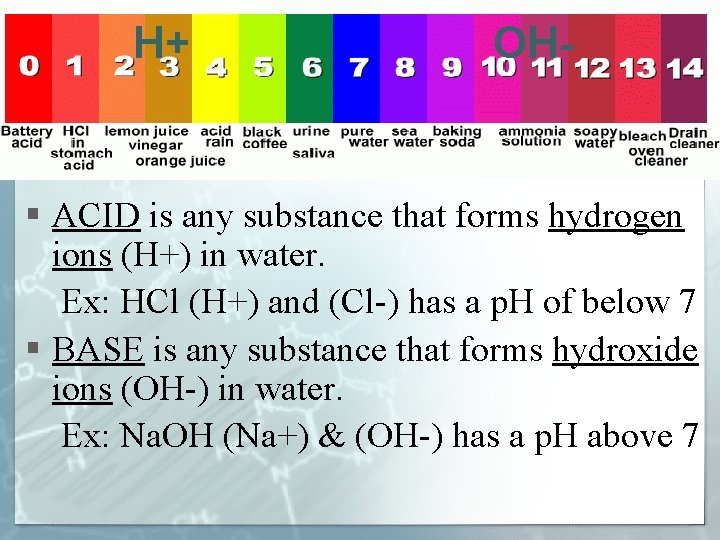
H+ OH- § ACID is any substance that forms hydrogen ions (H+) in water. Ex: HCl (H+) and (Cl-) has a p. H of below 7 § BASE is any substance that forms hydroxide ions (OH-) in water. Ex: Na. OH (Na+) & (OH-) has a p. H above 7

§ Buffers=dissolved compounds that control p. H in the body ( HOMEOSTASIS) § Buffers are weak acids or bases that can react with strong acids or bases to prevent sharp sudden changes in p. H.

Importance of Acids and Bases to Biological Systems § Chemical reactions in organisms depend on the p. H of the environment Ex: Pepsidase is an enzyme that works best in the acidic human stomach

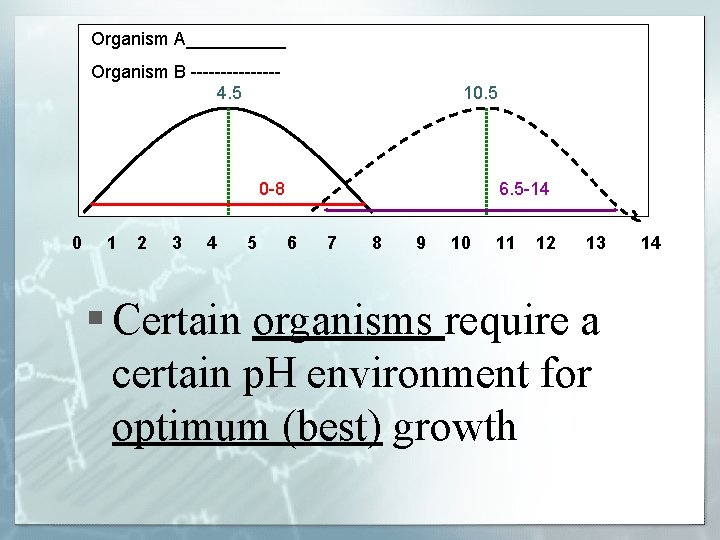
Organism A_____ Organism B -------4. 5 10. 5 0 -8 0 1 2 3 4 5 6. 5 -14 6 7 8 9 10 11 12 13 § Certain organisms require a certain p. H environment for optimum (best) growth 14

Life Substances 1. Organic compounds are derived from living things and contain Carbon, must have Carbon and Hydrogen to be organic 2. Inorganic compounds are derived from nonliving things (ex: Water, Carbon Dioxide)

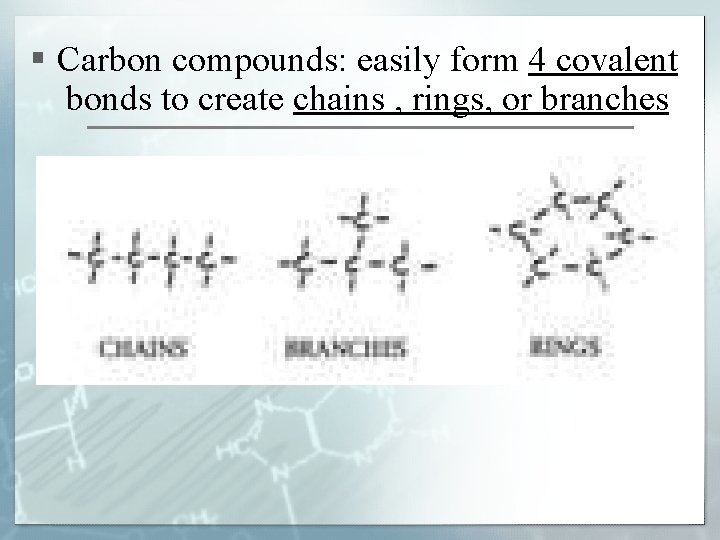
§ Carbon compounds: easily form 4 covalent bonds to create chains , rings, or branches

• Polymerization: when a large compound (polymer) is produced from smaller compounds (monomers) as the smaller compounds are joined together. • Macromolecules: large polymers

§ Condensation Reaction (dehydration synthesis) to make or build, water is produced § Hydrolysis to split, water is added

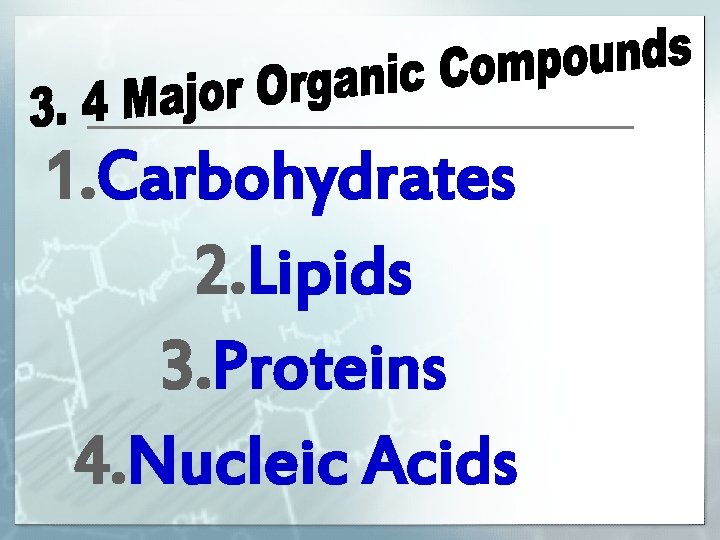
1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids

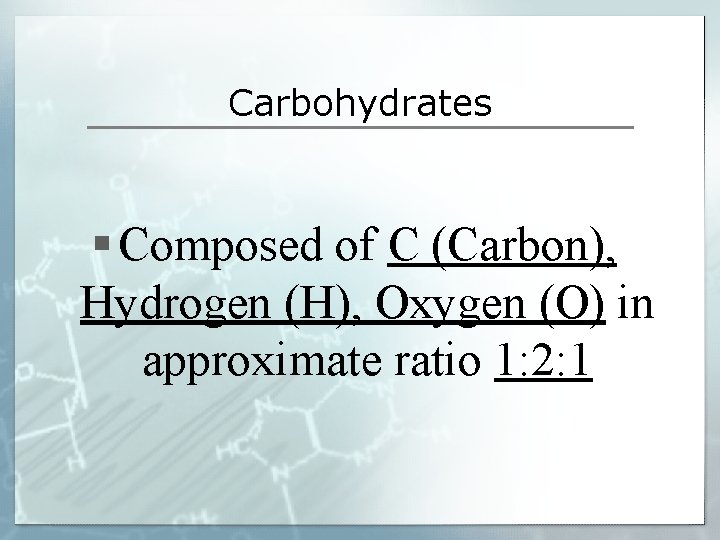
Carbohydrates § Composed of C (Carbon), Hydrogen (H), Oxygen (O) in approximate ratio 1: 2: 1

Monosaccharide: single (simple) sugar Molecular formula for all 3: C 6 H 12 O 6 § GLUCOSE-Produced by plants through photosynthesis § FRUCTOSE-found in fruits § GALACTOSE-found in milk

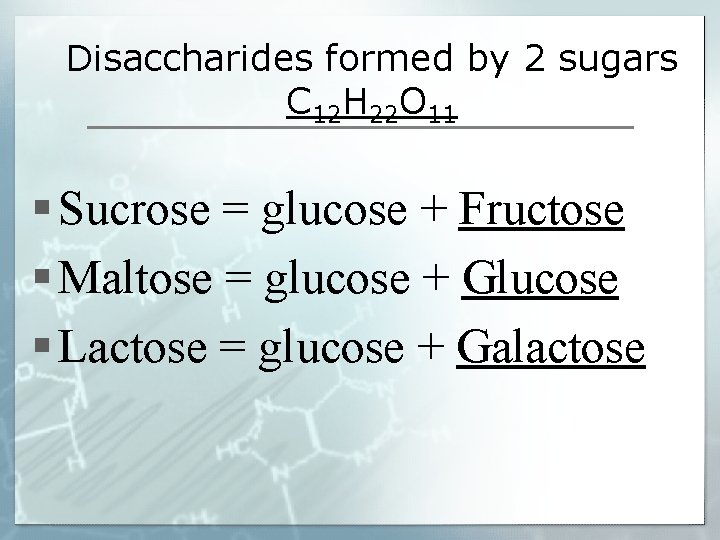
Disaccharides formed by 2 sugars C 12 H 22 O 11 § Sucrose = glucose + Fructose § Maltose = glucose + Glucose § Lactose = glucose + Galactose

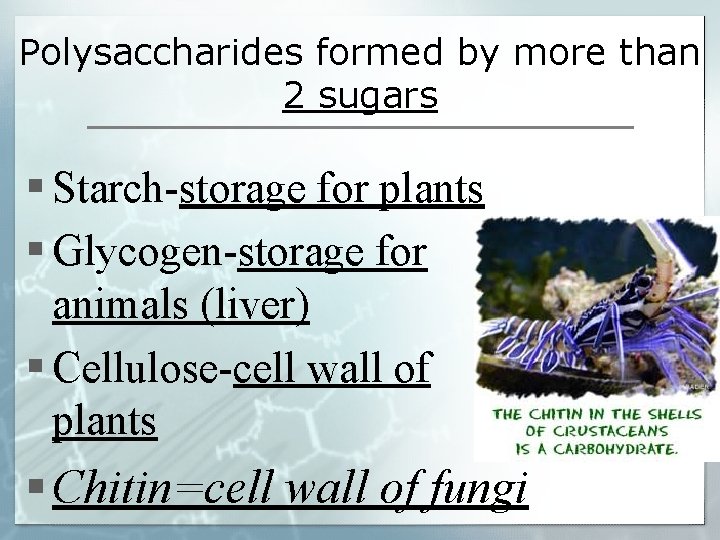
Polysaccharides formed by more than 2 sugars § Starch-storage for plants § Glycogen-storage for animals (liver) § Cellulose-cell wall of plants § Chitin=cell wall of fungi

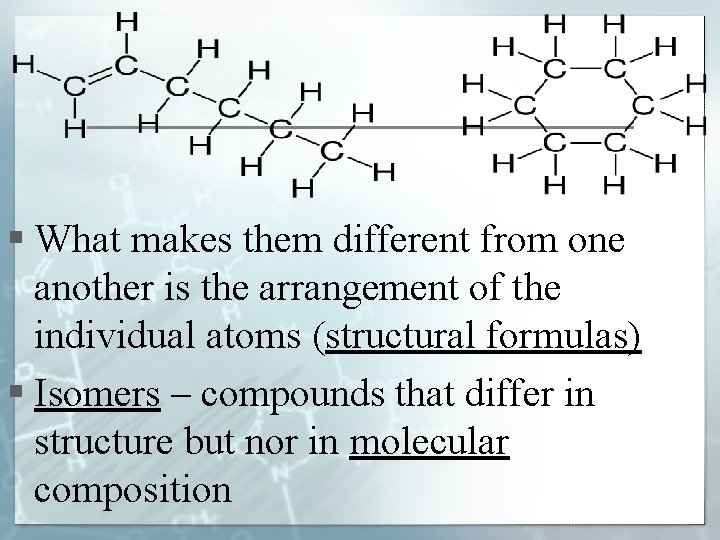
§ What makes them different from one another is the arrangement of the individual atoms (structural formulas) § Isomers – compounds that differ in structure but nor in molecular composition

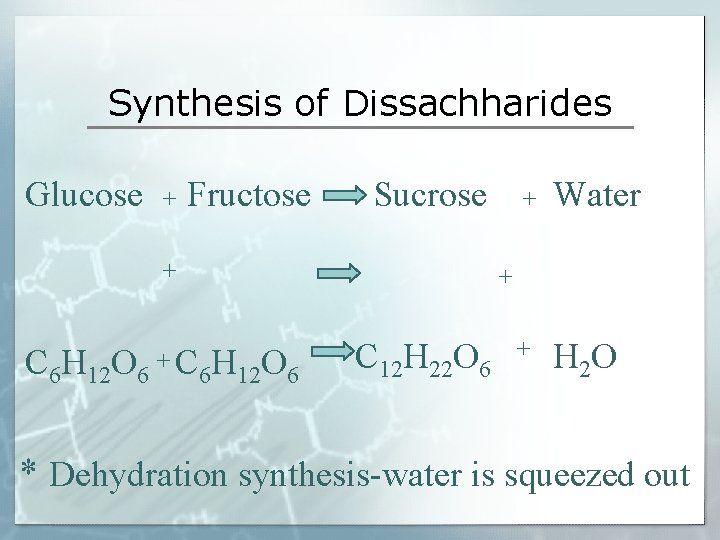
Synthesis of Dissachharides Glucose + Fructose Sucrose + C 6 H 12 O 6 +C + Water + H 2 O + 6 H 12 O 6 C 12 H 22 O 6 * Dehydration synthesis-water is squeezed out

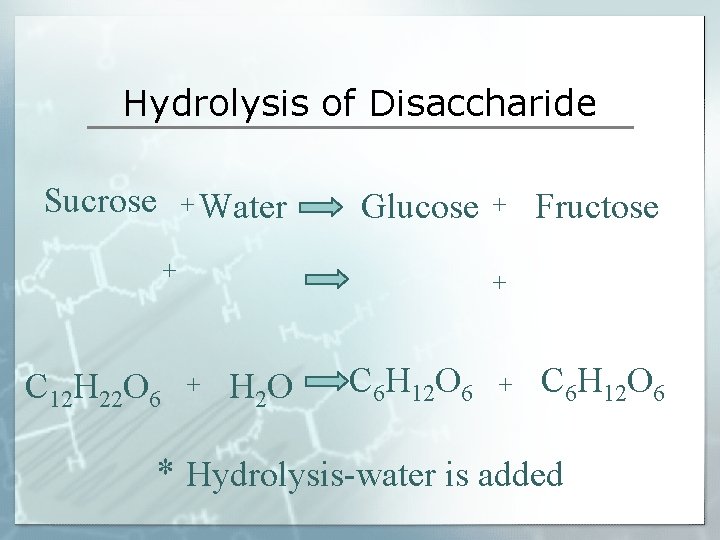
Hydrolysis of Disaccharide Sucrose + Water Glucose + C 12 H 22 O 6 + Fructose + + H 2 O C 6 H 12 O 6 + C 6 H 12 O 6 * Hydrolysis-water is added

Lipids: Fatty Compounds § Made of C, H, O w/ a greater # in C: H atoms and a smaller # of O atoms than carbohydrates (No uniform Ratio) § Ex: fats, oils, waxes (do Not dissolve in water)

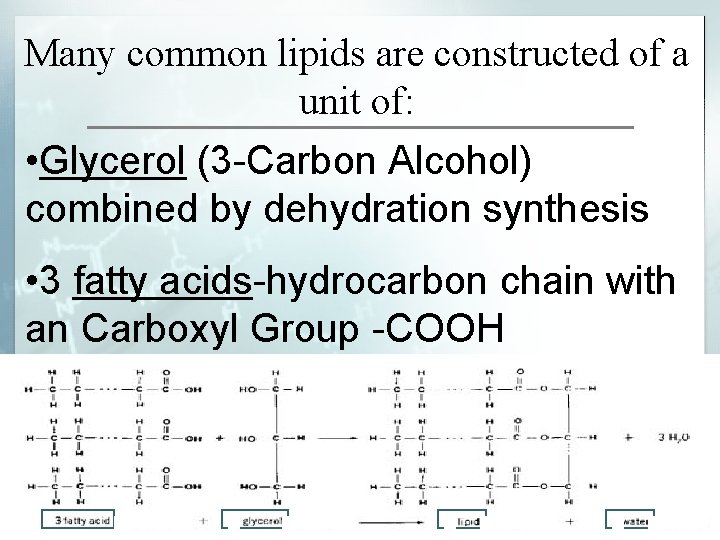
Many common lipids are constructed of a unit of: • Glycerol (3 -Carbon Alcohol) combined by dehydration synthesis • 3 fatty acids-hydrocarbon chain with an Carboxyl Group -COOH

3 fatty acids-hydrocarbon chain with an Carboxyl Group -COOH § Hydrophilic End (water loving-carboxyl end that is polar) § Hydrophobic End (water fearinghydrocarbon end that is nonpolar)

§ Functions: forms much of cell membrane to serve as a barrier between the inside and outside of the cell – energy storage for cells § Ex: waxes, triglycerides

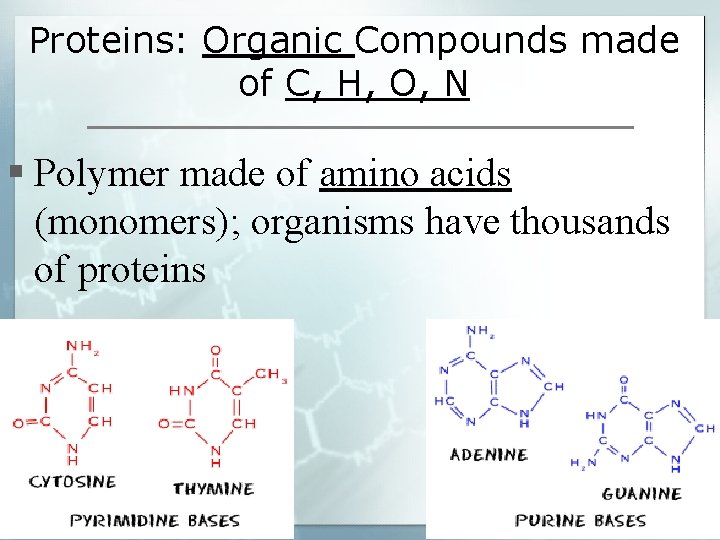
Proteins: Organic Compounds made of C, H, O, N § Polymer made of amino acids (monomers); organisms have thousands of proteins

Amino Acids: 20 different kinds that form proteins-has 5 Groups: a) Central C atom b) Single H atom c) Carboxyl Group (COOH) d) Amine Group (NH 2) e) R Group (repeating CH 2 + CH 2 of different lengths)

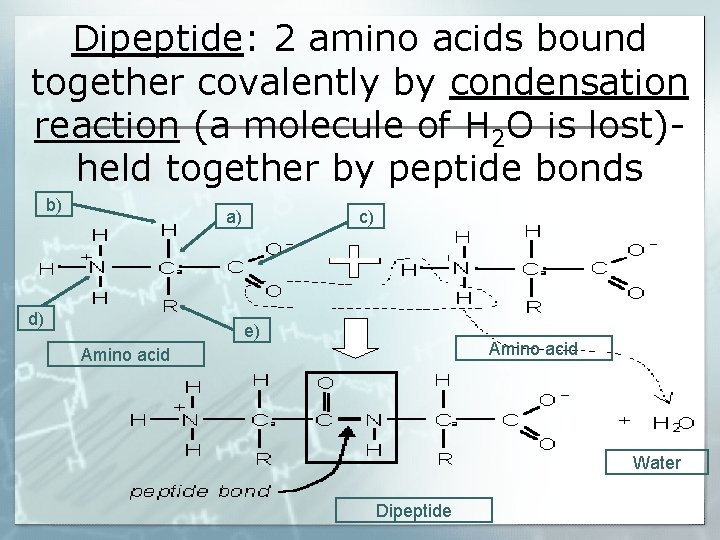
Dipeptide: 2 amino acids bound together covalently by condensation reaction (a molecule of H 2 O is lost)held together by peptide bonds b) a) d) c) e) Amino acid Water Dipeptide

Polypeptide: § A long chain of amino acids held together by peptide bonds § Ex of Proteins: Insulin (hormone), hemoglobin, and enzymes

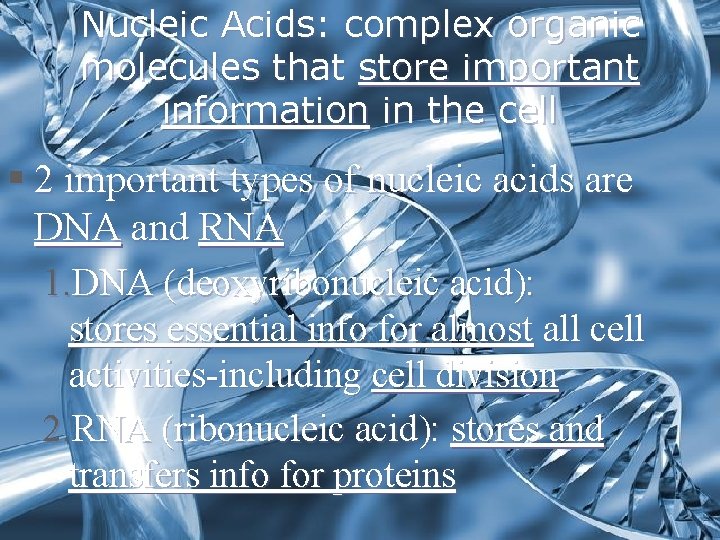
Nucleic Acids: complex organic molecules that store important information in the cell § 2 important types of nucleic acids are DNA and RNA 1. DNA (deoxyribonucleic acid): stores essential info for almost all cell activities-including cell division 2. RNA (ribonucleic acid): stores and transfers info for proteins

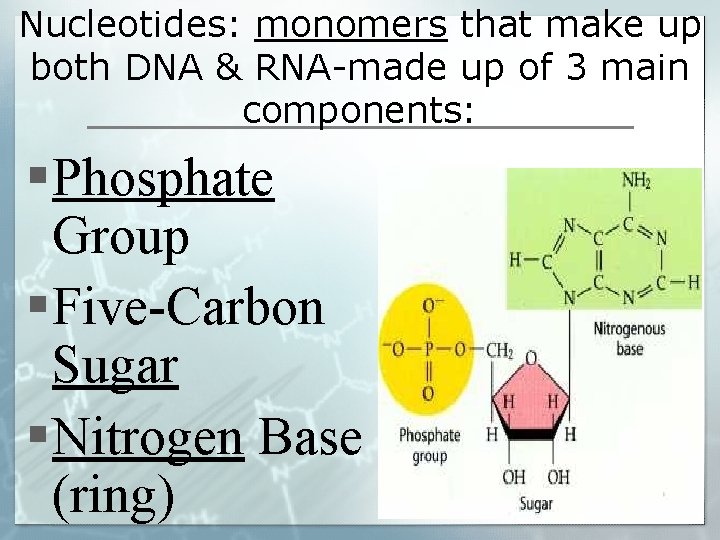
Nucleotides: monomers that make up both DNA & RNA-made up of 3 main components: § Phosphate Group § Five-Carbon Sugar § Nitrogen Base (ring)