Chapter 2 Chemistry Basic Chemistry 1 Elements Substances

Chapter 2: Chemistry
Basic Chemistry 1. Elements • Substances that CANNOT be broken down into simpler substances by chemical processes • Represented by symbols 1 or 2 letters Ex: oxygen (O) sodium (Na) chloride (Cl) hydrogen (H) nitrogen (N) iron (Fe)
Cont. Basic Chemistry 2. Compounds • Substances made of 2 or more elements chemically combined in definite proportions • Represented by formulas tells the number & kind of each atom Ex: water (H 2 O) salt (Na. Cl) calcium carbonate (Ca. CO 3) carbon dioxide (CO 2) glucose (C 6 H 12 O 6) • Organic compounds contains carbon-hydrogen bonds Ex: proteins, carbohydrates (CHO), lipids, & nucleic acids
Cont. Basic Chemistry 3. Atoms • Smallest unit of matter that still retains the properties of an element • Building blocks of matter • Subatomic Particles: a. Protons (+) charge - Found in nucleus (center of atom) b. Neutrons neutral - Found in nucleus c. Electrons (-) charge - Found outside of nucleus in energy levels - Always in constant motion - Important in chemical properties
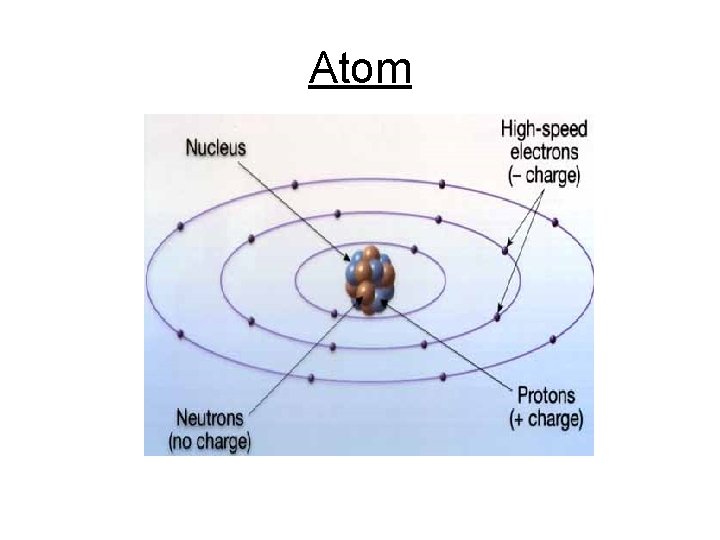
Atom
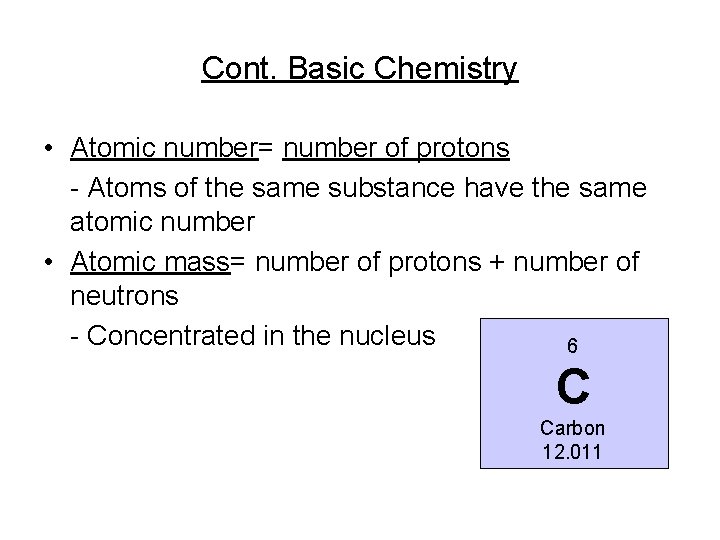
Cont. Basic Chemistry • Atomic number= number of protons - Atoms of the same substance have the same atomic number • Atomic mass= number of protons + number of neutrons - Concentrated in the nucleus 6 C Carbon 12. 011
Cont. Basic Chemistry 4. Ions • Atoms that have lost or gained electrons • Atoms will lose or gain electrons to achieve stability outer energy level filled - 1 st ring= maximum of 2 electrons - Outer shells= lucky #8 a. Anion atoms that gain electrons - Are negative ions Ex: Cl-, Flb. Cation atoms that lose electrons - Are positive ions Ex: K+, Na+, Ca+2
Cont. Basic Chemistry 5. Isotopes • Atoms of the same element that have different numbers of neutrons, but still the same number of protons - Changes the atomic mass, but not the atomic number Ex: 3 isotopes of carbon (atomic mass= 6) 12 -C, 13 -C, 14 -C • 50 naturally occurring radioactive isotopes unstable nucleus that breaks apart giving off radiation • Radioactive isotopes used: a) to determine age of rocks, fossils, & artifacts b) as tracers or tags shows where chemical reactions are occurring (PET) c) to preserve food & treat cancer
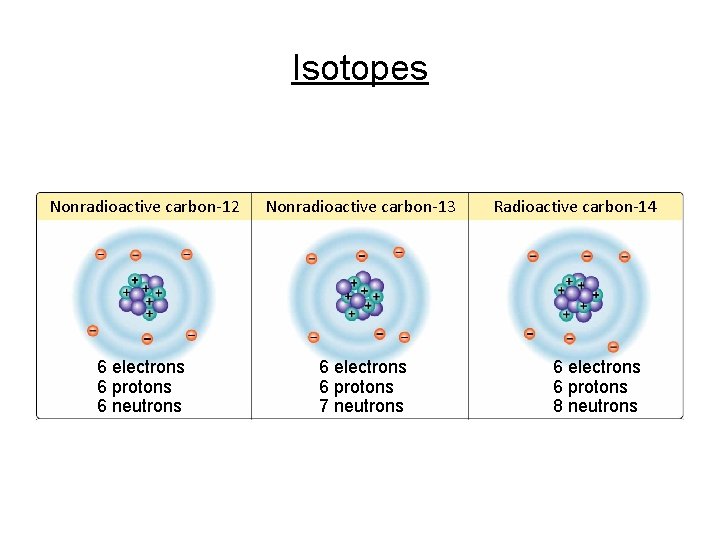
Isotopes Nonradioactive carbon-12 Nonradioactive carbon-13 6 electrons 6 protons 6 neutrons 6 electrons 6 protons 7 neutrons Radioactive carbon-14 6 electrons 6 protons 8 neutrons
Chemistry of Carbon 1. Organic compounds - Contains carbon-hydrogen bonds 2. Inorganic compounds - No carbon-hydrogen bonds • CARBON (atomic structure) reactive (unstable) atom - Must make 4 bonds to become stable - May bond w/ itself or other atoms in many ways, forming many kinds of organic compounds found in living things Ex: CHO, fats, proteins, nucleic acids

Formulas 1. Molecular Formula • List elements present • Shows number of atoms for each element Ex: CH 4, C 6 H 12 O 6 2. Structural Formula • List elements present • Shows number of atoms for each element • Show shape or pattern or arrangement of atoms methane
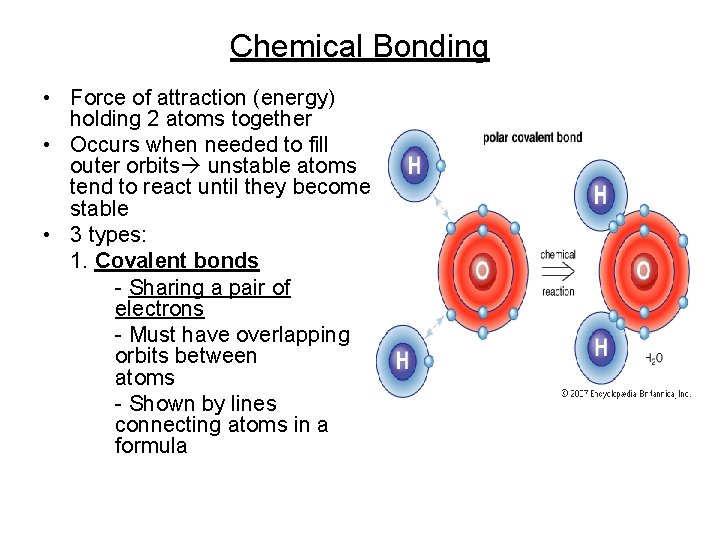
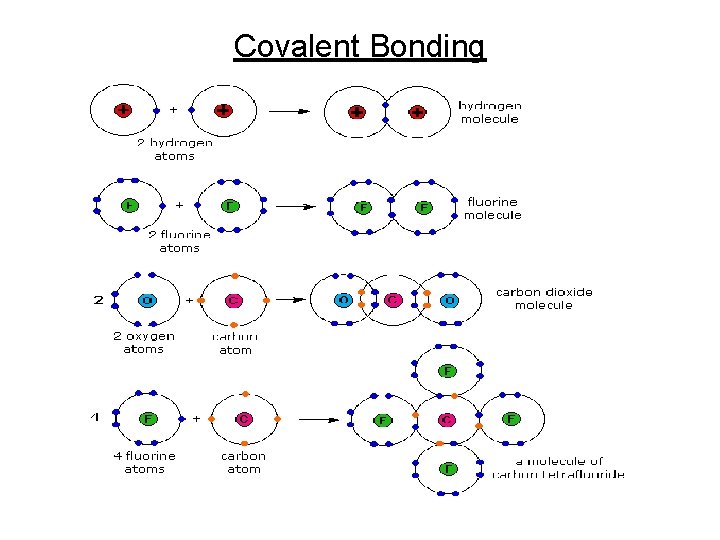
Chemical Bonding • Force of attraction (energy) holding 2 atoms together • Occurs when needed to fill outer orbits unstable atoms tend to react until they become stable • 3 types: 1. Covalent bonds - Sharing a pair of electrons - Must have overlapping orbits between atoms - Shown by lines connecting atoms in a formula

Orbital shells
Covalent Bonding
Cont. Chemical Bonding 2. Ionic bonds - Must involve a transfer of electrons - One atom loses an electron (+), while other gains an electron (-) - Bond will form between 2 oppositely charged ions - Shown by charge signs on ions of molecular formula Ex: Na+Cl-
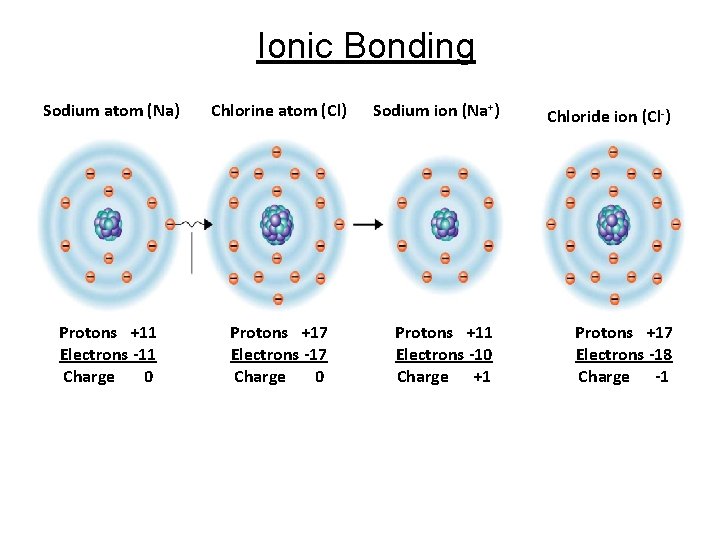
Ionic Bonding Sodium atom (Na) Chlorine atom (Cl) Protons +11 Electrons -11 Charge 0 Protons +17 Electrons -17 Charge 0 Sodium ion (Na+) Protons +11 Electrons -10 Charge +1 Chloride ion (Cl-) Protons +17 Electrons -18 Charge -1
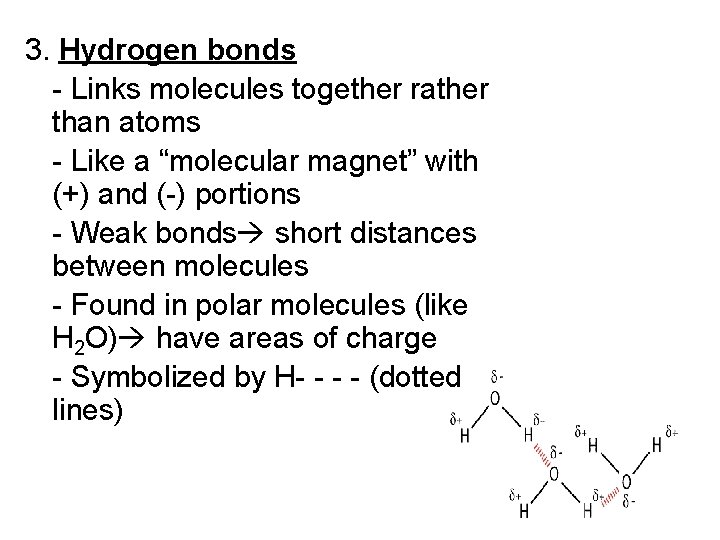
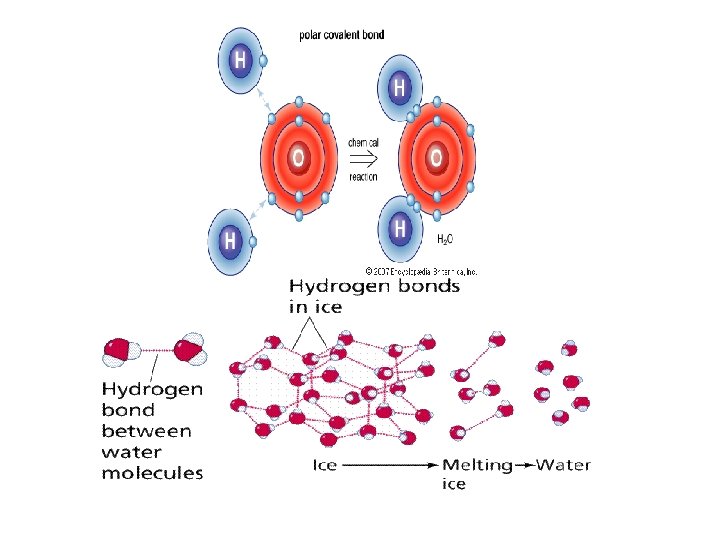
3. Hydrogen bonds - Links molecules together rather than atoms - Like a “molecular magnet” with (+) and (-) portions - Weak bonds short distances between molecules - Found in polar molecules (like H 2 O) have areas of charge - Symbolized by H- - (dotted lines)
Cont. Hydrogen bonds - Help to form shape of important biological molecules (DNA & protein) - Exhibits: a. Cohesion degree of “stickiness” between identical molecules b. Adhesion degree of “sticking” to different molecules c. Surface tension related to cohesion/ a measure of how difficult it is to stretch/ break the surface of a liquid

Properties of Water Adhesion Cohesion Surface Tension
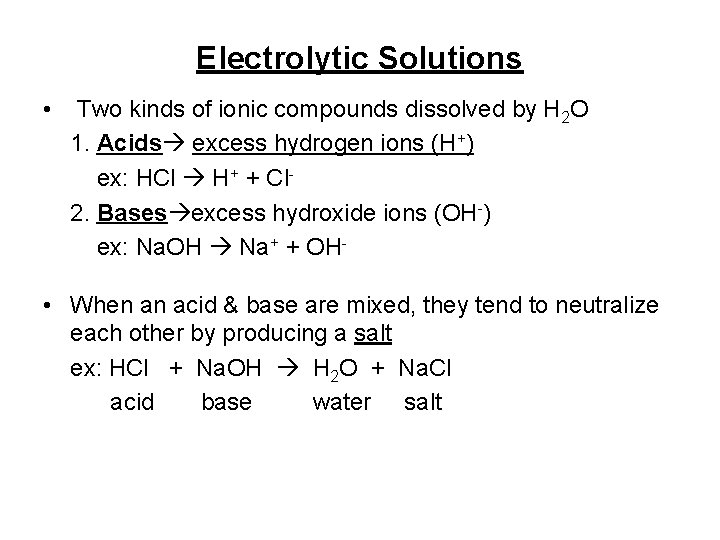
Electrolytic Solutions • Two kinds of ionic compounds dissolved by H 2 O 1. Acids excess hydrogen ions (H+) ex: HCl H+ + Cl 2. Bases excess hydroxide ions (OH-) ex: Na. OH Na+ + OH- • When an acid & base are mixed, they tend to neutralize each other by producing a salt ex: HCl + Na. OH H 2 O + Na. Cl acid base water salt
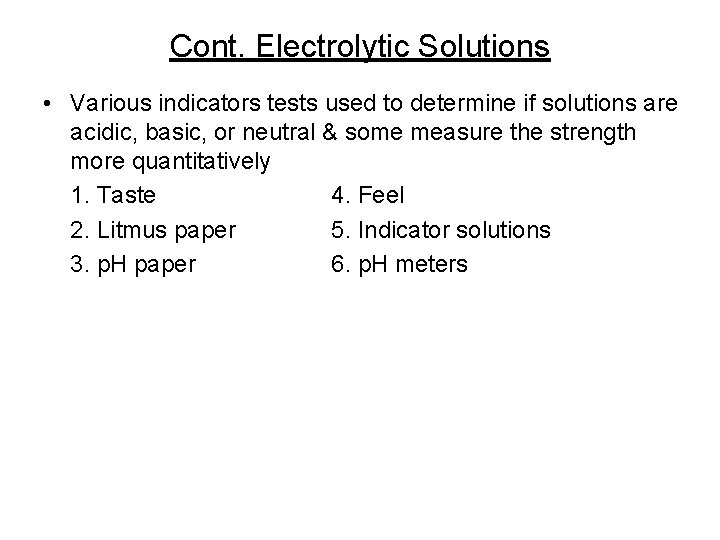
Cont. Electrolytic Solutions • Various indicators tests used to determine if solutions are acidic, basic, or neutral & some measure the strength more quantitatively 1. Taste 4. Feel 2. Litmus paper 5. Indicator solutions 3. p. H paper 6. p. H meters
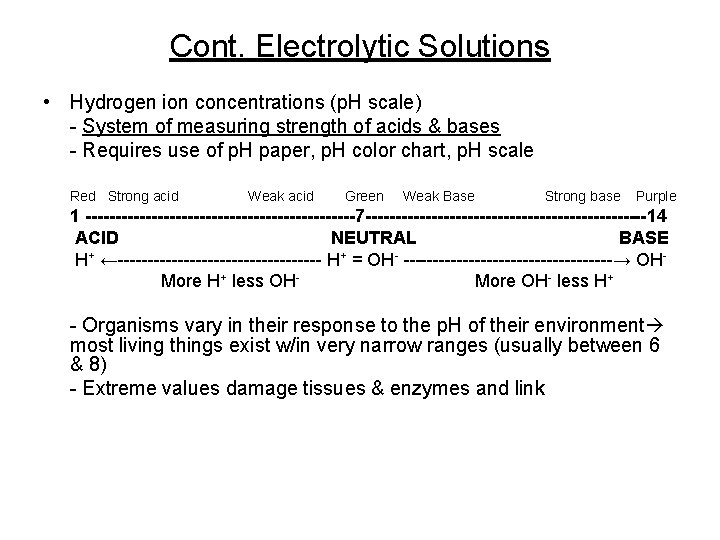
Cont. Electrolytic Solutions • Hydrogen ion concentrations (p. H scale) - System of measuring strength of acids & bases - Requires use of p. H paper, p. H color chart, p. H scale Red Strong acid Weak acid Green Weak Base Strong base Purple 1 -----------------------7 ------------------------14 ACID NEUTRAL BASE H+ ←----------------- H+ = OH- ------------------→ OHMore H+ less OHMore OH- less H+ - Organisms vary in their response to the p. H of their environment most living things exist w/in very narrow ranges (usually between 6 & 8) - Extreme values damage tissues & enzymes and link
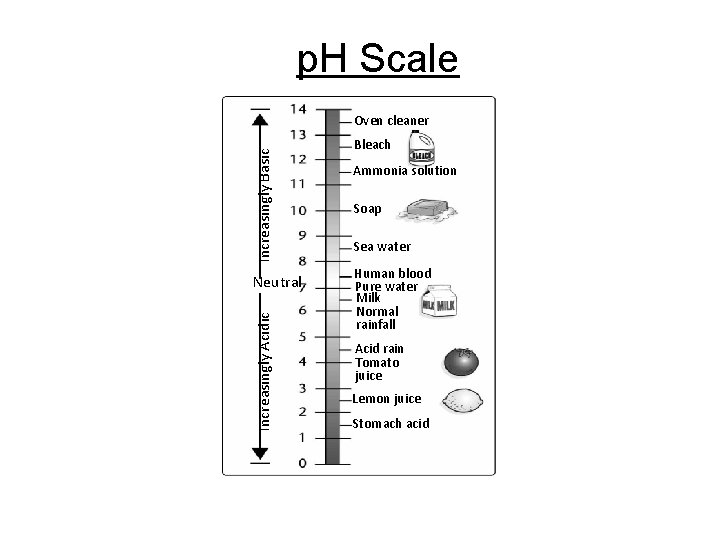
p. H Scale Section 2 -2 Increasingly Basic Oven cleaner Increasingly Acidic Neutral Bleach Ammonia solution Soap Sea water Human blood Pure water Milk Normal rainfall Acid rain Tomato juice Lemon juice Stomach acid

Cont. Ch 2: Organic Molecules

Size of Organic Molecules • Large organic molecules: 1. Carbohydrates (CHO) 2. Proteins 3. Lipids (Fats) 4. Nucleic Acids • All are found in living things • All are made up of many small repeating molecules (monomers) added to make a larger molecule (polymer)
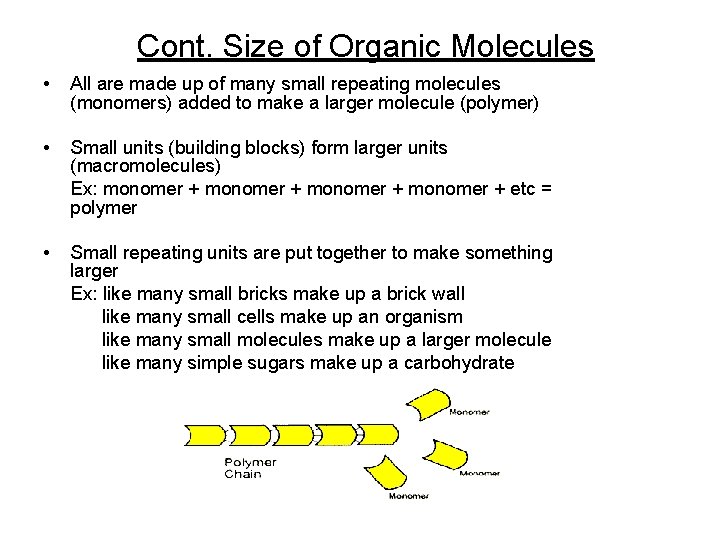
Cont. Size of Organic Molecules • All are made up of many small repeating molecules (monomers) added to make a larger molecule (polymer) • Small units (building blocks) form larger units (macromolecules) Ex: monomer + etc = polymer • Small repeating units are put together to make something larger Ex: like many small bricks make up a brick wall like many small cells make up an organism like many small molecules make up a larger molecule like many simple sugars make up a carbohydrate
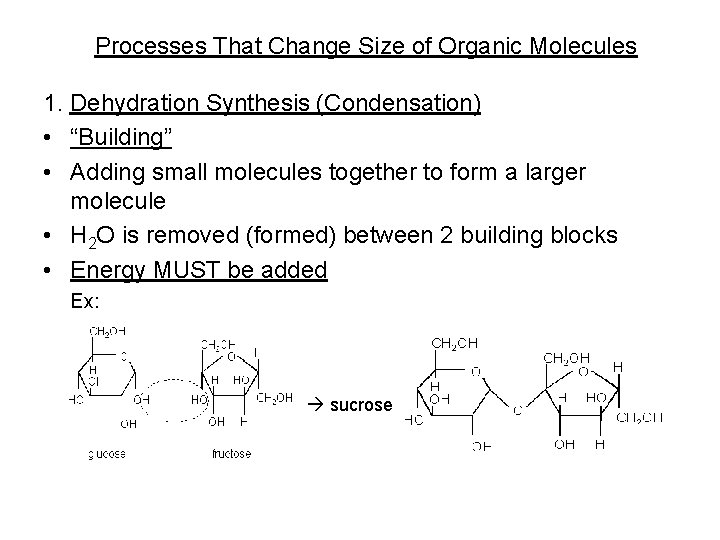
Processes That Change Size of Organic Molecules 1. Dehydration Synthesis (Condensation) • “Building” • Adding small molecules together to form a larger molecule • H 2 O is removed (formed) between 2 building blocks • Energy MUST be added Ex: sucrose
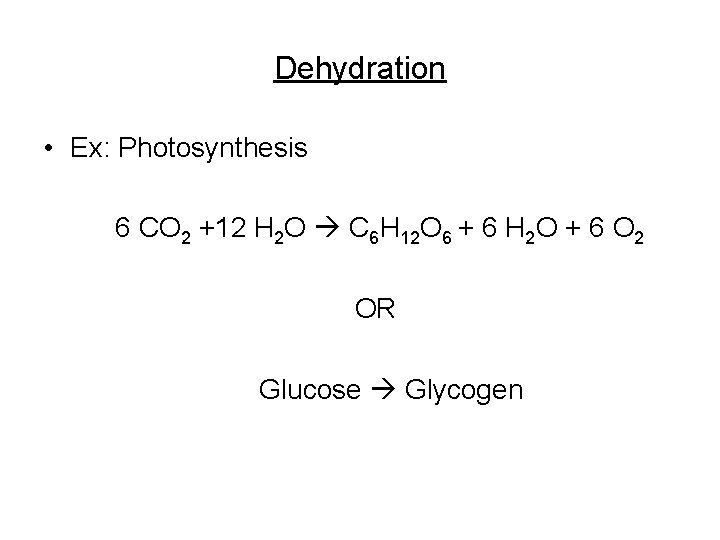
Dehydration • Ex: Photosynthesis 6 CO 2 +12 H 2 O C 6 H 12 O 6 + 6 H 2 O + 6 O 2 OR Glucose Glycogen
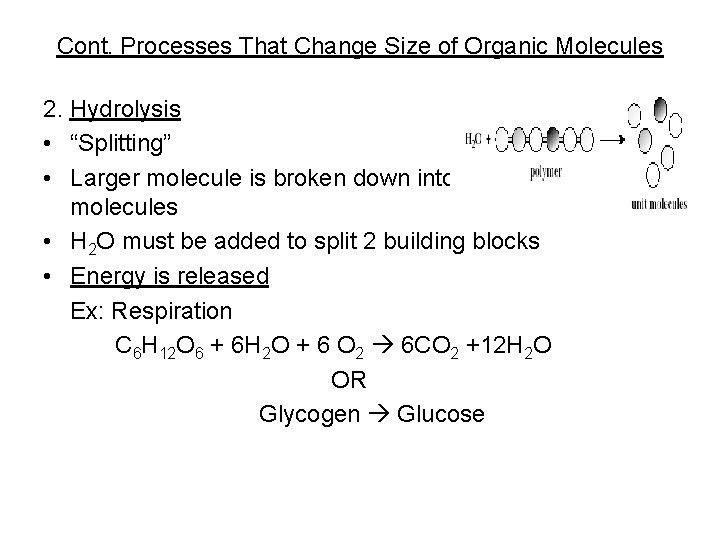
Cont. Processes That Change Size of Organic Molecules 2. Hydrolysis • “Splitting” • Larger molecule is broken down into smaller molecules • H 2 O must be added to split 2 building blocks • Energy is released Ex: Respiration C 6 H 12 O 6 + 6 H 2 O + 6 O 2 6 CO 2 +12 H 2 O OR Glycogen Glucose
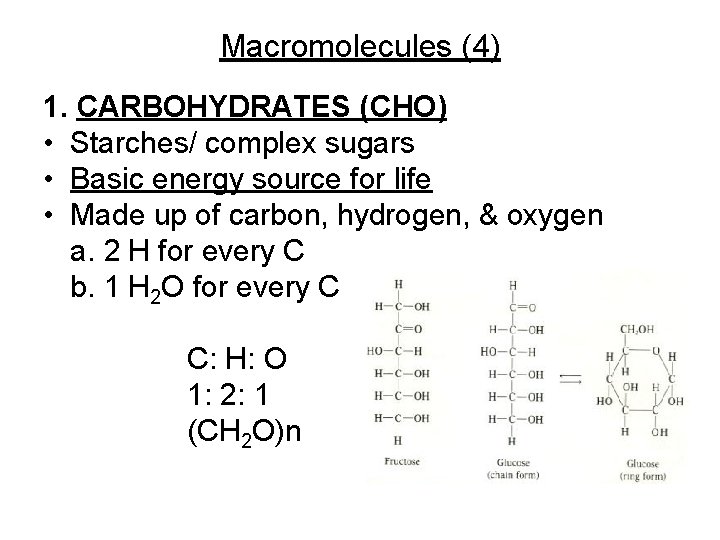
Macromolecules (4) 1. CARBOHYDRATES (CHO) • Starches/ complex sugars • Basic energy source for life • Made up of carbon, hydrogen, & oxygen a. 2 H for every C b. 1 H 2 O for every C C: H: O 1: 2: 1 (CH 2 O)n
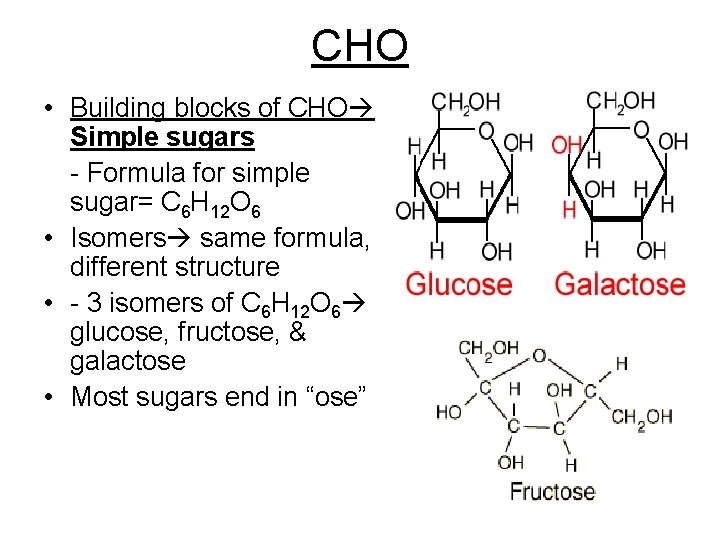
CHO • Building blocks of CHO Simple sugars - Formula for simple sugar= C 6 H 12 O 6 • Isomers same formula, different structure • - 3 isomers of C 6 H 12 O 6 glucose, fructose, & galactose • Most sugars end in “ose”
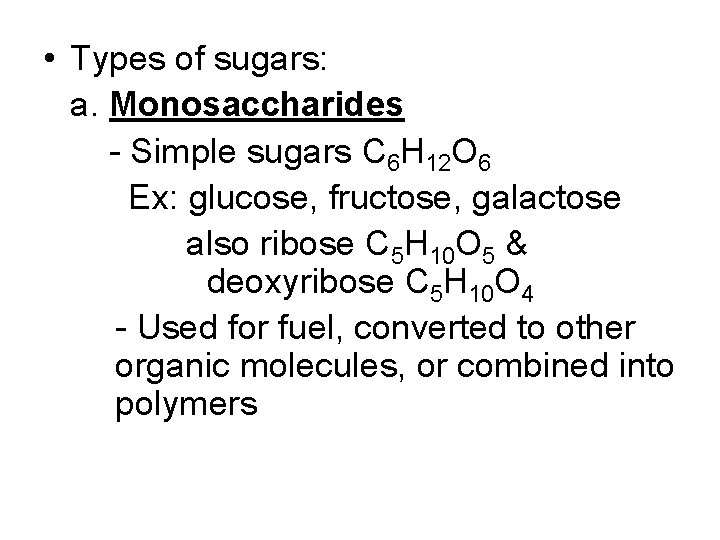
• Types of sugars: a. Monosaccharides - Simple sugars C 6 H 12 O 6 Ex: glucose, fructose, galactose also ribose C 5 H 10 O 5 & deoxyribose C 5 H 10 O 4 - Used for fuel, converted to other organic molecules, or combined into polymers
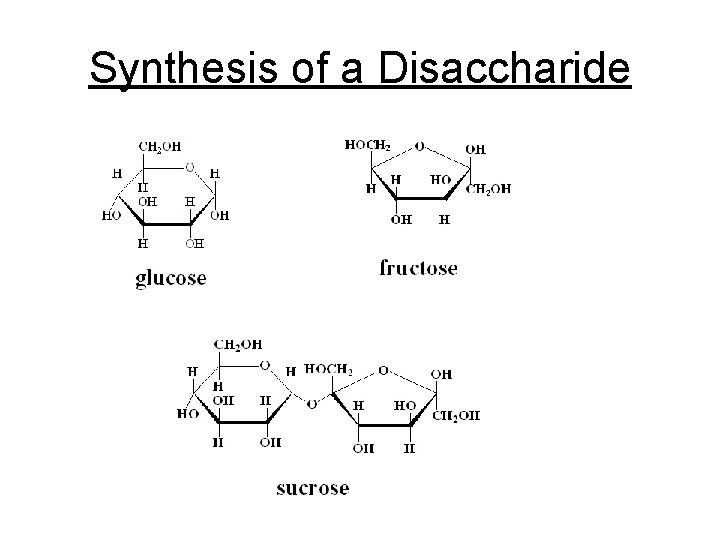
Synthesis of a Disaccharide
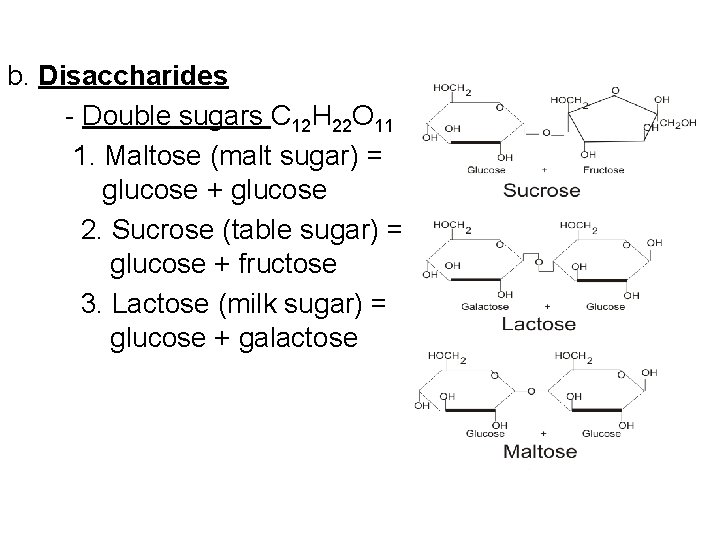
b. Disaccharides - Double sugars C 12 H 22 O 11 1. Maltose (malt sugar) = glucose + glucose 2. Sucrose (table sugar) = glucose + fructose 3. Lactose (milk sugar) = glucose + galactose =
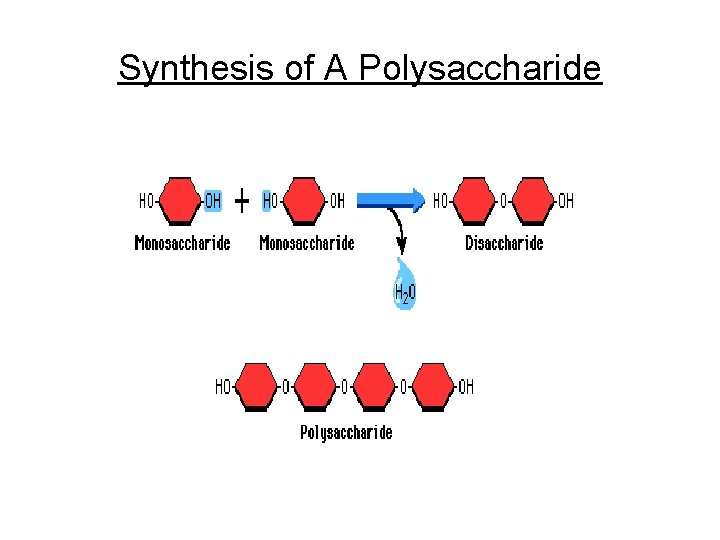
Synthesis of A Polysaccharide

c. Polysaccharides - Complex sugars 1. Cellulose plant cell walls 2. Glycogen animal starch (stored in liver & muscle cell) 3. Plant starch stored in plant vacuoles 4. Chitin exoskeletons of insects & crustaceans
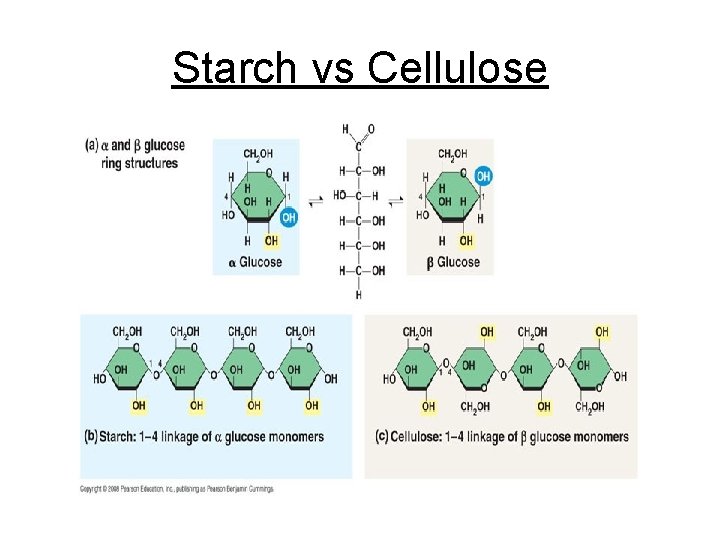
Starch vs Cellulose
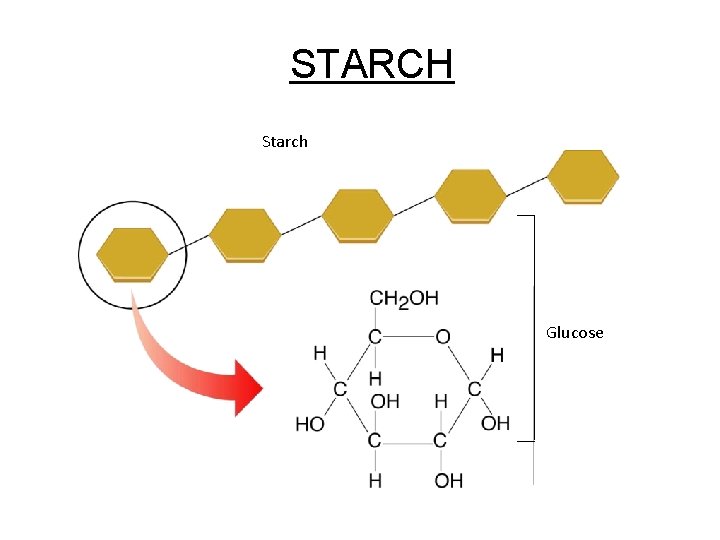
Figure 2 -13 A Starch Section 2 -3 STARCH Starch Glucose
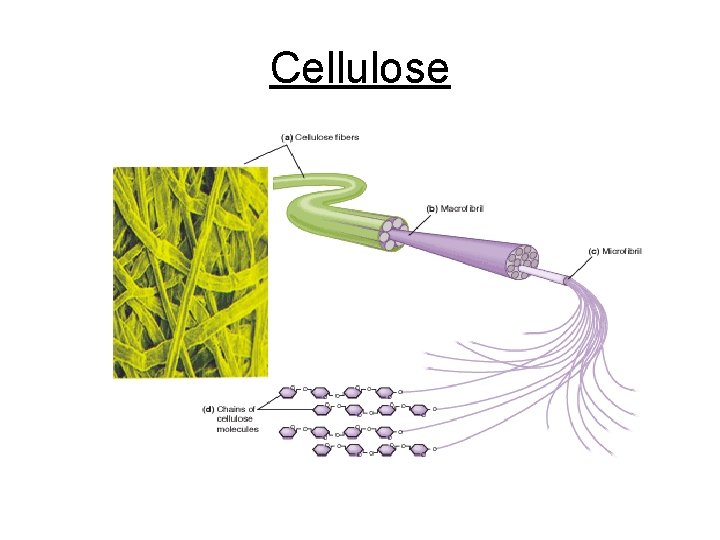
Cellulose
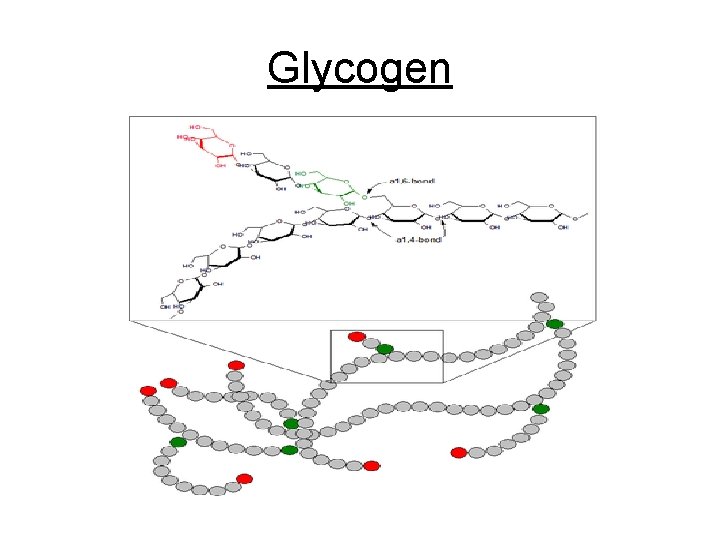
Glycogen
Cont. Macromolecules 2. PROTEINS • Polypeptides a chain of many amino acids • Makes up cell parts (membrane), cell enzymes, collagen, & some hormones account for variations between individuals of the same species, nutrients- provide energy • Made up of carbon, hydrogen, oxygen, & nitrogen
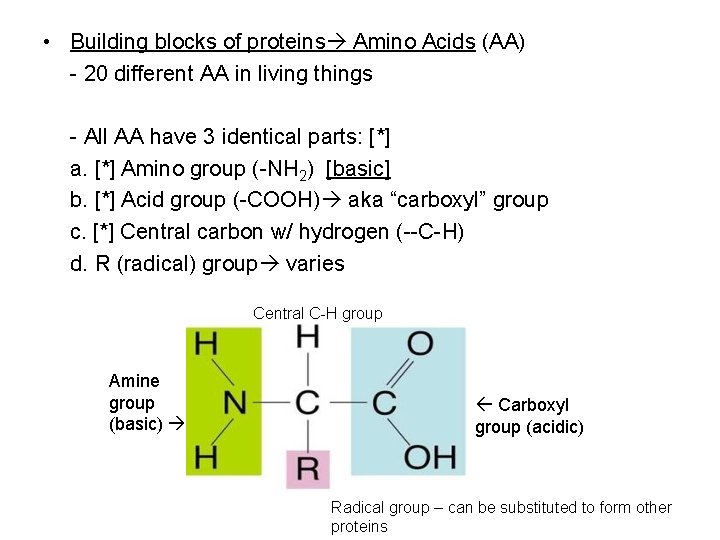
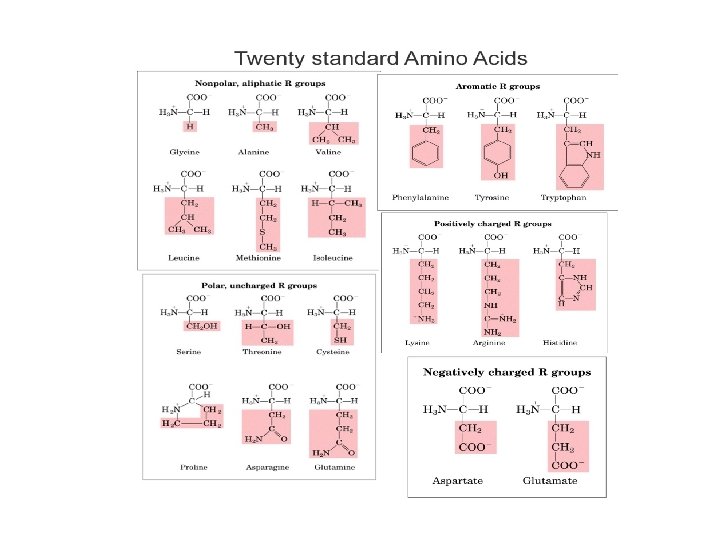
• Building blocks of proteins Amino Acids (AA) - 20 different AA in living things - All AA have 3 identical parts: [*] a. [*] Amino group (-NH 2) [basic] b. [*] Acid group (-COOH) aka “carboxyl” group c. [*] Central carbon w/ hydrogen (--C-H) d. R (radical) group varies Central C-H group Amine group (basic) Carboxyl group (acidic) Radical group – can be substituted to form other proteins
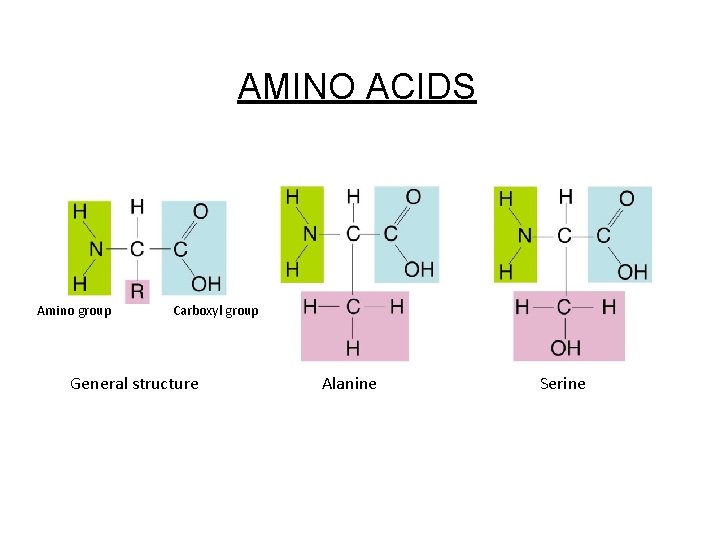
Figure 2 -16 Amino Acids Section 2 -3 Amino group AMINO ACIDS Carboxyl group General structure Alanine Serine
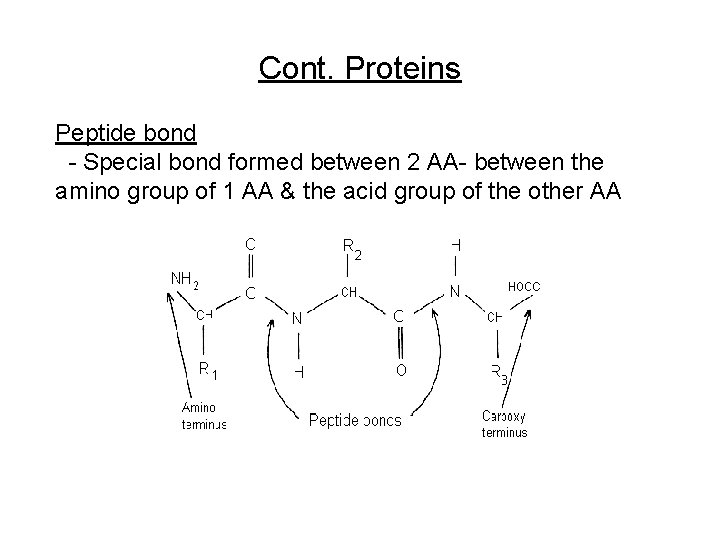
Cont. Proteins Peptide bond - Special bond formed between 2 AA- between the amino group of 1 AA & the acid group of the other AA
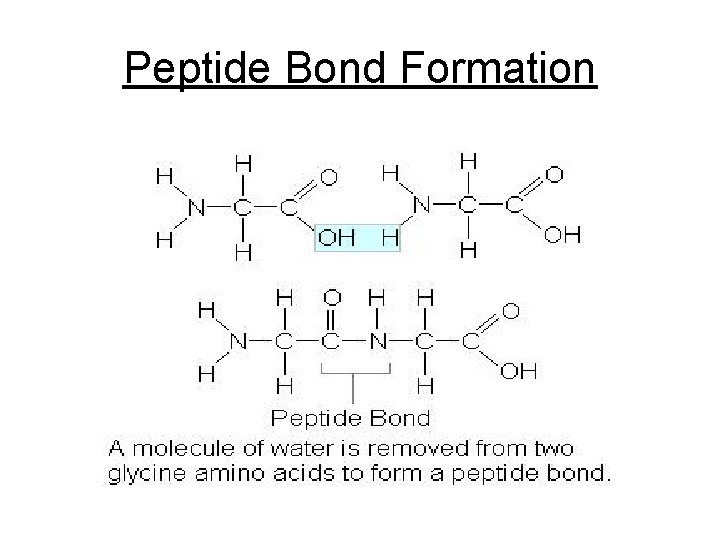
Peptide Bond Formation
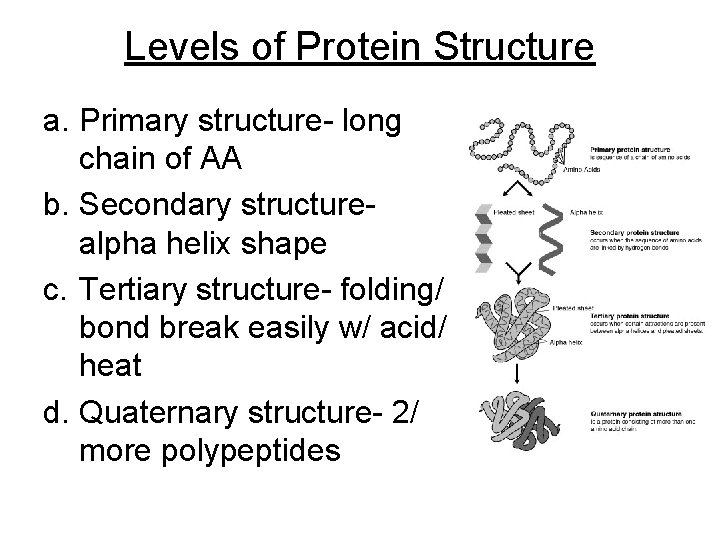
Levels of Protein Structure a. Primary structure- long chain of AA b. Secondary structurealpha helix shape c. Tertiary structure- folding/ bond break easily w/ acid/ heat d. Quaternary structure- 2/ more polypeptides
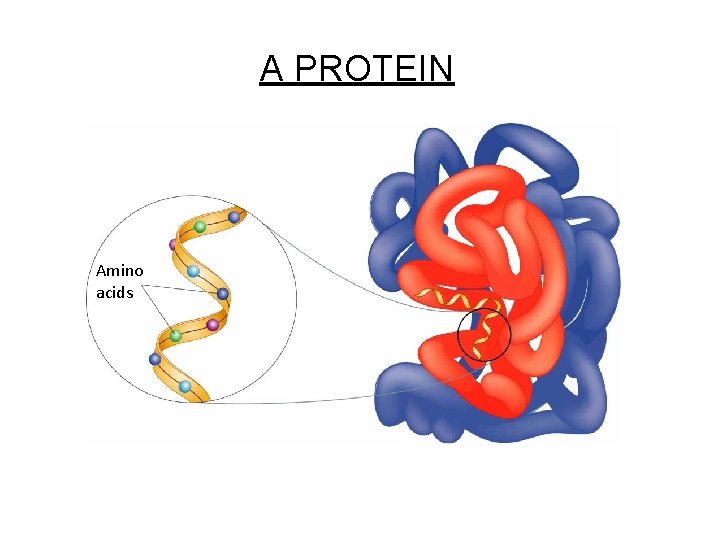
Figure 2 -17 A Protein Section 2 -3 Amino acids A PROTEIN

Protein
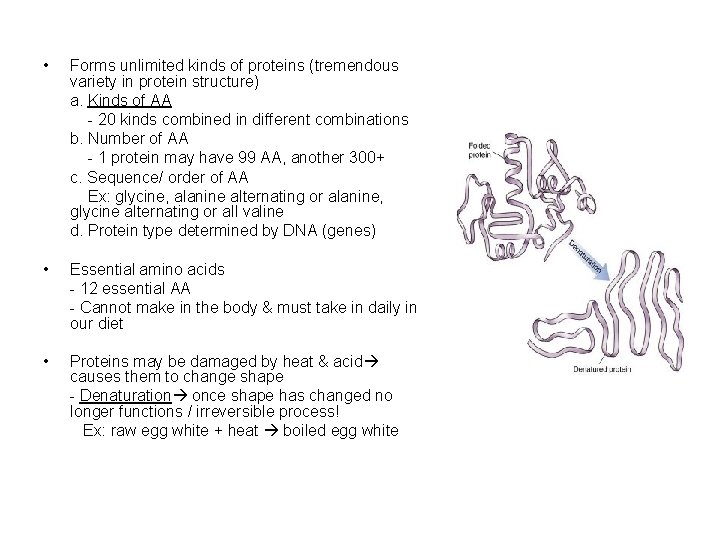
• Forms unlimited kinds of proteins (tremendous variety in protein structure) a. Kinds of AA - 20 kinds combined in different combinations b. Number of AA - 1 protein may have 99 AA, another 300+ c. Sequence/ order of AA Ex: glycine, alanine alternating or alanine, glycine alternating or all valine d. Protein type determined by DNA (genes) • Essential amino acids - 12 essential AA - Cannot make in the body & must take in daily in our diet • Proteins may be damaged by heat & acid causes them to change shape - Denaturation once shape has changed no longer functions / irreversible process! Ex: raw egg white + heat boiled egg white
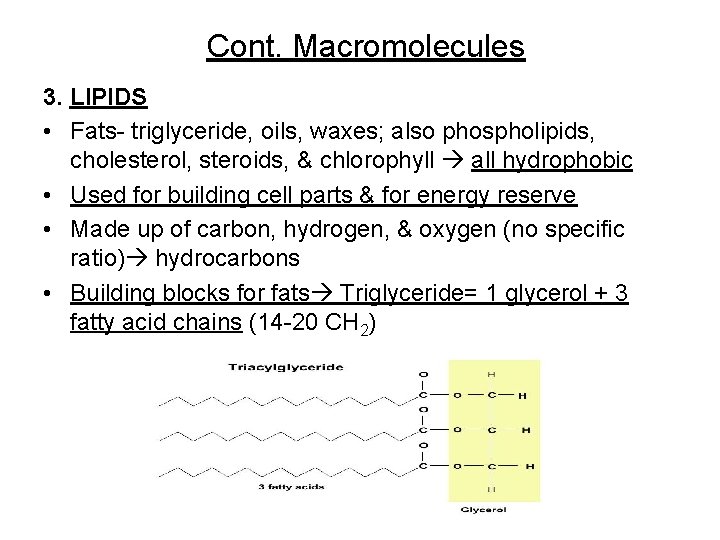
Cont. Macromolecules 3. LIPIDS • Fats- triglyceride, oils, waxes; also phospholipids, cholesterol, steroids, & chlorophyll all hydrophobic • Used for building cell parts & for energy reserve • Made up of carbon, hydrogen, & oxygen (no specific ratio) hydrocarbons • Building blocks for fats Triglyceride= 1 glycerol + 3 fatty acid chains (14 -20 CH 2)
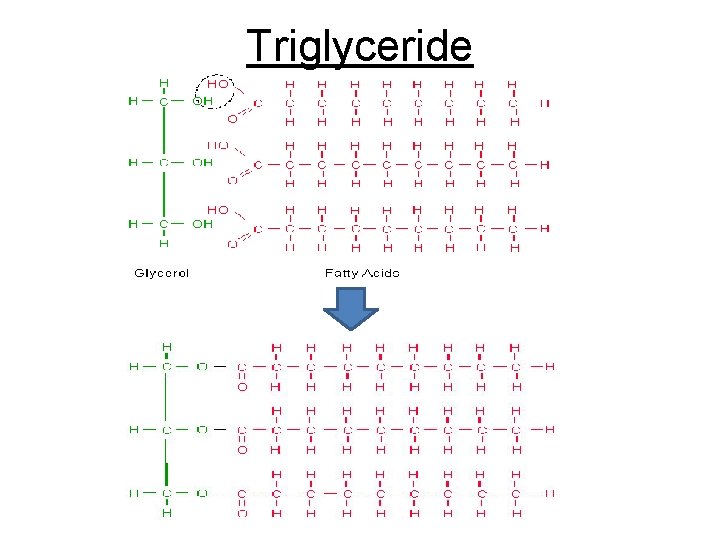
Triglyceride

Cont. Lipids • Types of fat: a. Saturated Fats - “Bad fats” - Every carbon is filled w/ hydrogen - NO double bonded carbons - Solid at room temperature - Animal fat/ lard hard to mix w/ H 2 O b. Unsaturated Fats - “Good fats” - Some carbons do NOT have hydrogens - 1 or more double bonded carbons - Liquid at room temperature - Vegetable oils
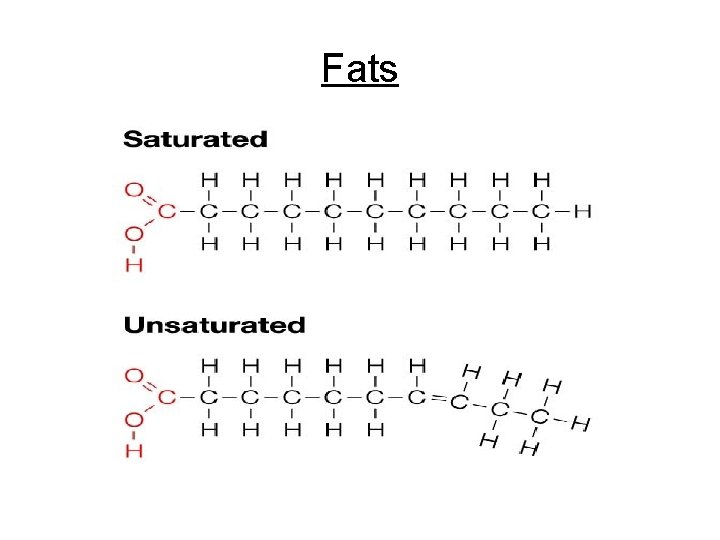
Fats
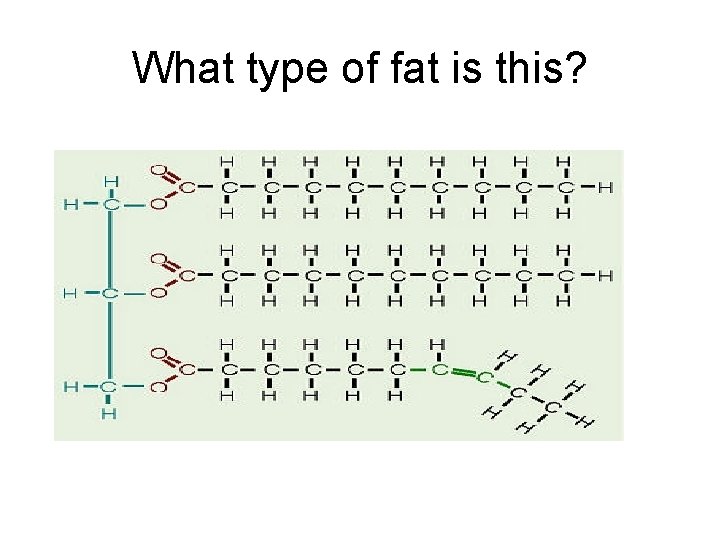
What type of fat is this?

Phospholipid • Similar to fat has only 2 fatty acids attached to glycerol instead of 3 • 3 rd hydroxyl group of glycerol is joined by phosphate group • Amphipathic- has polar (hydrophilic) & nonpolar regions (hydrophobic) • Major component of ALL cell membranes
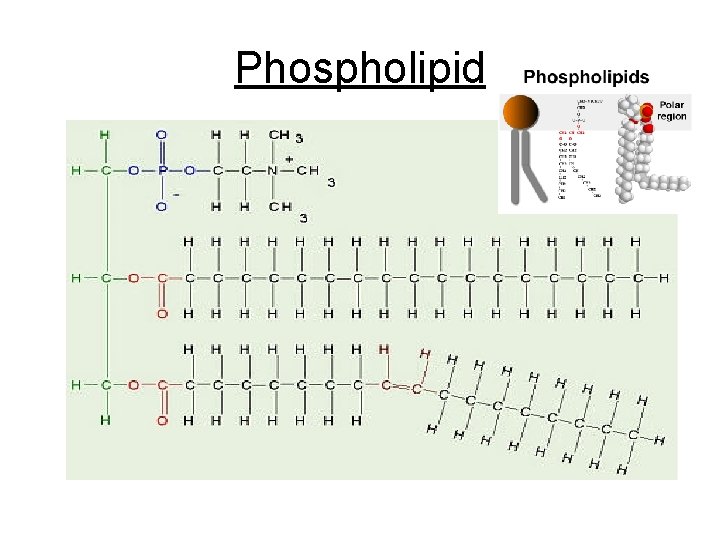
Phospholipid
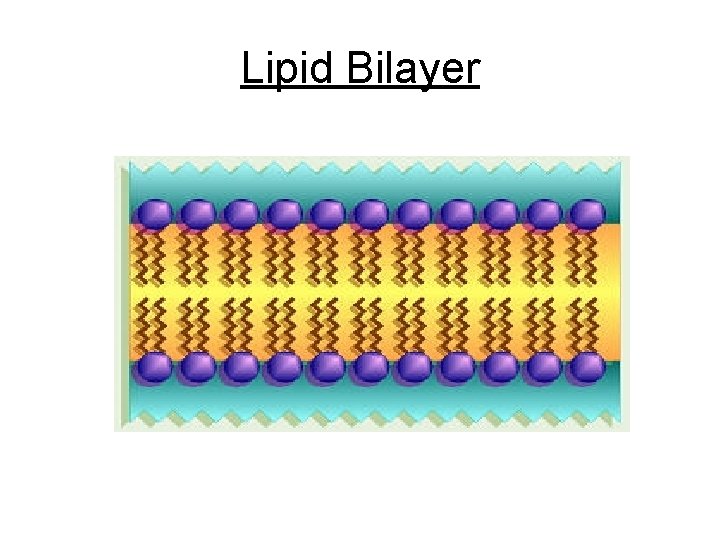
Lipid Bilayer
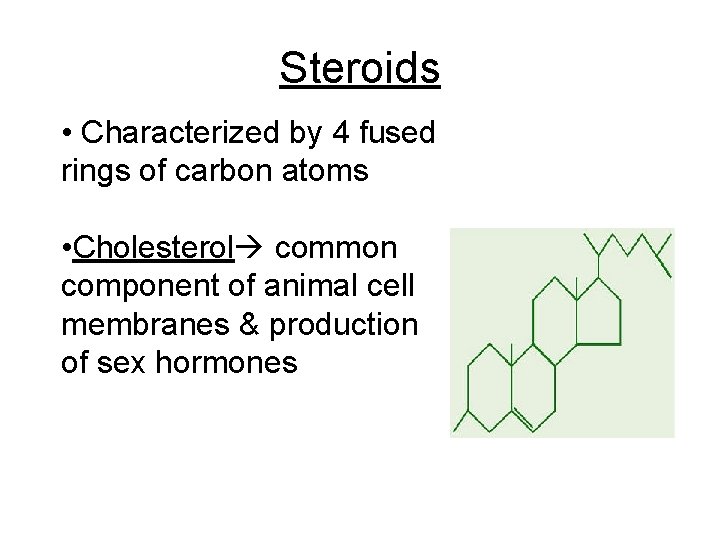
Steroids • Characterized by 4 fused rings of carbon atoms • Cholesterol common component of animal cell membranes & production of sex hormones
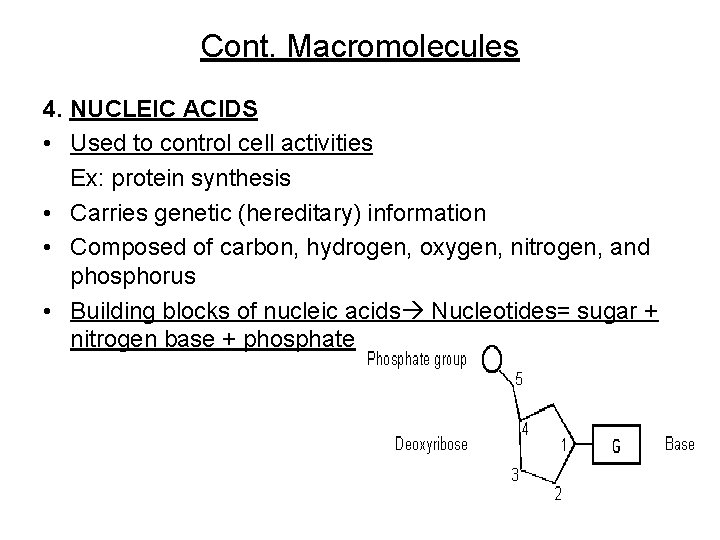
Cont. Macromolecules 4. NUCLEIC ACIDS • Used to control cell activities Ex: protein synthesis • Carries genetic (hereditary) information • Composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus • Building blocks of nucleic acids Nucleotides= sugar + nitrogen base + phosphate
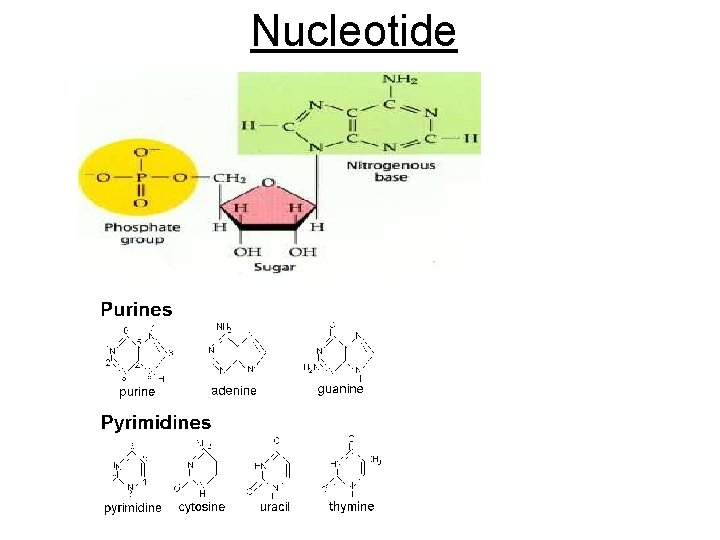
Nucleotide
Cont. Nucleic Acids • Types of nucleic acids: a. DNA (deoxyribonucleic acid) - Found mainly in the nucleus - Deoxyribose sugar b. RNA (ribonucleic acid) - Found in both nucleus & cytoplasm - Ribose sugar
Cont. Macromolecules 5. ENZYMES • All are proteins • Used as catalysts to start chemical reactions - Lower the amount of activation energy needed w/o increasing heat • Composed of carbon, hydrogen, oxygen, & nitrogen
Cont. Enzymes • Are specific 1 enzyme for each reaction • Active site specific part that matches shape w/ a substance “substrate” that enzyme acts on • Often ends in “ase” - maltase, lipase, amylase
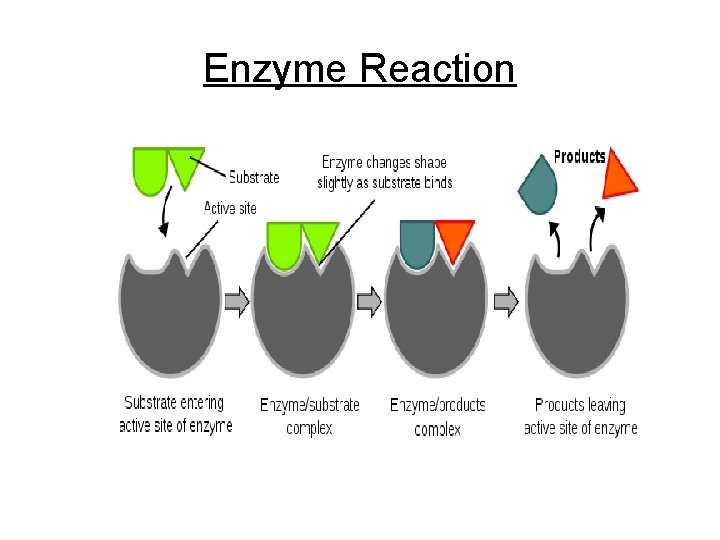
Enzyme Reaction
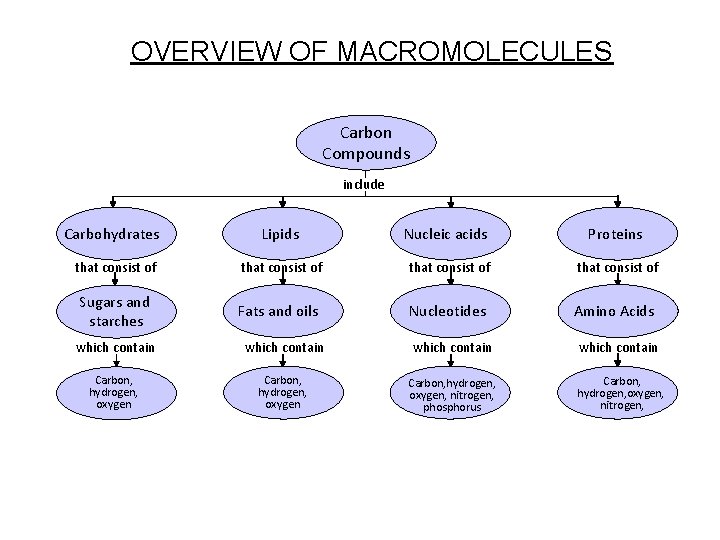
Concept Map OVERVIEW OF MACROMOLECULES Section 2 -3 Carbon Compounds include Carbohydrates Lipids Nucleic acids Proteins that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain Carbon, hydrogen, oxygen, nitrogen, phosphorus Carbon, hydrogen, oxygen, nitrogen,
- Slides: 67