Chapter 2 Chemical Compounds and Bonding Section 2

Chapter 2 Chemical Compounds and Bonding Section 2. 1 – Chemical Bonds and Ionic Bonding
Bonding From the trends studied in the last chapter, we can understand bonding. Bonding is the electrostatic attraction between 2 atoms or ions This is important because you rarely find substances as just pure elements, they usually exist as compounds. This is favored because the atoms are at a lower energy state, meaning they are more stable.
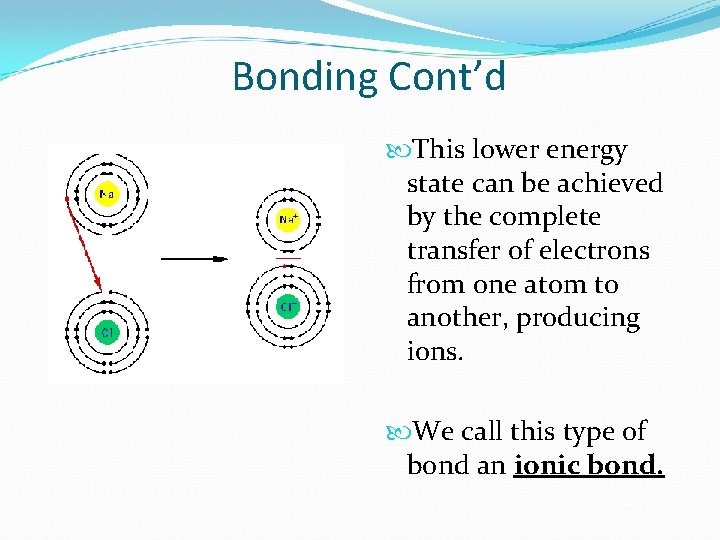
Bonding Cont’d This lower energy state can be achieved by the complete transfer of electrons from one atom to another, producing ions. We call this type of bond an ionic bond.
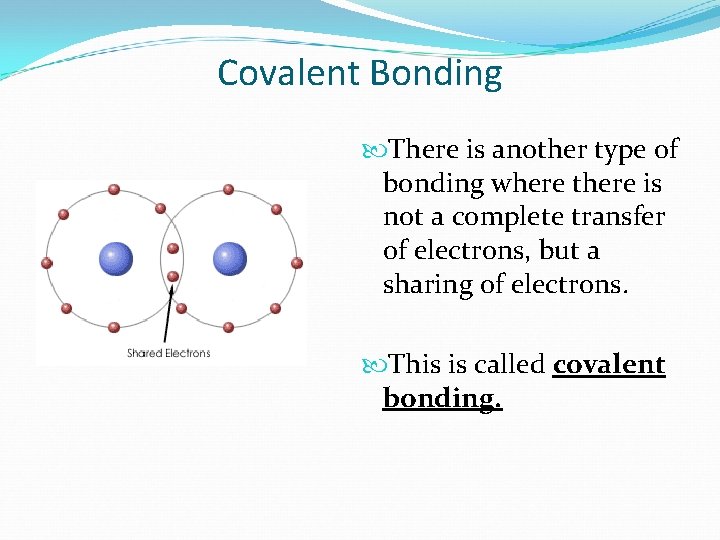
Covalent Bonding There is another type of bonding where there is not a complete transfer of electrons, but a sharing of electrons. This is called covalent bonding.

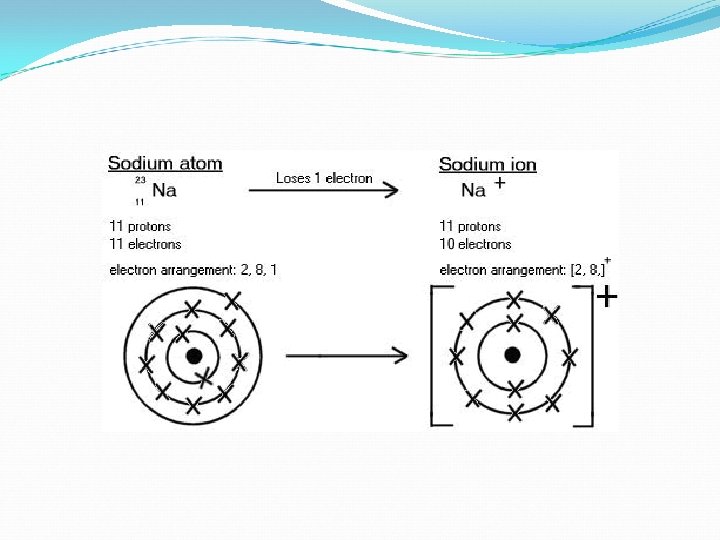
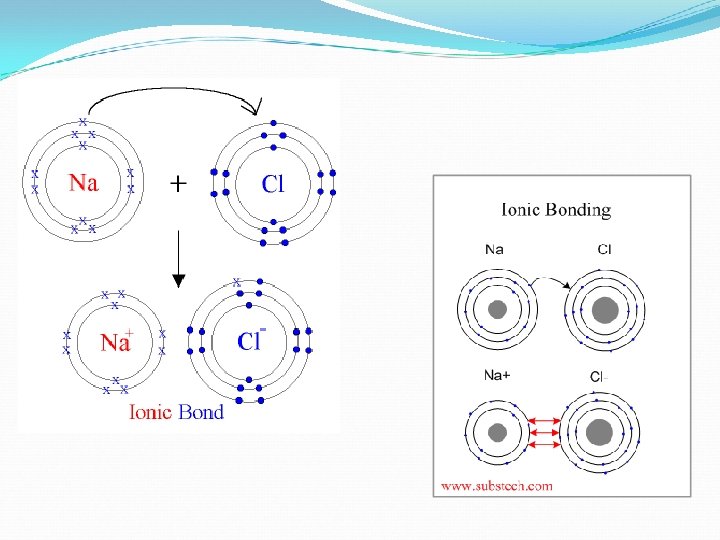
Electron Arrangement of Ions When atoms bond to form compounds, metals lose electrons. For example sodium, Na, loses 1 electron to become the Na+ ion. It then attains the electron arrangement of neon, the nearest noble gas, with 8 electrons in its outer shell. Because the sodium ion has the electron arrangement of neon, we say that sodium is isoelectronic with neon.
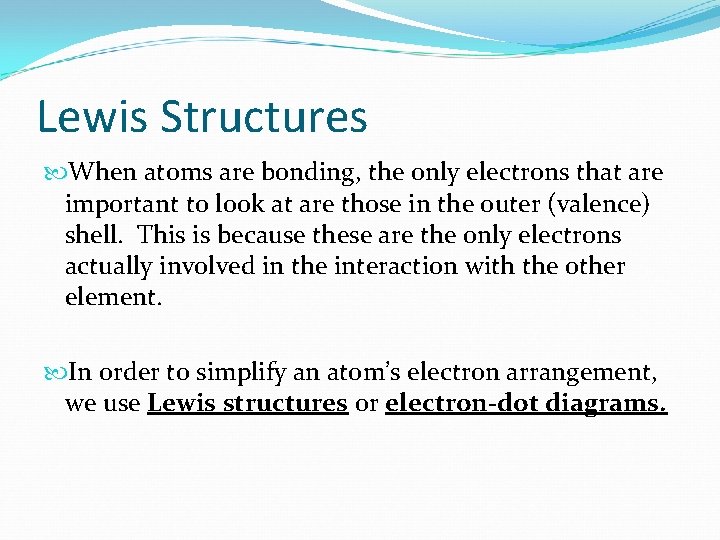
Lewis Structures When atoms are bonding, the only electrons that are important to look at are those in the outer (valence) shell. This is because these are the only electrons actually involved in the interaction with the other element. In order to simplify an atom’s electron arrangement, we use Lewis structures or electron-dot diagrams.
Lewis Structures Cont’d To draw a Lewis dot diagram, follow these rules: Write out the symbol of the element, this symbolizes the nucleus and inner electrons The electrons in the outer shell are drawn on the 4 sides of the symbol Dots are placed singly on each side of the symbol, then they are paired until you reach the number of outer electrons
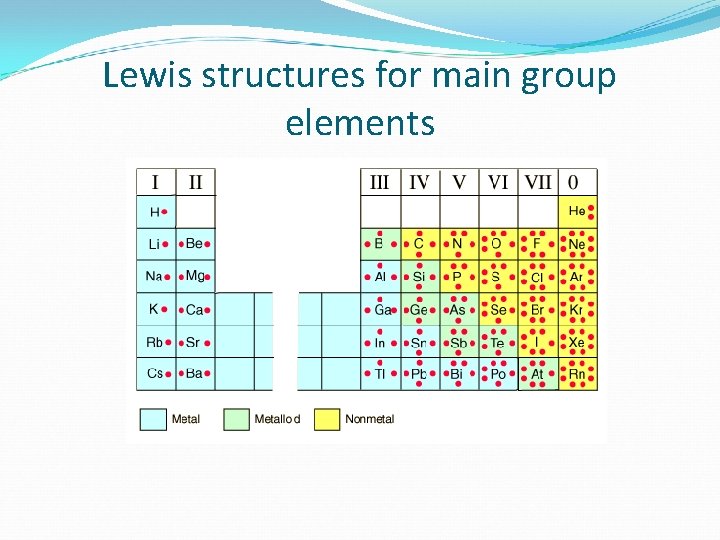
Lewis structures for main group elements

The Octet Rule: when atoms combine, electrons are transferred or shared so that each atom ends up with 8 electrons in their outermost shell. When they do this, they attain noble gas configurations.

Ionic Compounds Electrons cannot just appear or disappear from an atom so that it can become and ion and obtain a noble gas configuration. Elements with lower ionization energies (IE), the metals, will lose electrons to elements with high electron affinities (EA), the non-metals, who will gain these electrons.

Ionic Compounds Cont’d Once ions form, they carry either a positive or negative charge. Recall: metals that lose e- become positive ions (cation) and non-metals gain e- to become negative ions (anions). Because the ions have opposite charges, they are now attracted to each other and stick together like magnets. This is also known as electrostatic attractive forces.
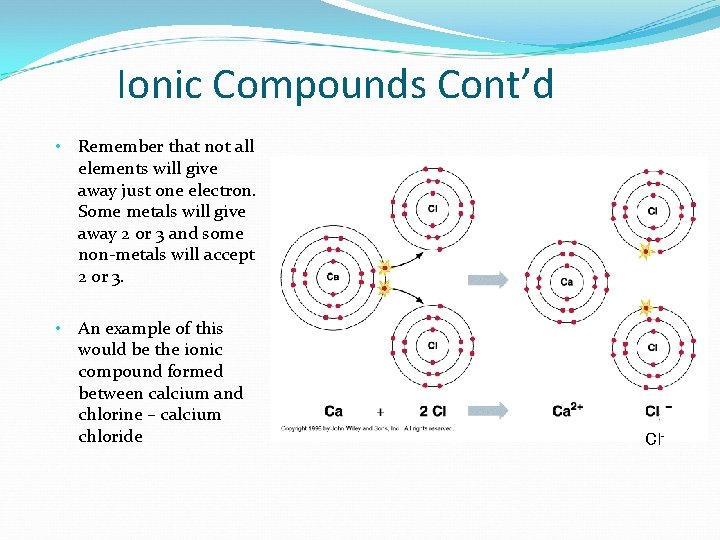
Ionic Compounds Cont’d • Remember that not all elements will give away just one electron. Some metals will give away 2 or 3 and some non-metals will accept 2 or 3. • An example of this would be the ionic compound formed between calcium and chlorine – calcium chloride Cl-

Ionic Bonding Video Clip http: //www. youtube. com/watch? v=upg. FUHp 6 ys&feature=related

Ionic Compounds Cont’d By forming ions, the properties of the substance that forms are different than the atom that were there initially. For example, sodium chloride, regular table salt, is a brittle solid that is essential for life on earth. This is very different from the soft metal and poisonous gas that make it up.
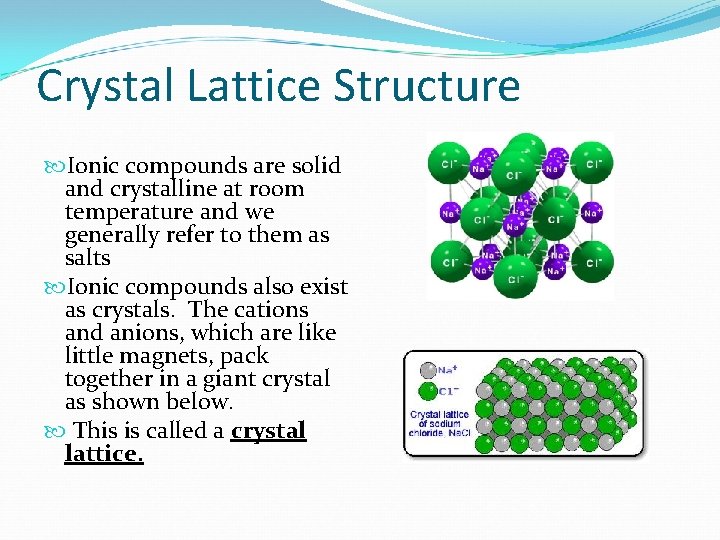
Crystal Lattice Structure Ionic compounds are solid and crystalline at room temperature and we generally refer to them as salts Ionic compounds also exist as crystals. The cations and anions, which are like little magnets, pack together in a giant crystal as shown below. This is called a crystal lattice.
Crystal Lattice Structure Cont’d Because ionic compounds exist as crystals, there are equal numbers of positive and negative charges, but it is impossible to say that a specific sodium ion belongs with a specific chloride ion as they are both surrounded by 6 other ions of opposite charge. Therefore, we refer to them in a specific ratio of how many ions are present. Chemists use the formula unit to represent the smallest unit of an ionic compound that still has properties of that compound. For example, calcium and chloride combine in a 1 to 2 ratio and the formula, from the formula unit, is Ca. Cl 2.

Properties of Ionic Compounds Melting an ionic compound or dissolving an ionic compound in water allows it to conduct electricity. This is due to the fact that when dissolved or melted, the substance will exist as ions that are free to move around and carry the electric current. When in the solid state, the ions are held in fixed positions which don’t carry current.

Properties of Ionic Compounds Cont’d Ionic compounds have high melting and boiling points because the ionic bonds holding the ions in place in the crystal lattice are very strong. (+ and – attraction force is very strong) A lot of energy needs to be put in to break these electrostatic forces before the ions will separate. . . thus hard to separate ions…high mp and bp Salts are also very brittle substances because they exist in a crystal lattice structure… alternating + and – show attraction but if a + and + or a – and- come close, the crystal shatters due to same charges repel
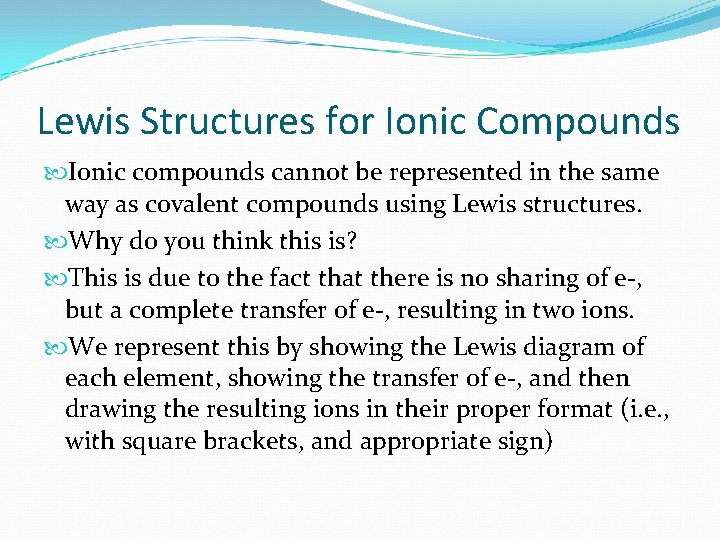
Lewis Structures for Ionic Compounds Ionic compounds cannot be represented in the same way as covalent compounds using Lewis structures. Why do you think this is? This is due to the fact that there is no sharing of e-, but a complete transfer of e-, resulting in two ions. We represent this by showing the Lewis diagram of each element, showing the transfer of e-, and then drawing the resulting ions in their proper format (i. e. , with square brackets, and appropriate sign)
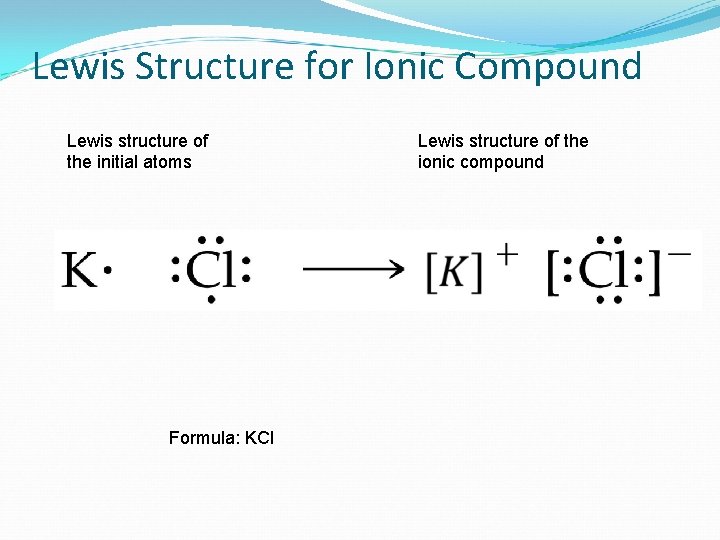
Lewis Structure for Ionic Compound Lewis structure of the initial atoms Formula: KCl Lewis structure of the ionic compound
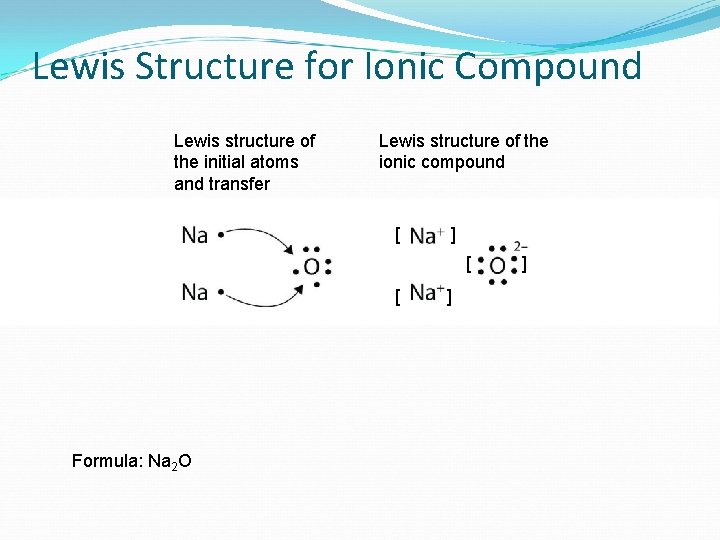
Lewis Structure for Ionic Compound Lewis structure of the initial atoms and transfer Lewis structure of the ionic compound [ ] [ [ Formula: Na 2 O ] ]

Chapter 2 Section 2. 2 – Covalent Bonding

Covalent Bonds We learned that a metal and a non-metal combine to form an ionic compound, but how do 2 non-metals react? 2 non-metals combine in what we call a molecular compound…with a covalent bond In these types of compounds, the electrons are not completely transferred from one substance to the other. In covalent compounds, the electrons are shared between 2 or more atoms.
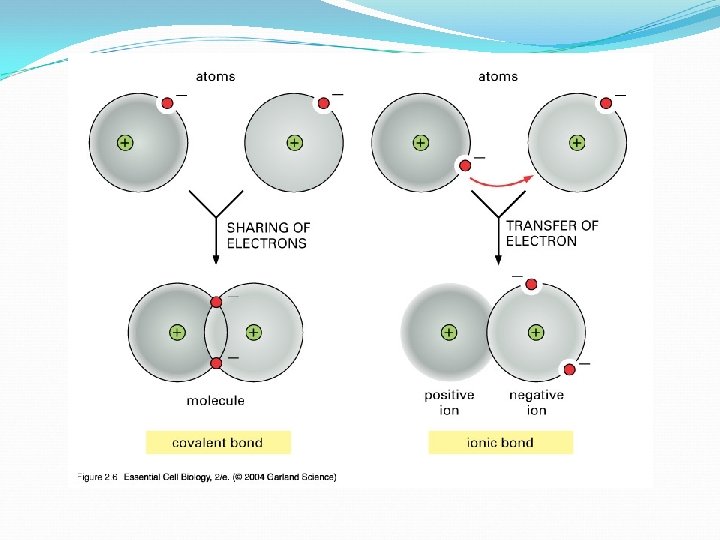
Covalent Bonds Cont’d In a covalent bond, the nucleus of each atom is electrostatically attracted to the shared electrons. The smallest part of a covalent compound is a molecule. What is the difference between a compound a molecule? ? If a molecule contains 2 atoms it is called a diatomic molecule. Remember HOFBr. INCl? ? If a molecule contains 3 atoms, it is a triatomic molecule.
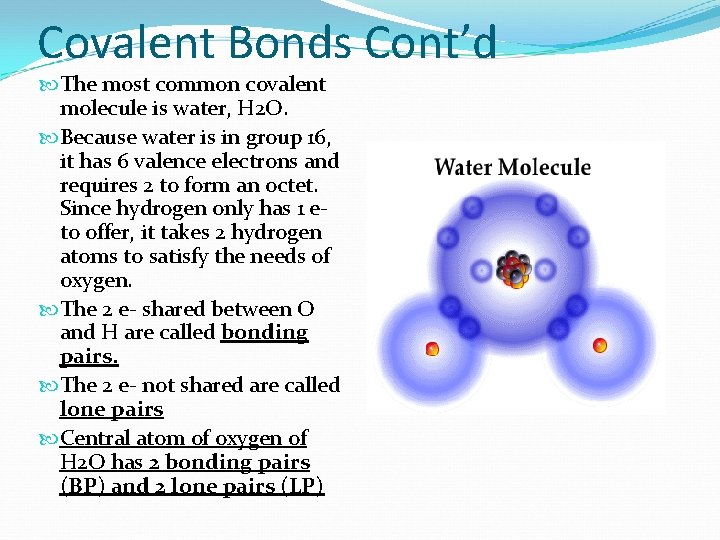
Covalent Bonds Cont’d The most common covalent molecule is water, H 2 O. Because water is in group 16, it has 6 valence electrons and requires 2 to form an octet. Since hydrogen only has 1 eto offer, it takes 2 hydrogen atoms to satisfy the needs of oxygen. The 2 e- shared between O and H are called bonding pairs. The 2 e- not shared are called lone pairs Central atom of oxygen of H 2 O has 2 bonding pairs (BP) and 2 lone pairs (LP)
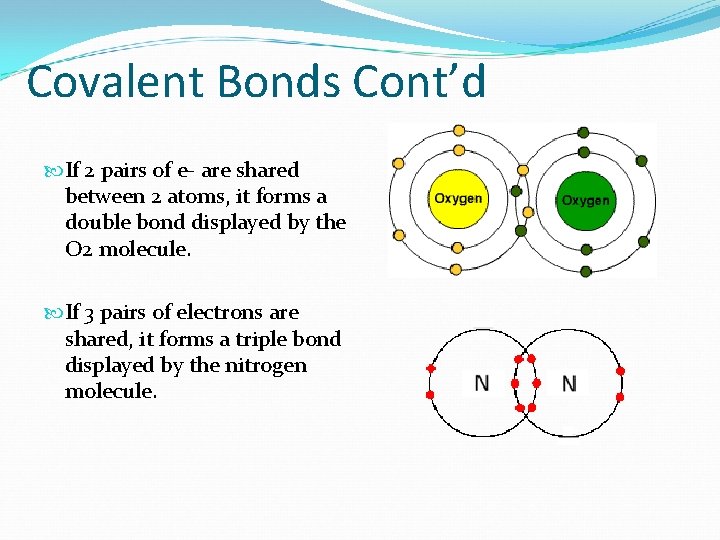
Covalent Bonds Cont’d If 2 pairs of e- are shared between 2 atoms, it forms a double bond displayed by the O 2 molecule. If 3 pairs of electrons are shared, it forms a triple bond displayed by the nitrogen molecule.
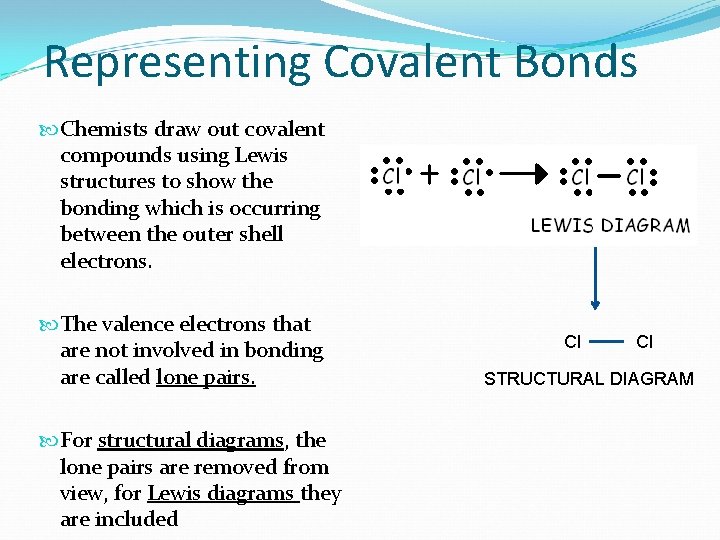
Representing Covalent Bonds Chemists draw out covalent compounds using Lewis structures to show the bonding which is occurring between the outer shell electrons. The valence electrons that are not involved in bonding are called lone pairs. For structural diagrams, the lone pairs are removed from view, for Lewis diagrams they are included Cl Cl STRUCTURAL DIAGRAM
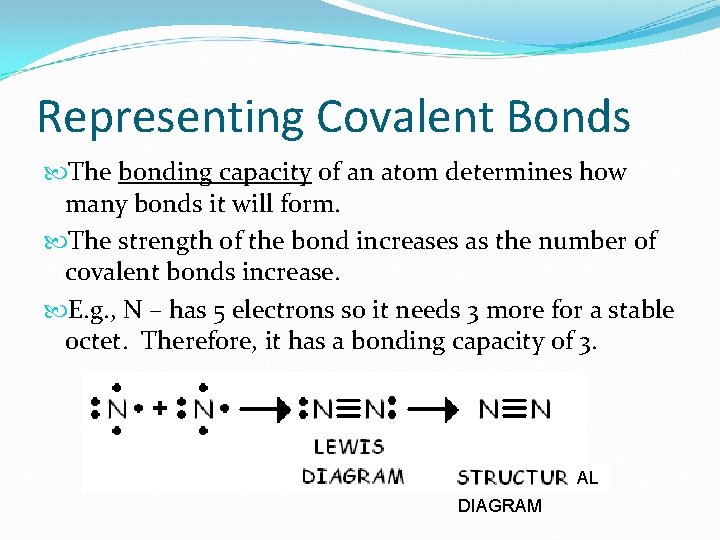
Representing Covalent Bonds The bonding capacity of an atom determines how many bonds it will form. The strength of the bond increases as the number of covalent bonds increase. E. g. , N – has 5 electrons so it needs 3 more for a stable octet. Therefore, it has a bonding capacity of 3. AL DIAGRAM
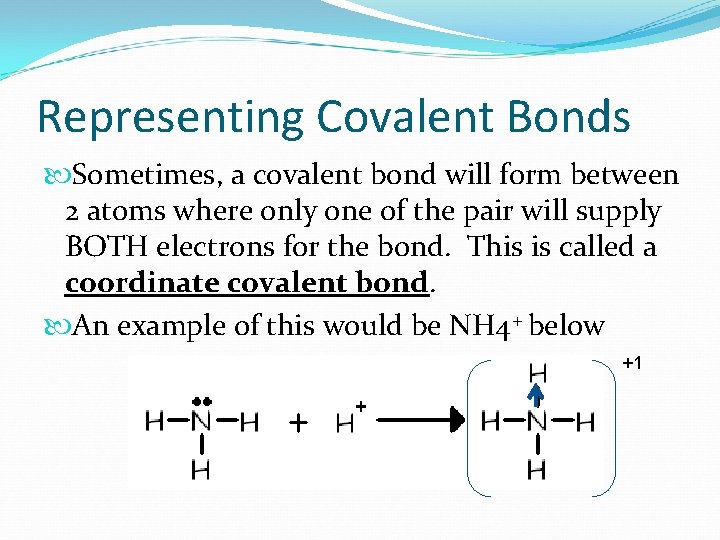
Representing Covalent Bonds Sometimes, a covalent bond will form between 2 atoms where only one of the pair will supply BOTH electrons for the bond. This is called a coordinate covalent bond. An example of this would be NH 4+ below +1
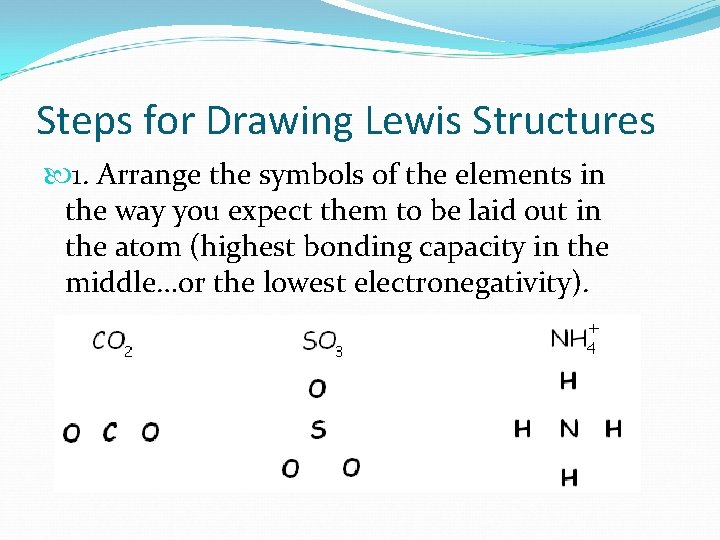
Steps for Drawing Lewis Structures 1. Arrange the symbols of the elements in the way you expect them to be laid out in the atom (highest bonding capacity in the middle…or the lowest electronegativity).
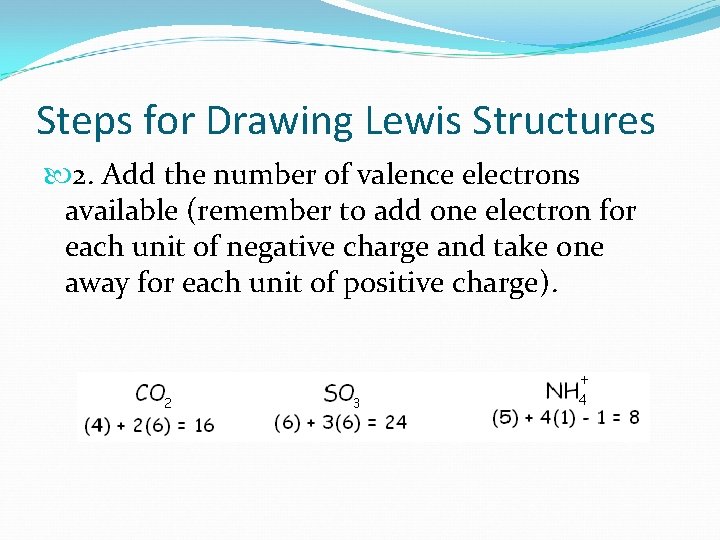
Steps for Drawing Lewis Structures 2. Add the number of valence electrons available (remember to add one electron for each unit of negative charge and take one away for each unit of positive charge).
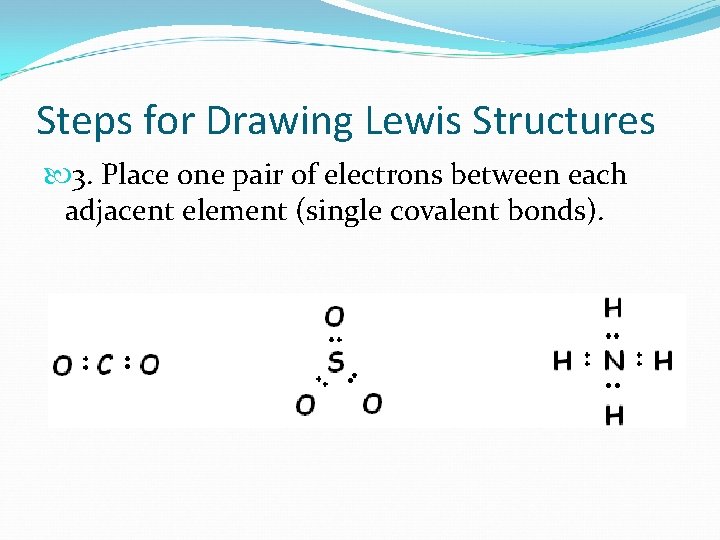
Steps for Drawing Lewis Structures 3. Place one pair of electrons between each adjacent element (single covalent bonds).
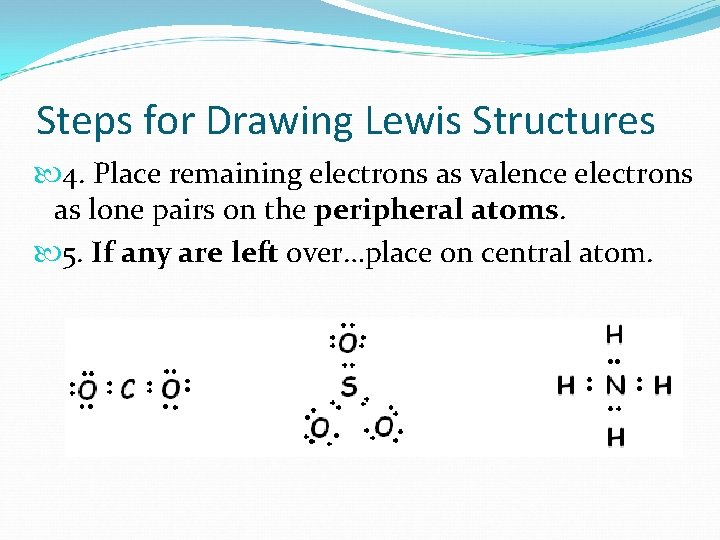
Steps for Drawing Lewis Structures 4. Place remaining electrons as valence electrons as lone pairs on the peripheral atoms. 5. If any are left over…place on central atom.
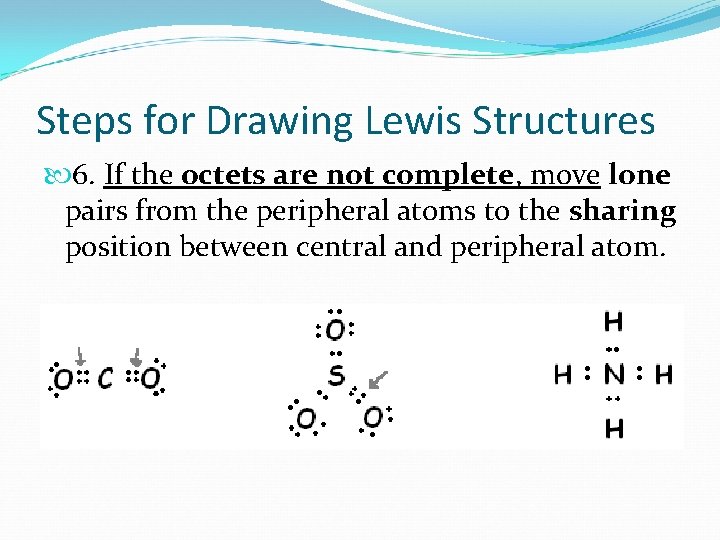
Steps for Drawing Lewis Structures 6. If the octets are not complete, move lone pairs from the peripheral atoms to the sharing position between central and peripheral atom.
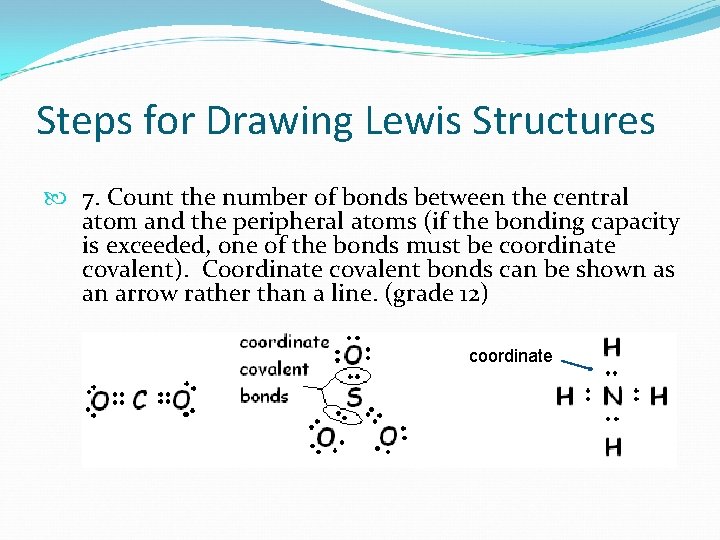
Steps for Drawing Lewis Structures 7. Count the number of bonds between the central atom and the peripheral atoms (if the bonding capacity is exceeded, one of the bonds must be coordinate covalent). Coordinate covalent bonds can be shown as an arrow rather than a line. (grade 12) coordinate
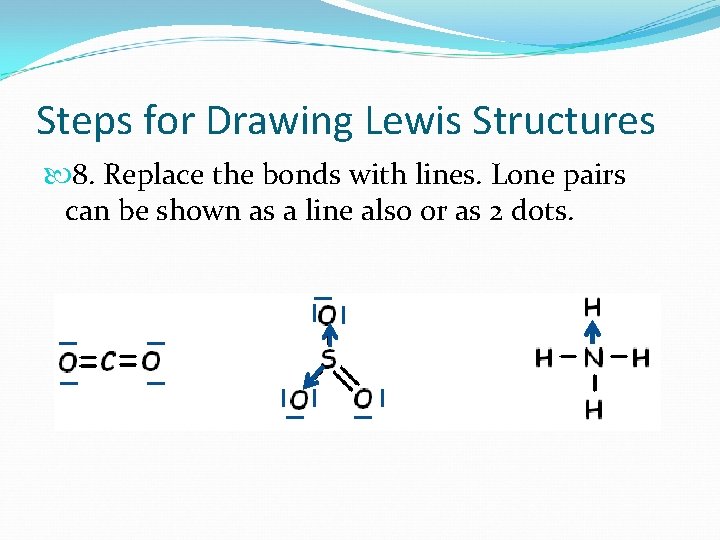
Steps for Drawing Lewis Structures 8. Replace the bonds with lines. Lone pairs can be shown as a line also or as 2 dots.
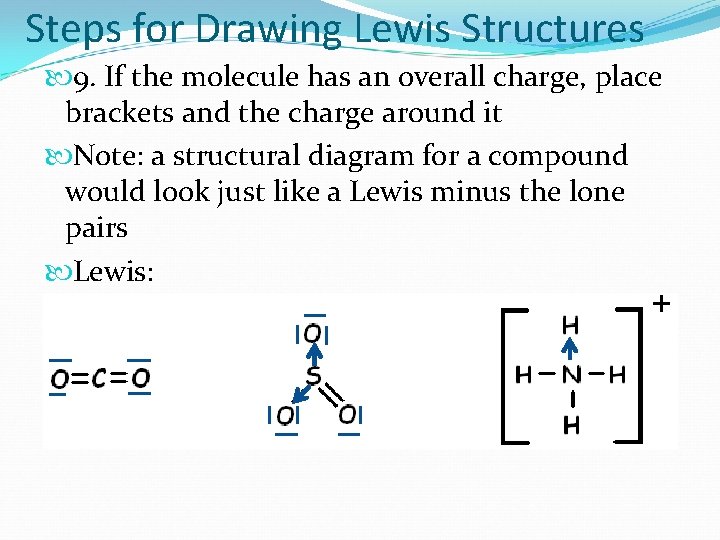
Steps for Drawing Lewis Structures 9. If the molecule has an overall charge, place brackets and the charge around it Note: a structural diagram for a compound would look just like a Lewis minus the lone pairs Lewis:

Exceeding the Octet Rule The octet rule is never exceeded for the covalent compounds that form from the second period of elements. However, the octet rule can be exceeded for elements in later periods (period 3 and higher), where more than 8 electrons can be held in the higher orbitals. Many compounds have been synthesized this way, and chemists have found that atoms will allow the addition of the same number of e- that they already possess in their outer shell. (thus doubling the number that they had originally…)
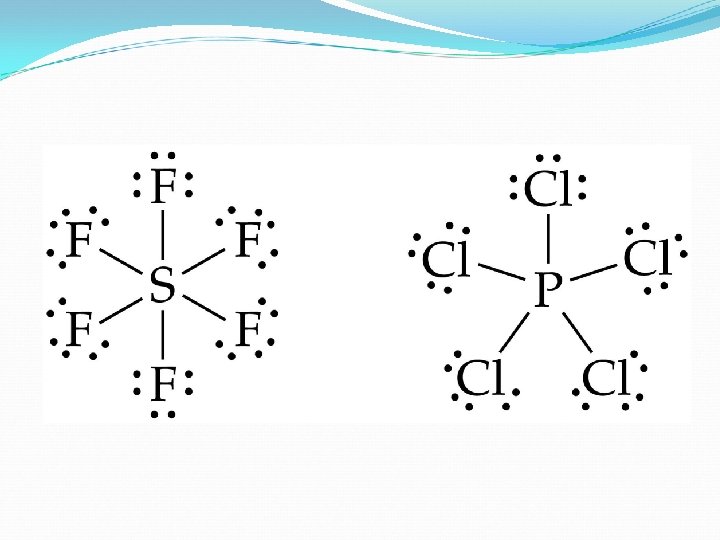

Not reaching the octet Other than hydrogen whose goal is for a duet (2), two other atoms are found to have less than the octet…beryllium, aluminum and boron. Thus in BH 3 , the central boron has 3 electrons of its own and 3 more from sharing reaching a total of 6 (hexet). Thus Be. H 2, the central beryllium has 2 electrons and 2 more from sharing thus reaching a total of 4 (quartet).
Properties of Molecular Compounds They can be either gases, such as CO 2, N 2 O, etc. , liquids, such as H 2 O, or solids, such as Si. O 2. They are different from ionic compounds which are always solids with high melting and boiling points. They can have low mp and low bp, often not soluble in water For example, water’s melting and boiling points are 0 °C and 100 °C respectively. Compare this to salt’s melting and boiling points of 804 °C and 1610 °C. Most all covalent substances do not produce conducting solutions, if they can be dissolved in water (e. g. , sugar). Many small molecular compounds are volatile…like a perfume…
Properties of Molecular Compounds However, it is important to note that these are generalizations based on the majority of substances and that there are exceptions. For example, HCl is a covalent gas that can be dissolved in water to produce an acidic compound in water that can conduct electricity. A decision on the type of bonding that occurs between 2 or more atoms is determined by evidence on solubility, melting and boiling points and conductivity. Molecular compounds will resemble ionic compounds in properties if the molecular compound is polar…see section that follows

Polar Covalent Bonds When we talk about covalent bonds, we are discussing the sharing of electrons between 2 atoms. It is important to know that the electrons are not always equally shared between the two. We can determine which atom, if any, has a stronger hold on the shared electrons based on the electronegativity scale (EN scale).
Polar Covalent Bonds Cont’d The atom with the higher electronegativity will attract the shared electrons more strongly. Because the electrons are held more strongly by one atom, they are closer to its nucleus, giving this atom a slight negative charge. The weaker atom therefore gets a slight positive charge. This slight difference in charge within a covalent molecule creates what is called a dipole. This is represented by the lower case delta.
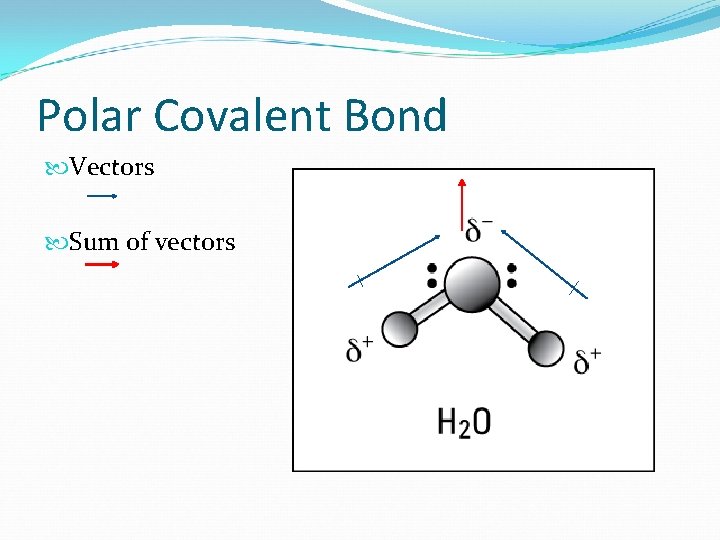
Polar Covalent Bond Vectors Sum of vectors
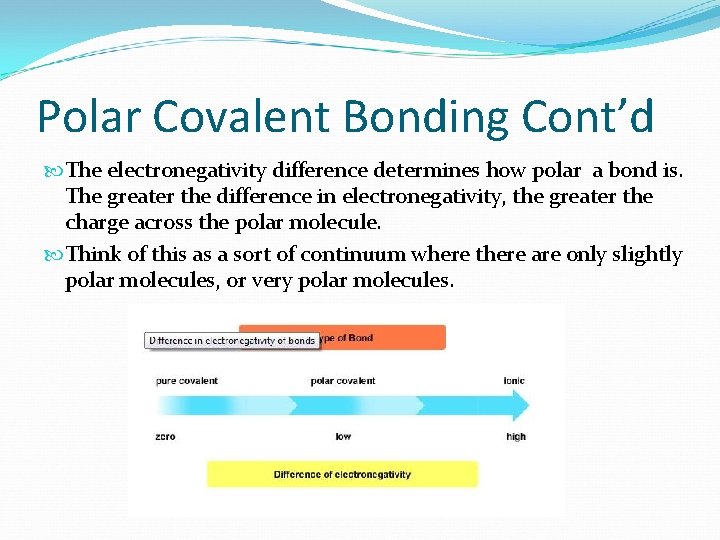
Polar Covalent Bonding Cont’d The electronegativity difference determines how polar a bond is. The greater the difference in electronegativity, the greater the charge across the polar molecule. Think of this as a sort of continuum where there are only slightly polar molecules, or very polar molecules.

EN Difference: ∆EN or END An EN difference of more than 1. 7 means that the bond is ionic. In this case the atom (non-metal) has such a strong nucleus, that it completely pulls away the other atom’s (metal) electron/s. An EN difference of less than 1. 7 but greater than 0. 4 is said to be polar (according to your textbook). An EN difference of less than or equal to 0. 4 is said to be non-polar covalent bond or a pure covalent bond. This generally happens in a molecule made of the same atom, like O 2. An END of 0 is an example of pure covalent.
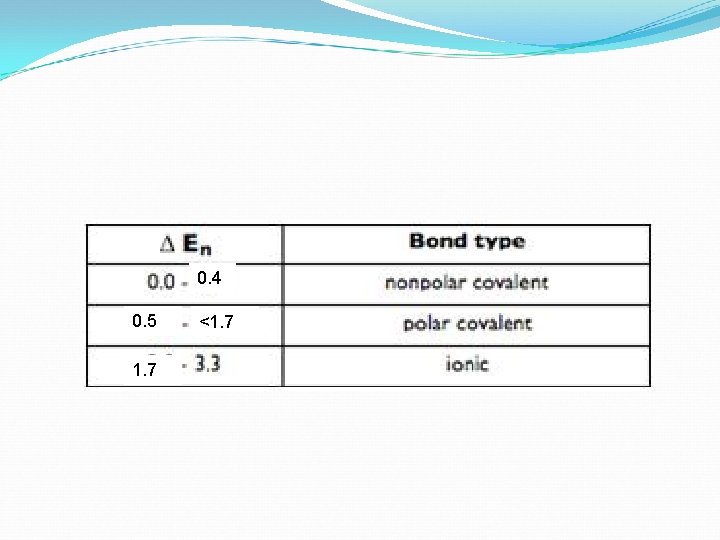
0. 4 0. 5 1. 7 <1. 7

Try It! Using p. 25 of your textbook, predict the type of bond that will form between the following pairs of atoms: a) P & Cl _____________ b) Li & Br _____________ c) C & H _____________ d) C & Cl _____________

A polar bond ≠ a polar molecule A polar molecule has 1) polar bonds AND 2) is not symmetrical It is important to know the shape of a molecule since a molecule with polar bonds will be a polar molecule ONLY if the polar bonds are NOT arranged symmetrically. By knowing the shape of the molecule, it is possible to know if the bonds are arranged symmetrically or not and thus make a final determination on the polarity of the whole molecule. The shape of the molecule is determined using the Lewis diagram and VSEPR Overall polarity is dependent on shape- if the molecule has symmetry, the symmetry cancel the polarity of the bonds…the molecule is nonpolar
How to tell the shape…VSEPR Use Valence Shell Electron Pair Repulsion and Lewis diagrams to predict shape Convert Lewis diagram to AXE notation and choose VSEPR shape that corresponds All AXn. E 0 shapes are naturally symmetrical and the molecule with AXn. E 0 notation would show asymmetry only if the X’s were different Almost all AXn. E 1 shapes would be asymmetrical Learn all the shapes…see handout
- Slides: 55