Chapter 2 C Biochemistry Biosynthesis What is biosynthesis
Chapter 2 C Biochemistry

Biosynthesis • What is biosynthesis? – Living organisms making organic compounds that are required for life

Organic Compounds • What is an organic compound? – Compound made with carbon and large quantities of hydrogen and usually oxygen – Found in living things

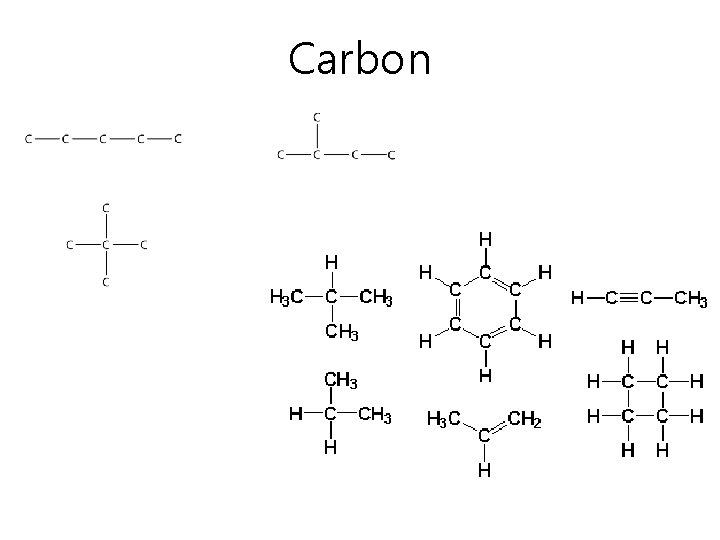
Why is carbon special? • Carbon has 6 electrons and can easily bond with other carbon atoms. – These form long chains or even rings of carbon – Called a carbon backbone – Can form single, double, or triple bonds with other carbons
Carbon

Carbohydrates • What are carbohydrates? – Organic compounds that contain carbon, hydrogen, and oxygen (only!) – Energy storing and structural substances • We will study 3 types of carbohydrates – Monosaccharides – Disaccharides – Polysaccharides

Carbohydrates • First- what is a saccharide? – A simple sugar • What does mono mean? – One • What does di mean? – Two • What does poly mean? – Three or more

Carbohydrates • What is a monosaccharide? – The basic unit of carbohydrates, made of only 1 simple sugar – Good example: glucose • The food we eat gets digested into glucose • Transferred to cells through the blood • Our body uses it for energy

Carbohydrates • What is a disaccharide? – 2 monosaccharides joined together (by enzymes) – One way 2 are joined together- dehydration (taking away H 2 O) • Can they be separated again? – Yes! By hydrolysis (add H 2 O) – Good example: sucrose (table sugar)
Carbohydrates • What is a polysaccharide? – Large molecule of monosaccharides together – 4 important polysaccharides • • Starch Glycogen Cellulose Chitin

Carbohydrates • Starch – Plants use it to store food – Major energy source for humans • Glycogen – Animal starch – The starch is eaten, then made into glycogen for storage in animals/ humans

Carbohydrates • Cellulose – Much larger than starches – Made of loooooong chains of glucose – Make up a plant’s cell wall • Chitin – Almost identical to cellulose, but with an extra group attached – Makes the shells of shrimp and crabs
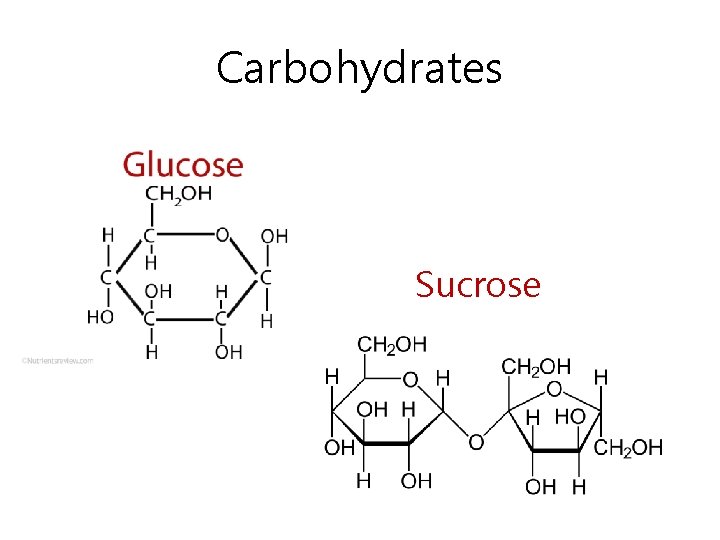
Carbohydrates Sucrose
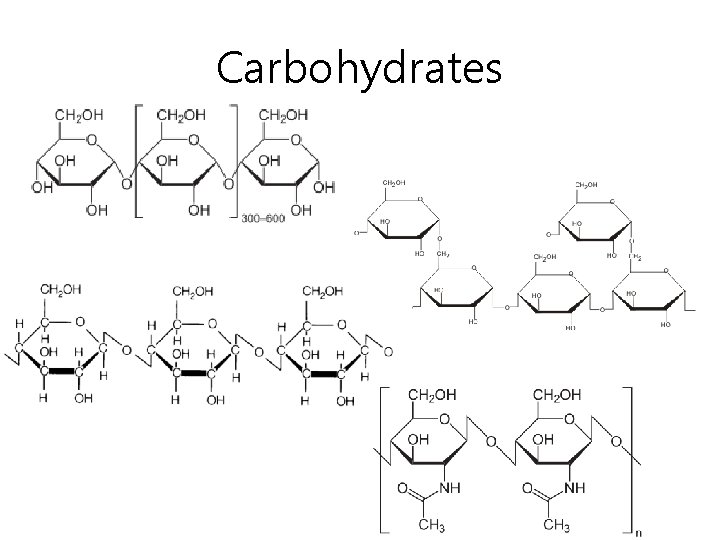
Carbohydrates

Lipids • What are lipids? – Organic substances slightly soluble in water, but very soluble in organic solvents • What is soluble? – Can be dissolved – Often help with structure or energy – 4 main type of lipids • • Fatty acids Triglycerides Phospholipids Sterols

Lipids • Fatty acids – Made of unbranched (straight) chains of 1428 carbon atoms with a –COOH (carboxyl) on the end – Carboxyl end is hydrophilic (attracted to water), other end is hydrophobic (repelled by water)

Lipids • Triglycerides – Most abundant type of lipid – Main function is energy storage – Formed with 3 or more fatty acids attached to a glycerin (3 carbon alcohol) – Can be 2 things: saturated or unsaturated • Saturated- all carbons have 2 hydrogrens (only single bonds) • Unsaturated- one or more carbons are double bonded
Lipids • Phospholipids – Composed of 2 fatty acid chains on a glycerol molecule, PLUS a group that has a phosphate (P) – Have a hydrophobic and hydrophilic end • Sterols – Carbon backbone of 4 rings and a side chain
Proteins • Proteins- mostly carbon, hydrogen, oxygen, and nitrogen – Sometimes phosphorus, sulfur, a few others • Amino Acids- building blocks of proteins – 20 different amino acids – Contain an amino group (-NH 2) and a carboxyl group (-COOH)
Proteins • Special bond– Peptide bond- connects the carboxyl from one amino acid to the amino group of another – Polypeptide chain- chain of amino acids bonded by peptide bonds
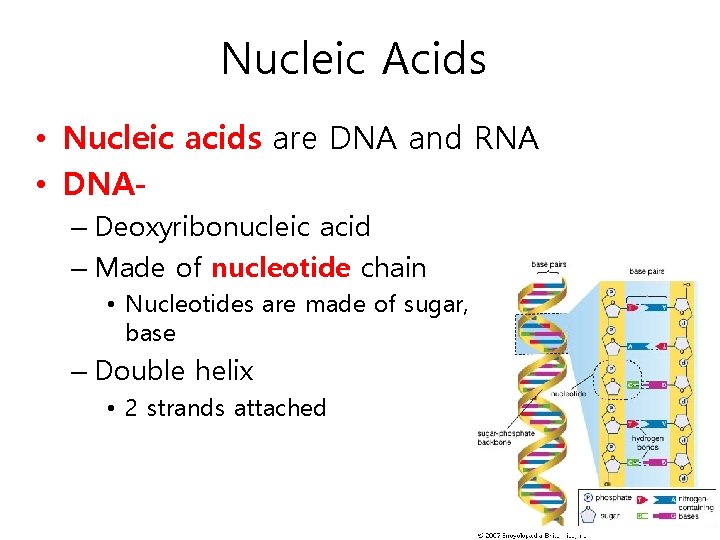
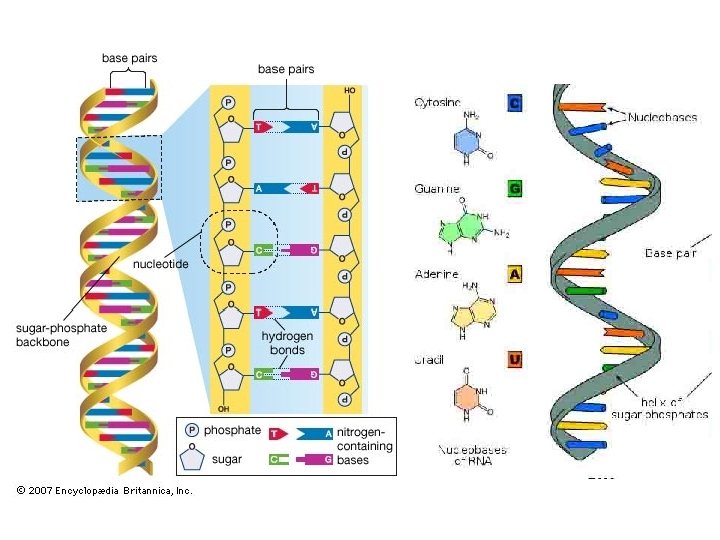
Nucleic Acids • Nucleic acids are DNA and RNA • DNA– Deoxyribonucleic acid – Made of nucleotide chain • Nucleotides are made of sugar, phosphate and a base – Double helix • 2 strands attached
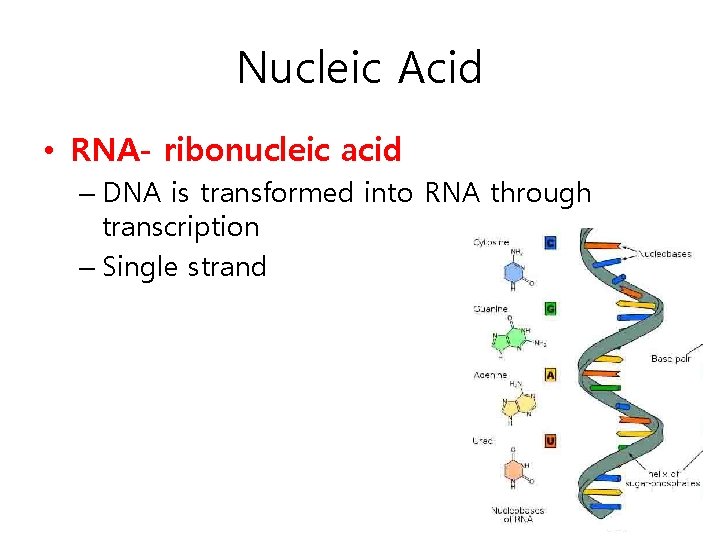
Nucleic Acid • RNA- ribonucleic acid – DNA is transformed into RNA through transcription – Single strand
- Slides: 23