Chapter 2 B Energy and Matter Part 2

Chapter 2 B: Energy and Matter Part 2 Thermal Energy, Temperature, Heat, and measurements

Kinetic Energy • Particles can be moving any way – Straight lines, vibrating, spinning • The total of all motions is: – Total Kinetic Energy (KE) – Formula: KE = ½ mv 2 – The mass (m) is important, but the velocity (v) or the speed of a particle is more important
Temperature • How does this relate to temperature? – The sum of the kinetic and potential energy is measured as temperature – It’s proportional to the average kinetic energy of the particles in a substance – Measure of “hotness” or “coldness”
Thermal Energy • Temperature and thermal energy are NOT the same • The sum of all the kinetic energy of an object’s particles is thermal energy – And heat is the amount of thermal energy transferred between objects – The measure of the speed at which the particles move is the temperature.
Chemical Reactions • In chemistry, reactions either give thermal energy or get thermal energy • If it gives off thermal energy, it is an exothermic reaction – Example: burning gas, firecracker • If it receives thermal energy, it is an endothermic reaction – Example: baking bread, photosynthesis

Measuring • How do we measure temperature and thermal energy? – There are 3 scales • Fahrenheit scale- used mostly in America • Celsius Scale • Kelvin Scale
Measuring • Celsius – 2 reference points: • Freezing water- 0°C • Boiling Water- 100°C – Good for looking at temperature – But bad for calculations (negative temperature doesn’t work)

Measuring • Kelvin – Was created so there is a scale with no negatives - 2 reference points • 0°C = 273. 16 K • Absolute Zero = 0 K – Theoretical point when all motion stops – Third law of thermodynamics says you can’t reach it – Ever

Measuring • How do we go between them? • Celsius ↔ Kelvin – T is Kelvin – tc is Celsius T = tc + 273. 15° tc = T - 273. 15°
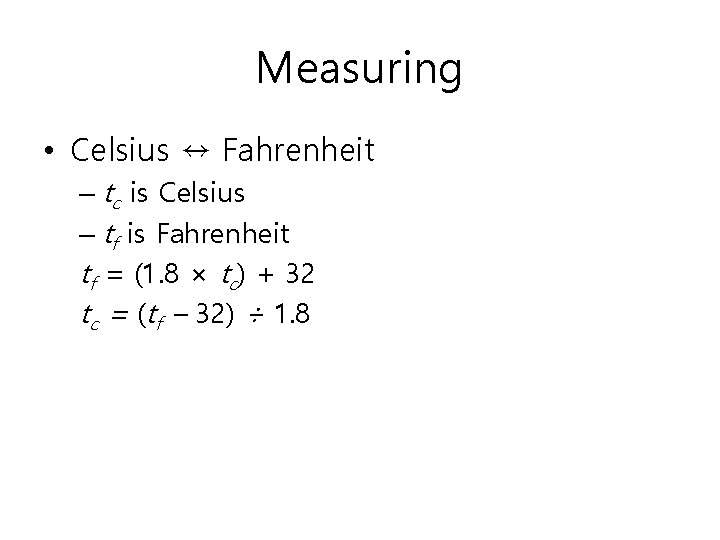
Measuring • Celsius ↔ Fahrenheit – tc is Celsius – tf is Fahrenheit tf = (1. 8 × tc) + 32 tc = (tf – 32) ÷ 1. 8

Example Problems a) While performing an experiment, you collected a sample of gas in a bottle immersed in 32. 50°C water. What is this temperature in Kelvins? T = tc + 273. 15° 32. 50 + 273. 15 = 305. 65 K

Examples Problems b) The temperature of seawater jetting from a “black smoker” vent on the bottom of the ocean on the Mid-Atlantic Ridge was measured at 671. 50 K. What is this temperature in degrees Celsius? tc = T - 273. 15° 671. 5 – 273. 15 = 398. 35 °C
- Slides: 12