Chapter 2 Atoms Molecules and Ions Three Fundamental










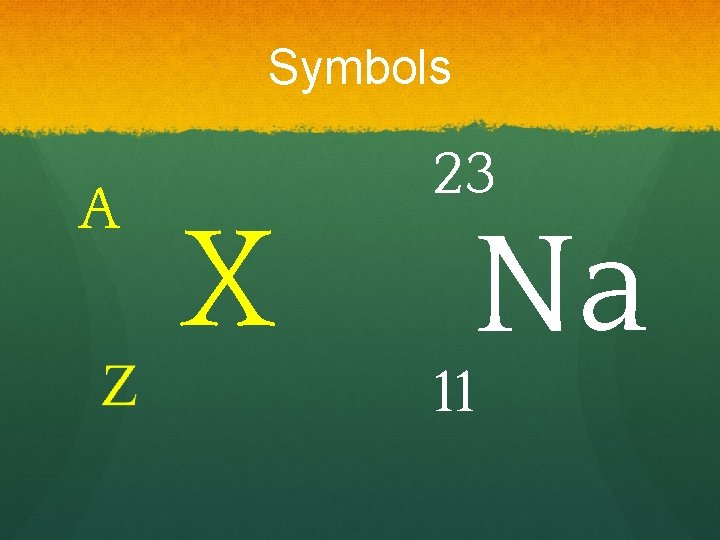























- Slides: 63

Chapter 2 Atoms, Molecules, and Ions

Three Fundamental Chemical Laws Law of definite proportion (Proust): A given compound always contains exactly the same proportion of elements by mass. (It has a constant composition) Law of multiple proportions (Dalton): When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers.

What ? ! • Water has 8 g of oxygen per 1 g of hydrogen. • Hydrogen peroxide has 16 g of oxygen per 1 g of hydrogen. • 16/8 = 2/1 • Small whole number ratios.

John Dalton ) A Quaker schoolmaster (became a teacher at the age of 12) who studied all sciences, but made his greatest contributions in chemistry. Developed Atomic Theory and Law of Multiple Proportions. Atomic Theory helped to explain many of the observations that scientists were making. Law of Multiple Proportions helped to explain that 2 elements could combine to form more than 1 compound; for example CO and CO 2.

Dalton’s Atomic Theory ● 1. Elements are made up of atoms which are indivisible. ● 2. Atoms of the same element are identical. Atoms of different elements are different. ● 3. Compounds are formed when atoms combine. Each compound has a specific number and kind of atom. ● 4. Chemical reactions are a rearrangement of atoms. Atoms are not created or destroyed.

Indivisible? ● Well, Dalton did this work in the early 1800’s. ● We know that atoms are composed of protons, neutrons and electrons. Dalton didn’t know about them—they hadn’t been discovered yet! ● HOWEVER, the atom is “the smallest part of an element that retains the properties of that element. ” ● So an atom of gold is still gold and is different from an atom of carbon.

JJ Thompson In 1897, Thompson discovered the electron. Electrons are negatively charged and have almost no mass at all, compared to a proton. Thompson revised Dalton’s model of the atom with one of his own, called the “Plum Pudding Model. ”

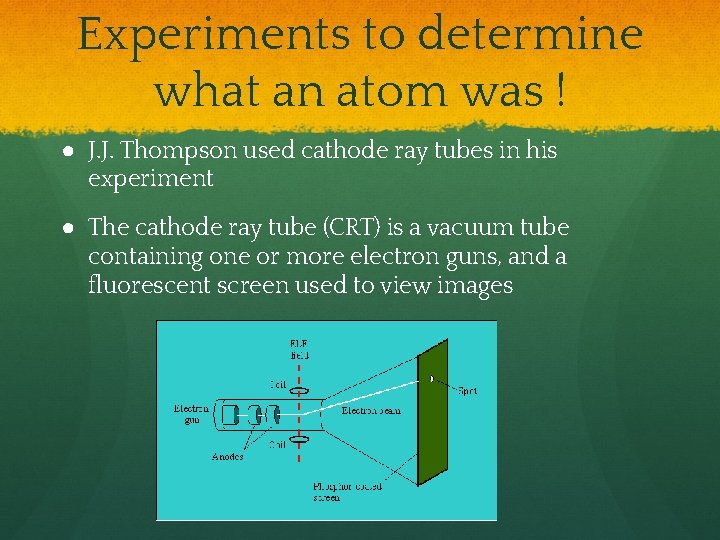
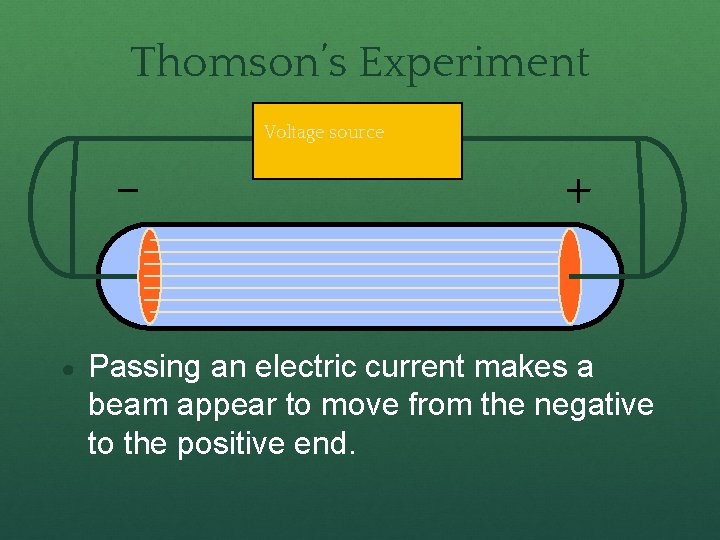
Experiments to determine what an atom was ! ● J. J. Thompson used cathode ray tubes in his experiment ● The cathode ray tube (CRT) is a vacuum tube containing one or more electron guns, and a fluorescent screen used to view images

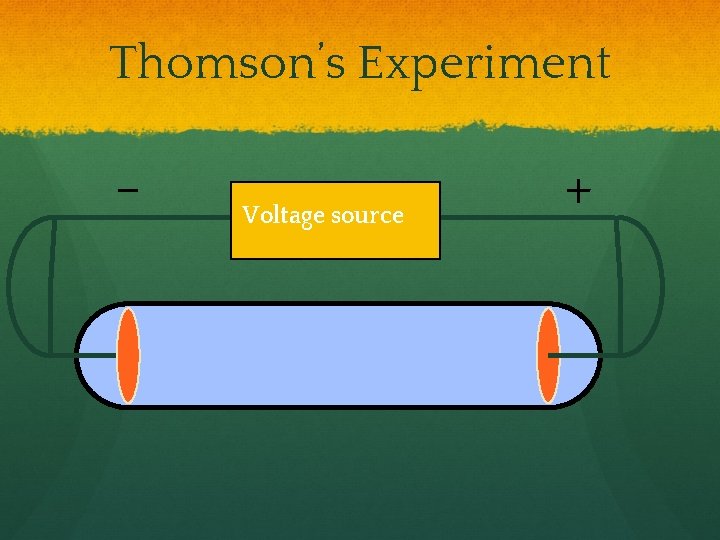
Thomson’s Experiment - Voltage source +

Thomson’s Experiment - Voltage source +

Thomson’s Experiment Voltage source ● + Passing an electric current makes a beam appear to move from the negative to the positive end.

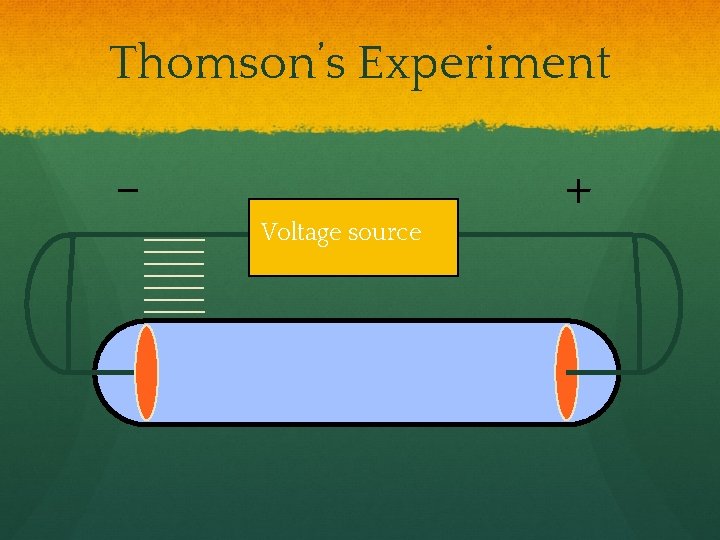
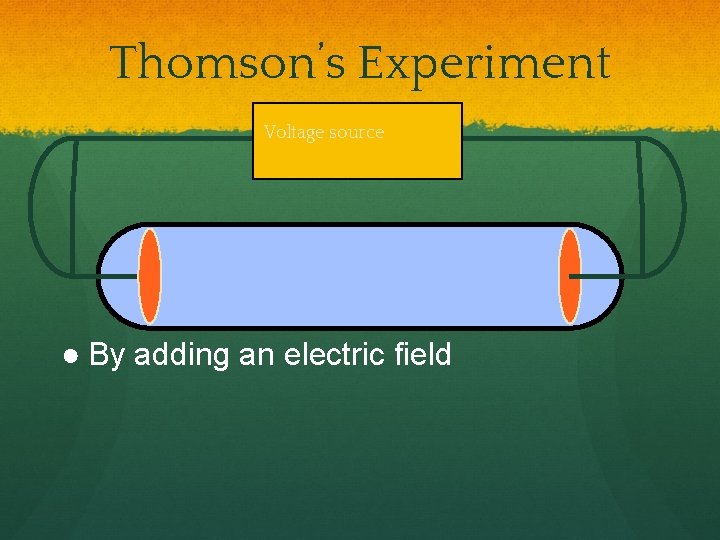
Thomson’s Experiment Voltage source ● By adding an electric field

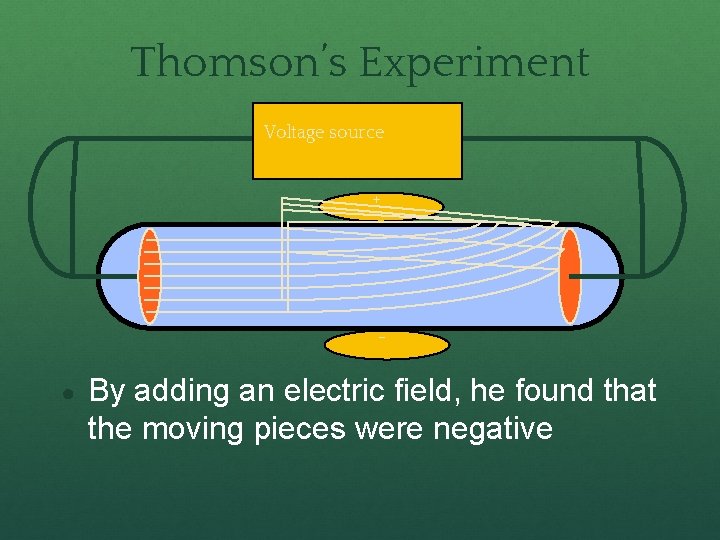
Thomson’s Experiment Voltage source + - ● By adding an electric field, he found that the moving pieces were negative

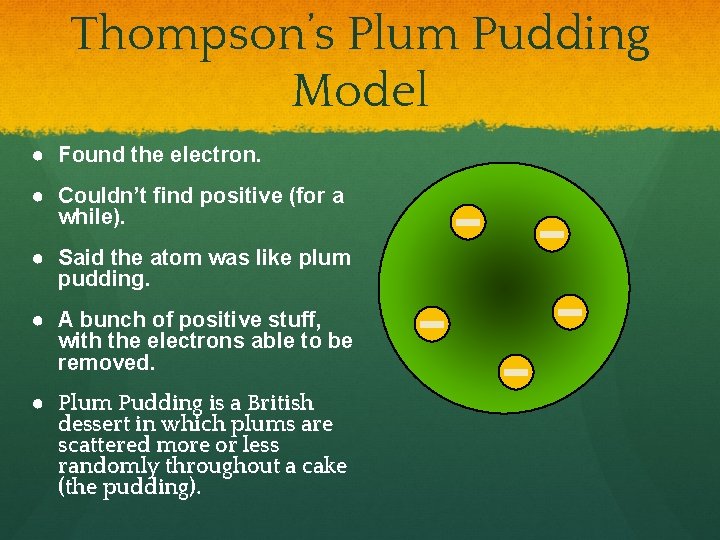
Thompson’s Plum Pudding Model ● Found the electron. ● Couldn’t find positive (for a while). ● Said the atom was like plum pudding. ● A bunch of positive stuff, with the electrons able to be removed. ● Plum Pudding is a British dessert in which plums are scattered more or less randomly throughout a cake (the pudding).

Ernest Rutherford The Plum Pudding Model wouldn’t last long, because one of JJ’s former students did some experiments that forced the model to be revised again. Rutherford was from New Zealand, and like his mentor, Thompson, also won a Nobel Prize for his work. His “work” was the famous “gold foil” experiments, where he was researching alpha particles. As sometimes happened, Rutherford didn’t set out to discover what he actually did.

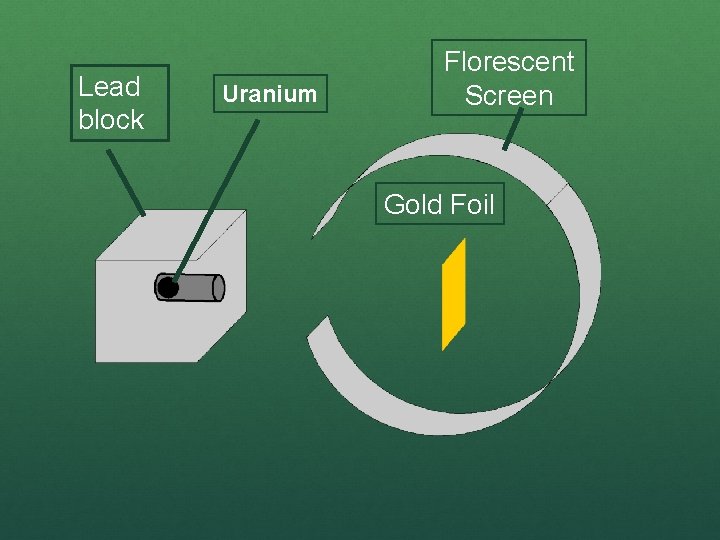
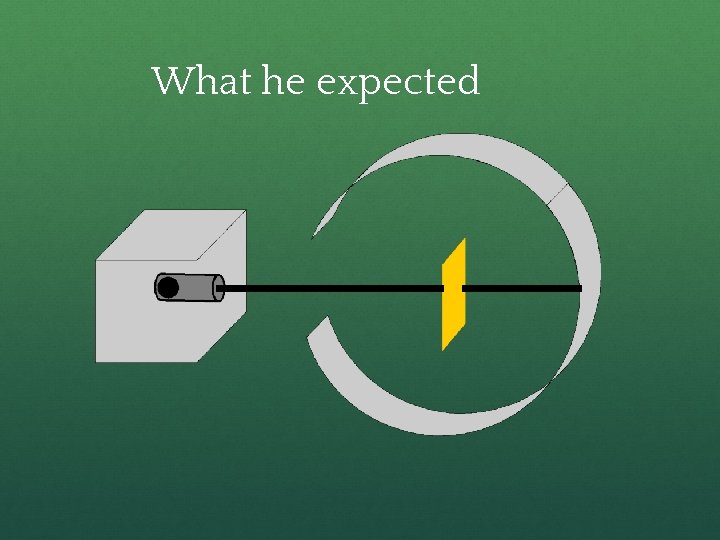
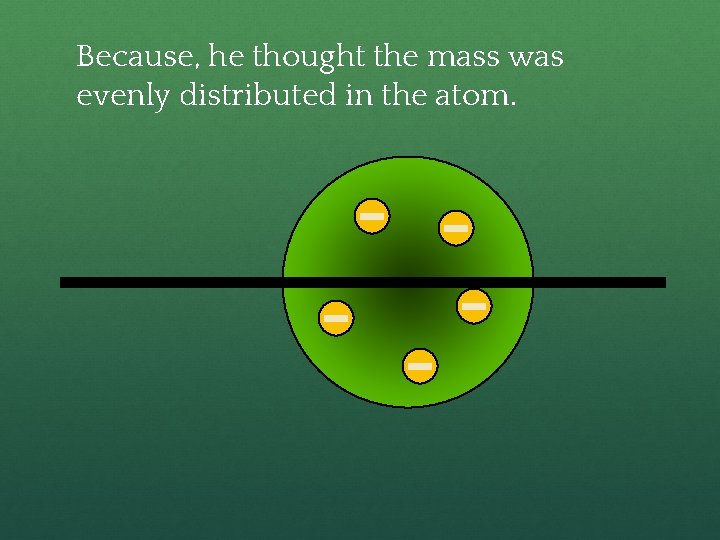
Rutherford’s Experiment ● Used uranium to produce alpha particles. ● Aimed alpha particles at gold foil by drilling hole in lead block. ● Since the mass is evenly distributed in gold atoms alpha particles should go straight through. ● Used gold foil because it could be made atoms thin.

Lead block Uranium Florescent Screen Gold Foil

What he expected

Because, he thought the mass was evenly distributed in the atom.

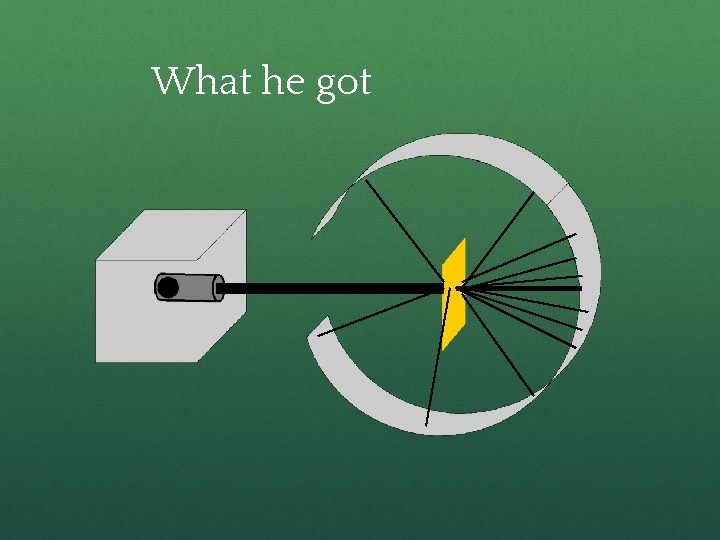
What he got

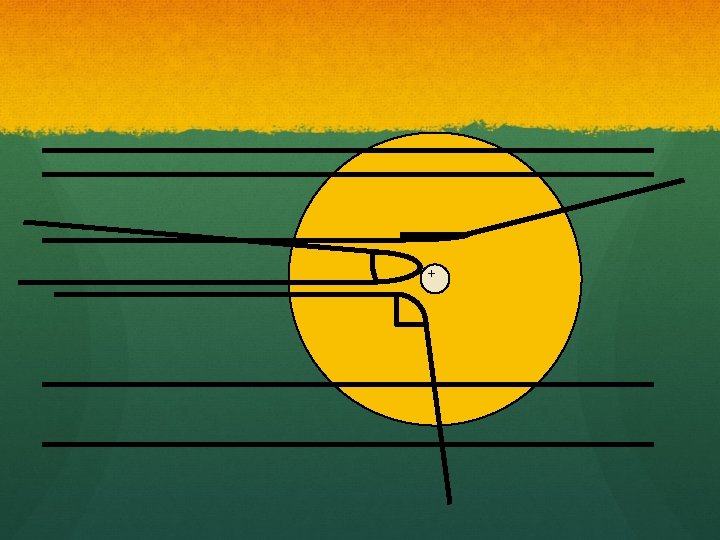
How he explained it ● Atom is mostly empty ● Small dense, positive piece at center. ● Alpha particles are deflected by it if they get close enough. +


Neils Bohr Rutherford’s nuclear model only really lasted for about 3 years, before Neils Bohr (who, oh by the way, also won a Nobel Prize for this) revised it again. Bohr asked a question: if the electrons are rotating around the nucleus, why don’t they “run out of energy. ” As they did, they would come closer and closer, attracted by the opposite charge of the nucleus, and eventually collapse onto the nucleus, destroying the atom in the process. Soccer goalie on Denmark’s 1908 Olympic team AND a Nobel Prize winner!! This doesn’t happen, and Bohr answered why. His model is usually called “the Planetary model, ” because in his model, electrons “orbit” the nucleus much as our planets orbit the Sun.

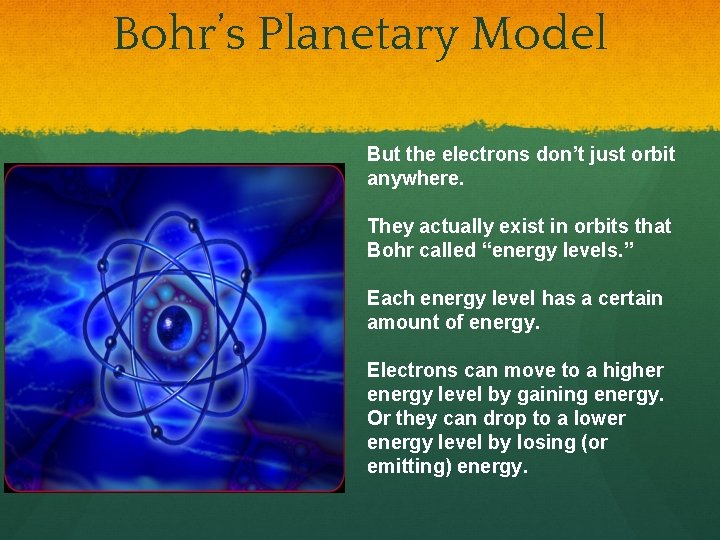
Bohr’s Planetary Model But the electrons don’t just orbit anywhere. They actually exist in orbits that Bohr called “energy levels. ” Each energy level has a certain amount of energy. Electrons can move to a higher energy level by gaining energy. Or they can drop to a lower energy level by losing (or emitting) energy.

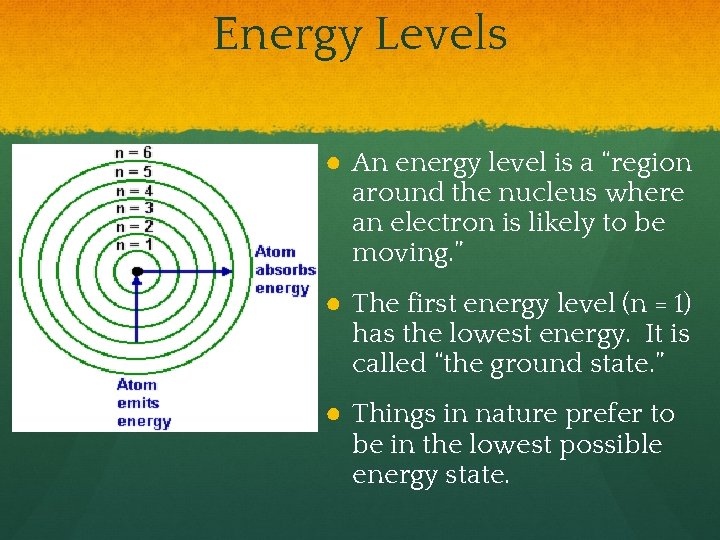
Energy Levels ● An energy level is a “region around the nucleus where an electron is likely to be moving. ” ● The first energy level (n = 1) has the lowest energy. It is called “the ground state. ” ● Things in nature prefer to be in the lowest possible energy state.

The Modern Model of the Atom ● Many scientists (Louis De. Broglie, Max Planck, Albert Einstein, Erwin Schroedinger, and many others) worked on the model of the atom. ● Actually, they weren’t working on the model of the atom. They were just working on interesting scientific problems. But they all made contributions to our current understanding of the atom. ● Quantum mechanics is the “modern” model of the atom. By the early 1930 s, it had been “born. ” It’s the model we still use today.

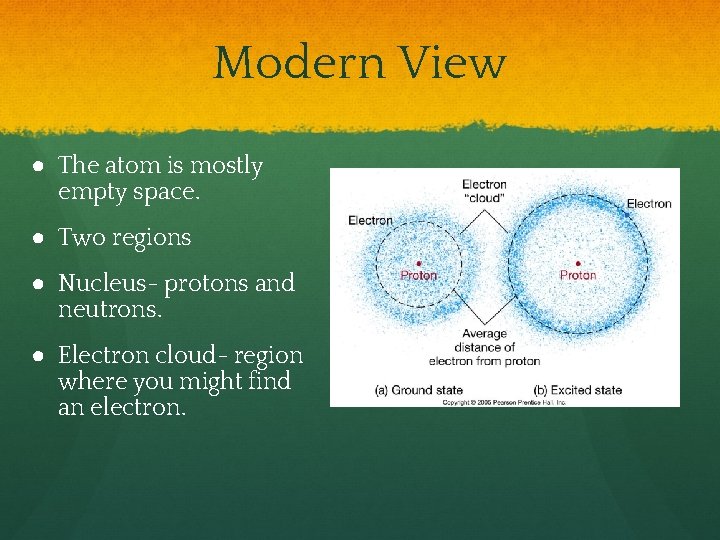
Modern View ● The atom is mostly empty space. ● Two regions ● Nucleus- protons and neutrons. ● Electron cloud- region where you might find an electron.

Sub-Atomic Particles ● Z - atomic number = number of protons determines type of atom. ● A - mass number = number of protons + neutrons. ● Number of protons = number of electrons if neutral.
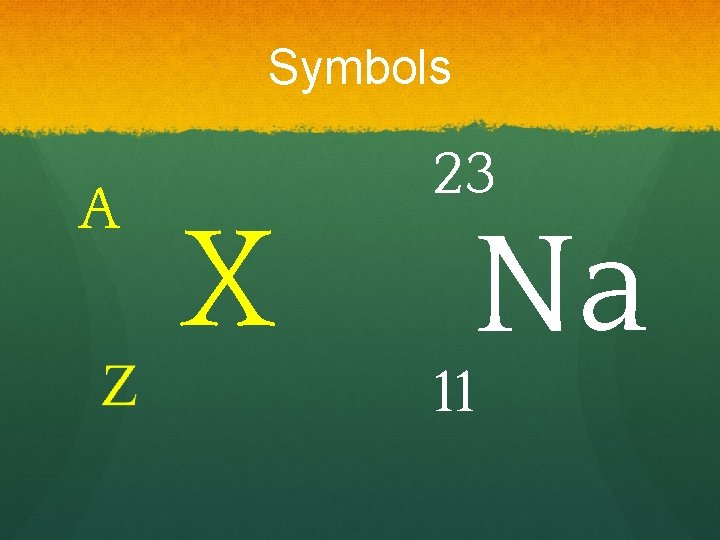
Symbols A X 23 Na 11

Chemical Bonds ● The forces that hold atoms together. ● Covalent bonding - sharing electrons. ● Makes molecules. ● Chemical formula- the number and type of atoms in a molecule. ● C 2 H 6 - 2 carbon atoms, 6 hydrogen atoms,

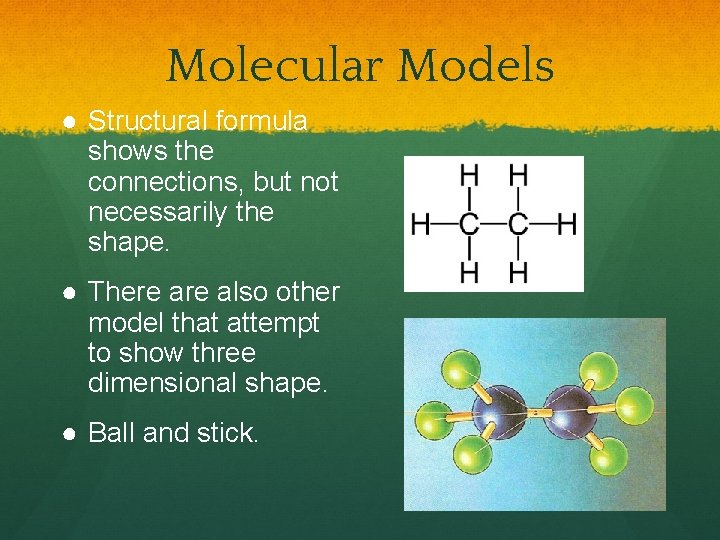
Molecular Models ● Structural formula shows the connections, but not necessarily the shape. ● There also other model that attempt to show three dimensional shape. ● Ball and stick.

Ions and Polyatomic Ions ● Atoms or groups of atoms with a charge. ● Cations- positive ions - created by losing electrons(s). ● Anions- negative ions - created by gaining electron(s). ● Ionic bonding- held together by the opposite charges. ● Ionic solids are called salts.


Metals ● Conductors ● Lose electrons ● Malleable and ductile

Nonmetals ● Brittle ● Gain electrons ● Covalent bonds

Semi-metals or Metalloids

Alkali Metals

Alkaline Earth Metals

Halogens

Transition metals

Noble Gases

Inner Transition Metals

Naming compounds Two types ● Ionic - metal and non metal or polyatomics. ● Covalent- we will just learn the rules for 2 nonmetals.

Ionic compounds ● If the cation is monoatomic- Name the metal (cation) just write the name. ● If the cation is polyatomic- name it. ● If the anion is monoatomic- name it but change the ending to –ide. ● If the anion is poly atomic- just name it.

Ionic Compounds ● Have to know what ions they form ● From the table, polyatomic, or figure it out ● Ca. S ● K 2 S ● Al. PO 4 ● K 2 SO 4 ● Fe. S ● Co. I 3

Ionic Compounds ● Fe 2(C 2 O 4) ● Mg. O ● Mn. O ● KMn. O 4 ● NH 4 NO 3 ● Hg 2 Cl 2 ● Cr 2 O 3

Ionic Compounds ● KCl. O 4 ● Na. Cl. O 3 ● YBr. O 2 ● Cr(Cl. O)6

Naming Covalent Compounds ● Two words, with prefixes ● Prefixes tell you how many. ● mono, di, tri, tetra, penta, hexa, septa, nona, deca ● First element whole name with the appropriate prefix, except mono ● Second element, -ide ending with appropriate prefix ● Practice

Naming Covalent Compounds ● CO 2 ● CO ● CCl 4 ● N 2 O 4 ● Xe. F 6 ● N 4 O 4 ● P 2 O 10

Writing Formulas ● Two sets of rules, ionic and covalent ● To decide which to use, decide what the first word is. ● If is a metal or polyatomic use ionic. ● If it is a non-metal use covalent.

Ionic Formulas ● Charges must add up to zero. ● Get charges from table, name of metal ion, or memorized from the list. ● Use parenthesis to indicate multiple polyatomics.

Ionic Formulas ● Sodium nitride ● sodium- Na is always +1 ● nitride - ide tells you it comes from the table ● nitride is N-3

Ionic Formulas ● Sodium nitride ● sodium- Na is always +1 ● Nitride - ide tells you it comes from the table ● nitride is N-3 ● Doesn’t add up to zero. +1 Na -3 N

Ionic Formulas ● Sodium nitride ● sodium- Na is always +1 ● nitride - ide tells you it comes from the table ● nitride is N-3 ● Doesn’t add up to zero ● Need 3 Na +1 Na -3 N Na 3 N

Ionic Compounds ● Sodium sulfite ● calcium iodide ● Lead (II) oxide ● Lead (IV) oxide ● Mercury (I) sulfide ● Barium chromate ● Aluminum hydrogen sulfate ● Cerium (IV) nitrite

Covalent compounds ● The name tells you how to write the formula ● duh ● Sulfur dioxide ● diflourine monoxide ● nitrogen trichloride ● diphosphorus pentoxide

Acids ● Substances that produce H+ ions when dissolved in water. ● All acids begin with H. ● Two types of acids: ● Oxyacids ● Non-oxyacids

Naming acids ● If the formula has oxygen in it ● write the name of the anion, but change ● ate to -ic acid ● ite to -ous acid ● Watch out for sulfuric and sulfurous ● H 2 Cr. O 4 ● HMn. O 4 ● HNO 2

Naming acids ● If the acid doesn’t have oxygen ● add the prefix hydro● change the suffix -ide to -ic acid ● HCl ● H 2 S ● HCN

Formulas for acids ● Backwards from names. ● If it has hydro- in the name it has no oxygen ● Anion ends in -ide ● No hydro, anion ends in -ate or -ite ● Write anion and add enough H to balance the charges.

Formulas for acids ● hydrofluoric acid ● dichromic acid ● carbonic acid ● hydrophosphoric acid ● hypofluorous acid ● perchloric acid ● phosphorous acid

Hydrates ● Some salts trap water crystals when they form crystals. ● These are hydrates. ● Both the name and the formula needs to indicate how many water molecules are trapped. ● In the name we add the word hydrate with a prefix that tells us how many water molecules.

Hydrates ● In the formula you put a dot and then write the number of molecules. ● Calcium chloride dihydrate = Ca. Cl 2∙ 2Η 2Ο ● Chromium (III) nitrate hexahydrate = Cr(NO 3)3∙ 6 H 2 O