Chapter 2 Atoms and Elements Electron Arrangement and










- Slides: 21

Chapter 2 Atoms and Elements Electron Arrangement and Periodic Law Lecture. PLUS Timberlake 1

Characteristics of Electrons l Extremely small mass l Located outside the nucleus l Moving at extremely high speeds in a sphere l Have specific energy levels Lecture. PLUS Timberlake 2

Energy of Electrons l When atoms are heated, bright lines appear called line spectra l Electrons in atoms arranged in discrete levels. l An electron absorbs energy to “jump” to a higher energy level. l When an electron falls to a lower energy level, energy is emitted. Lecture. PLUS Timberlake 3

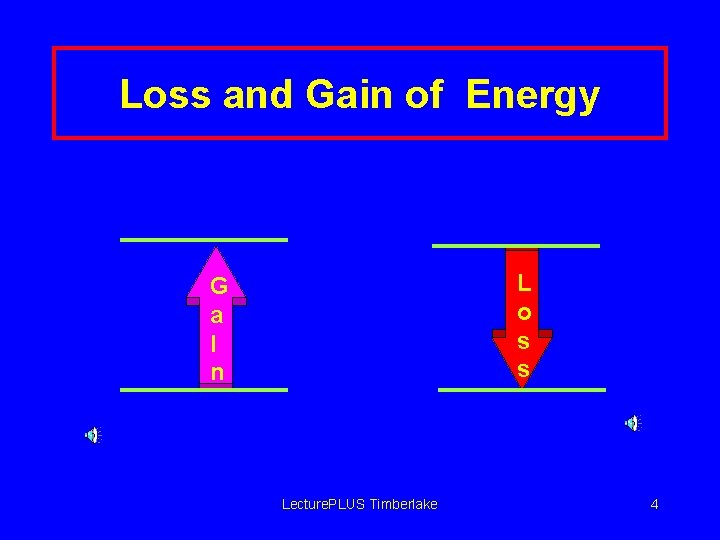
Loss and Gain of Energy L o s s G a I n Lecture. PLUS Timberlake 4

Learning Check EA 1 Answer with 1) Energy absorbed 2) Energy emitted 3) No change in energy A. What energy change takes place when an electron in a hydrogen atom moves from the first (n=1) to the second shell (n=2)? B. What energy change takes place when the electron moves from the third shell to the second shell? Lecture. PLUS Timberlake 5

Solution EA 1 A. 1) Energy absorbed B. 2) Energy emitted Lecture. PLUS Timberlake 6

Bohr Model • First model of the electron structure • Gives levels where an electron is most likely to be found • Incorrect today, but a key in understanding the atom Lecture. PLUS Timberlake 7

Quantum Mechanics Describes the arrangement and space occupied by electrons in atoms Lecture. PLUS Timberlake 8

Electron Levels (Shells) l Contain electrons that are similar in energy and distance from nucleus l Low energy electrons are closest to the nucleus l Identify by numbers 1, 2, 3, 4, 5, 6…. . l The first shell (1) is lowest in energy, 2 nd level next and so on 1<2<3<4 Lecture. PLUS Timberlake 9

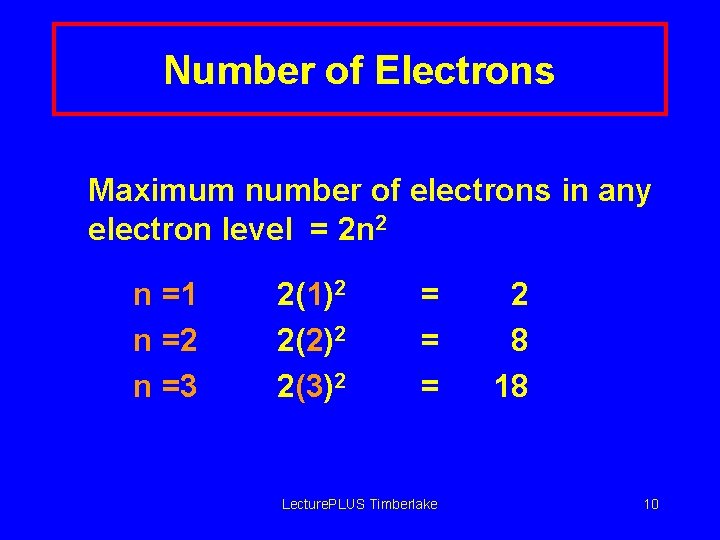
Number of Electrons Maximum number of electrons in any electron level = 2 n 2 n =1 n =2 n =3 2(1)2 2(2)2 2(3)2 = = = Lecture. PLUS Timberlake 2 8 18 10

Order of Electron Filling All electrons in the same energy level have similar energy. Shell 1 Shell 2 Shell 3 2 electrons 8 electrons 18 electrons (8 first, later 10) Order of filling for the first 20 electrons Shell 1 2 e 2 8 e 3 8 e Lecture. PLUS Timberlake 4 2 e 11

Electron Configuration l Lists the shells containing electrons l Written in order of increasing energy Element Shell 1 2 3 He 2 C 2 4 F 2 7 Ne 2 8 Al 2 8 3 Cl 2 8 7 Lecture. PLUS Timberlake 12

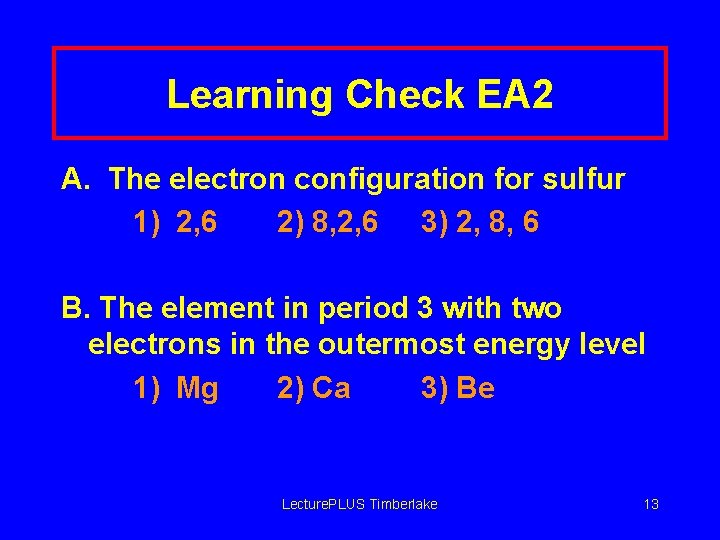
Learning Check EA 2 A. The electron configuration for sulfur 1) 2, 6 2) 8, 2, 6 3) 2, 8, 6 B. The element in period 3 with two electrons in the outermost energy level 1) Mg 2) Ca 3) Be Lecture. PLUS Timberlake 13

Solution EA 2 A. The electron configuration for sulfur 3) 2, 8, 6 B. The element in period 3 with two electrons in the outermost energy level 1) Mg Lecture. PLUS Timberlake 14

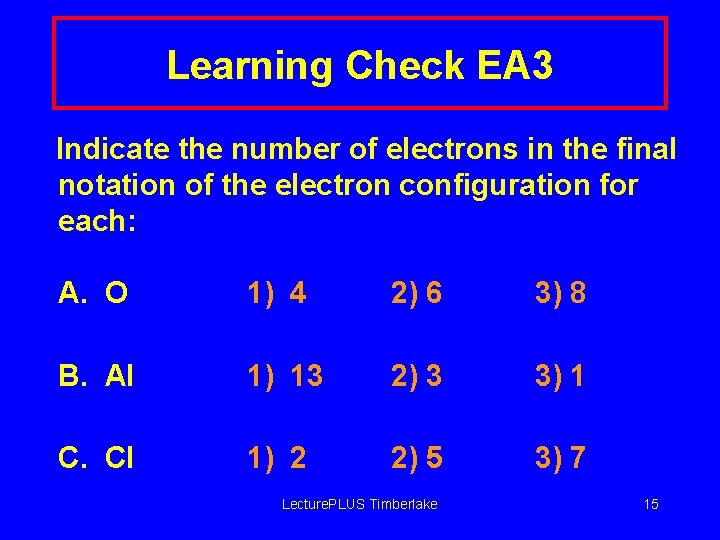
Learning Check EA 3 Indicate the number of electrons in the final notation of the electron configuration for each: A. O 1) 4 2) 6 3) 8 B. Al 1) 13 2) 3 3) 1 C. Cl 1) 2 2) 5 3) 7 Lecture. PLUS Timberlake 15

Solution EA 3 Indicate the number of electrons in the final notation of the electron configuration for each: A. O 2) 6 B. Al 2) 3 C. Cl 3) 7 Lecture. PLUS Timberlake 16

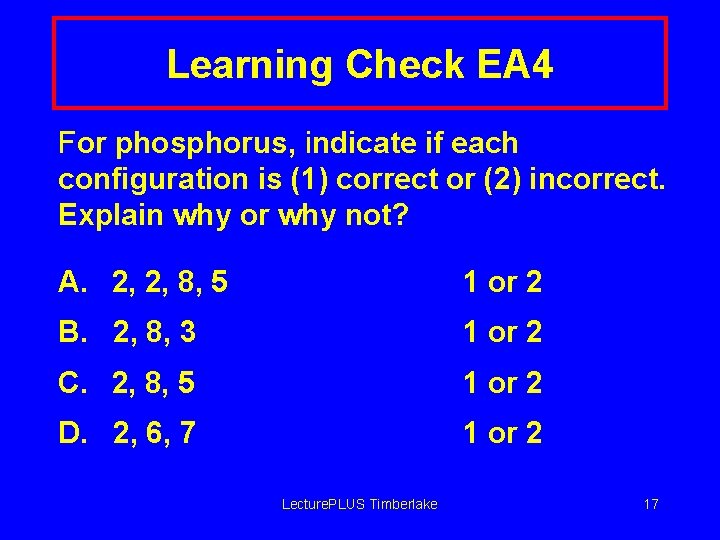
Learning Check EA 4 For phosphorus, indicate if each configuration is (1) correct or (2) incorrect. Explain why or why not? A. 2, 2, 8, 5 1 or 2 B. 2, 8, 3 1 or 2 C. 2, 8, 5 1 or 2 D. 2, 6, 7 1 or 2 Lecture. PLUS Timberlake 17

Solution EA 4 For phosphorus, indicate if each configuration is (1) correct or (2) incorrect. Explain why or why not? A. 2, 2, 8, 5 2 Shell 2 holds 8 e- B. 2, 8, 3 2 P has 15 electrons C. 2, 8, 5 1 Correct arrangement D. 2, 6, 7 2 Shell 2 holds 8 e. Lecture. PLUS Timberlake 18

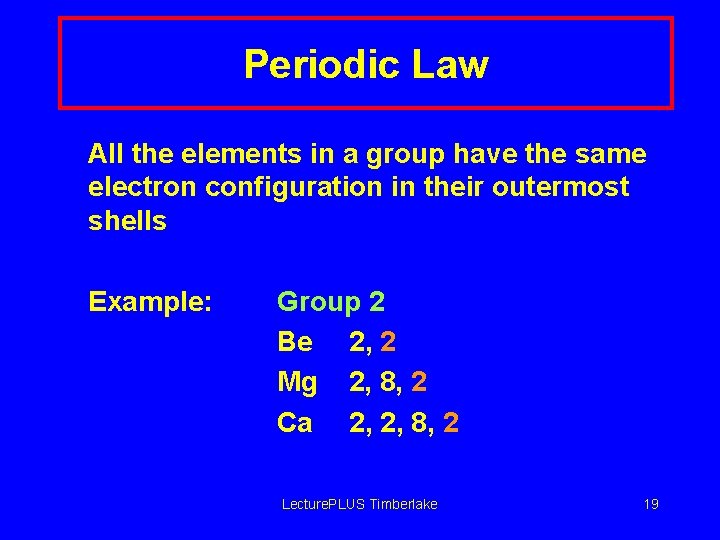
Periodic Law All the elements in a group have the same electron configuration in their outermost shells Example: Group 2 Be 2, 2 Mg 2, 8, 2 Ca 2, 2, 8, 2 Lecture. PLUS Timberlake 19

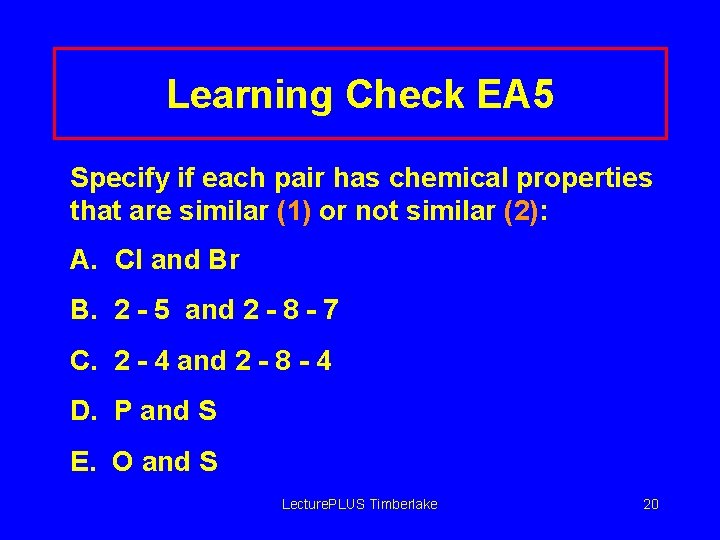
Learning Check EA 5 Specify if each pair has chemical properties that are similar (1) or not similar (2): A. Cl and Br B. 2 - 5 and 2 - 8 - 7 C. 2 - 4 and 2 - 8 - 4 D. P and S E. O and S Lecture. PLUS Timberlake 20

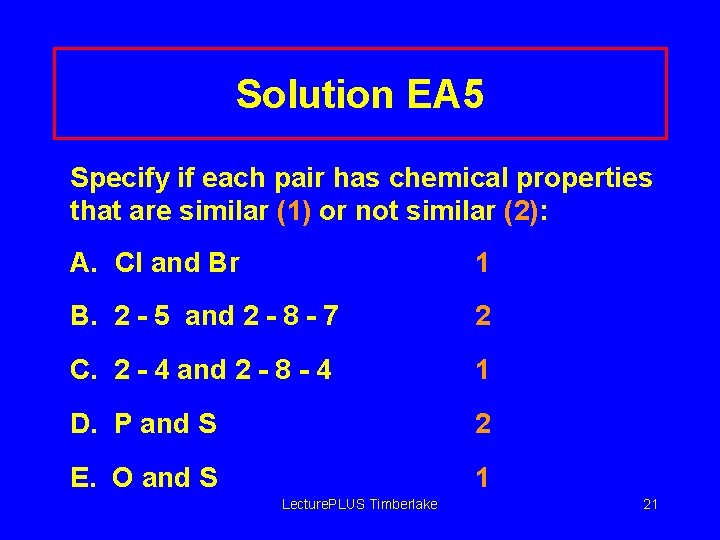
Solution EA 5 Specify if each pair has chemical properties that are similar (1) or not similar (2): A. Cl and Br 1 B. 2 - 5 and 2 - 8 - 7 2 C. 2 - 4 and 2 - 8 - 4 1 D. P and S 2 E. O and S 1 Lecture. PLUS Timberlake 21