Chapter 2 Atomic Structure Interatomic Bonding ISSUES TO
Chapter 2: Atomic Structure & Interatomic Bonding ISSUES TO ADDRESS. . . • What promotes bonding? • What types of bonds are there? • What properties are inferred from bonding? 1
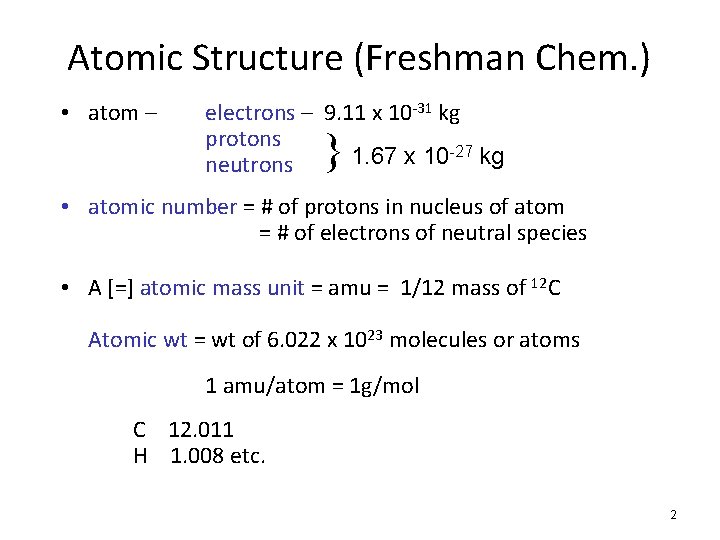
Atomic Structure (Freshman Chem. ) • atom – electrons – 9. 11 x 10 -31 kg protons 1. 67 x 10 -27 kg neutrons } • atomic number = # of protons in nucleus of atom = # of electrons of neutral species • A [=] atomic mass unit = amu = 1/12 mass of 12 C Atomic wt = wt of 6. 022 x 1023 molecules or atoms 1 amu/atom = 1 g/mol C 12. 011 H 1. 008 etc. 2
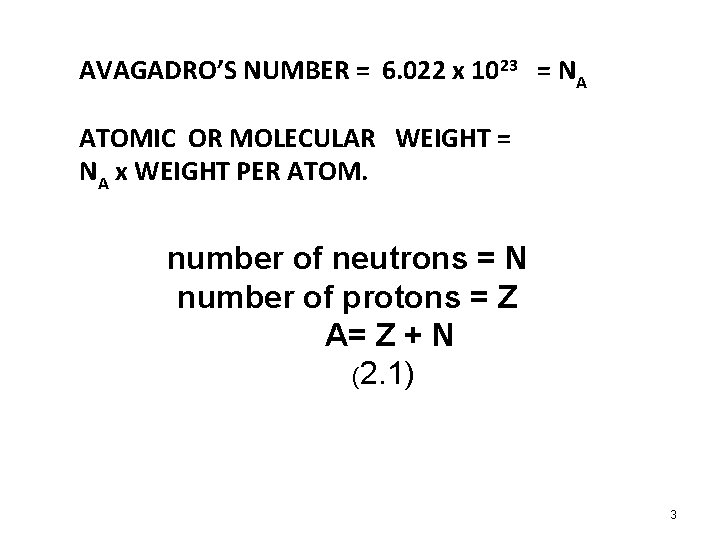
AVAGADRO’S NUMBER = 6. 022 x 1023 = NA ATOMIC OR MOLECULAR WEIGHT = NA x WEIGHT PER ATOM. number of neutrons = N number of protons = Z A= Z + N (2. 1) 3

Atomic Structure • Valence electrons determine all of the following properties 1) 2) 3) 4) Chemical Electrical Thermal Optical 4

BOHR ATOM 5
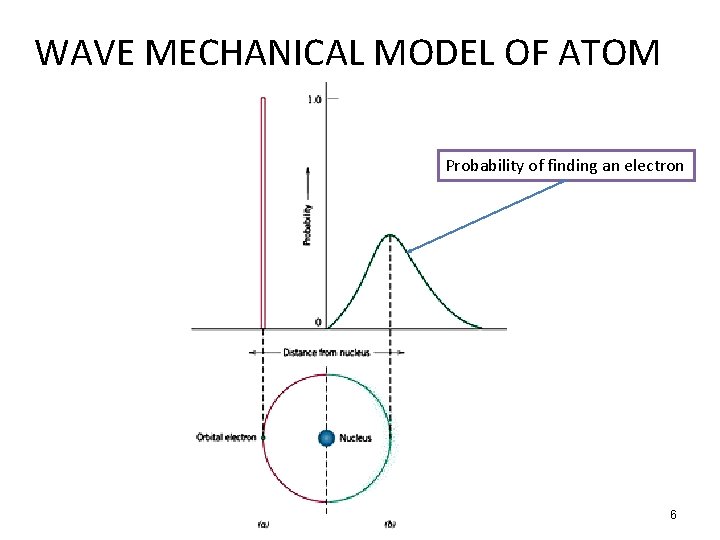
WAVE MECHANICAL MODEL OF ATOM Probability of finding an electron 6

Electronic Structure • Electrons have wavelike and particulate properties. – This means that electrons are in orbitals defined by a probability. – Each orbital at discrete energy level is determined by quantum numbers. Quantum # Designation Impact n = principal (energy level-shell)K, L, M, N, O (1, 2, 3, etc. ) size l = subsidiary (orbitals) s, p, d, f (0, 1, 2, 3, …, n -1) shape ml = magnetic 1, 3, 5, 7 (-l to +l) no. of states ms = spin ½, -½ 7
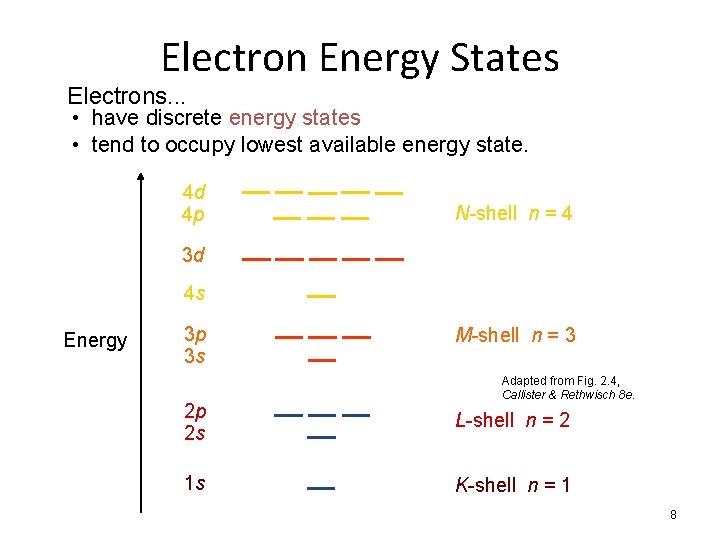
Electron Energy States Electrons. . . • have discrete energy states • tend to occupy lowest available energy state. 4 d 4 p N-shell n = 4 3 d 4 s Energy 3 p 3 s M-shell n = 3 Adapted from Fig. 2. 4, Callister & Rethwisch 8 e. 2 p 2 s L-shell n = 2 1 s K-shell n = 1 8
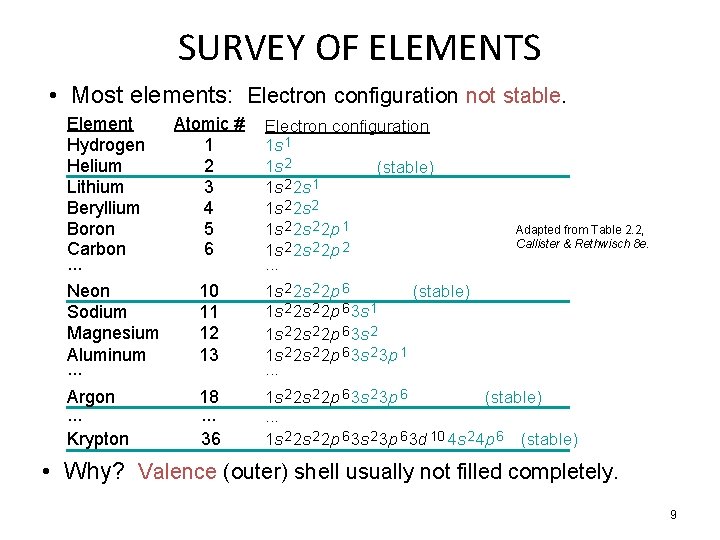
SURVEY OF ELEMENTS • Most elements: Electron configuration not stable. Element Atomic # Hydrogen 1 Helium 2 Lithium 3 Beryllium 4 Boron 5 Carbon 6. . . Neon 10 Sodium 11 Magnesium 12 Aluminum 13. . . Electron configuration 1 s 1 1 s 2 (stable) 1 s 2 2 s 1 1 s 2 2 s 2 2 p 1 1 s 2 2 p 2. . . Argon. . . Krypton 1 s 2 2 p 6 3 s 2 3 p 6 (stable). . . 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 (stable) 18. . . 36 Adapted from Table 2. 2, Callister & Rethwisch 8 e. 1 s 2 2 p 6 (stable) 1 s 2 2 p 6 3 s 1 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1. . . • Why? Valence (outer) shell usually not filled completely. 9

Electron Configurations • Valence electrons – those in unfilled shells • Filled shells more stable • Valence electrons are most available for bonding and tend to control the chemical properties – example: C (atomic number = 6) 1 s 2 2 s 2 2 p 2 valence electrons 10

Electrons in different shells 11
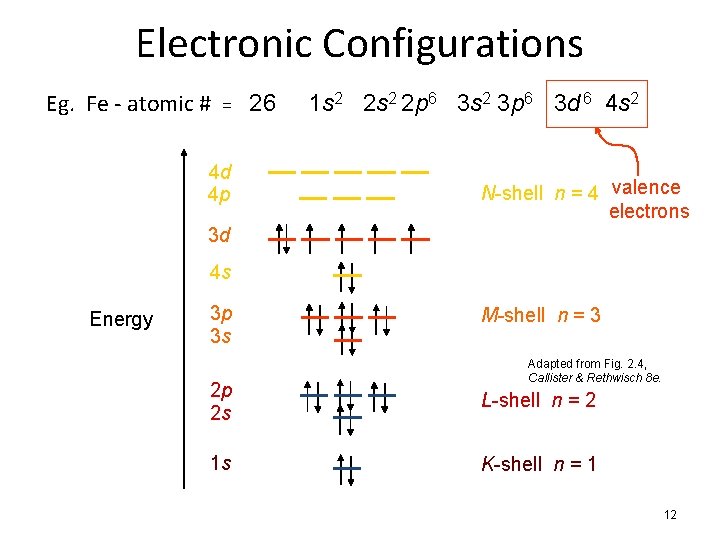
Electronic Configurations Eg. Fe - atomic # = 26 4 d 4 p 1 s 2 2 p 6 3 s 2 3 p 6 3 d 6 4 s 2 N-shell n = 4 valence electrons 3 d 4 s Energy 3 p 3 s M-shell n = 3 Adapted from Fig. 2. 4, Callister & Rethwisch 8 e. 2 p 2 s L-shell n = 2 1 s K-shell n = 1 12

give up 1 egive up 2 egive up 3 e- • Columns: Similar Valence Structure accept 2 eaccept 1 einert gases The Periodic Table H He Li Be O F Ne Na Mg S Cl Ar K Ca Sc Rb Sr Y Cs Ba Se Br Kr Te I Adapted from Fig. 2. 6, Callister & Rethwisch 8 e. Xe Po At Rn Fr Ra Electropositive elements: Readily give up electrons to become + ions. Electronegative elements: Readily acquire electrons to become - ions. 13
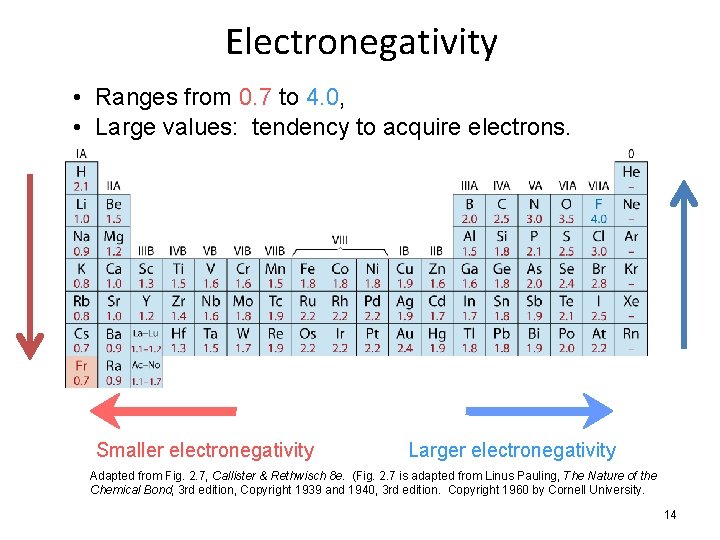
Electronegativity • Ranges from 0. 7 to 4. 0, • Large values: tendency to acquire electrons. Smaller electronegativity Larger electronegativity Adapted from Fig. 2. 7, Callister & Rethwisch 8 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. 14
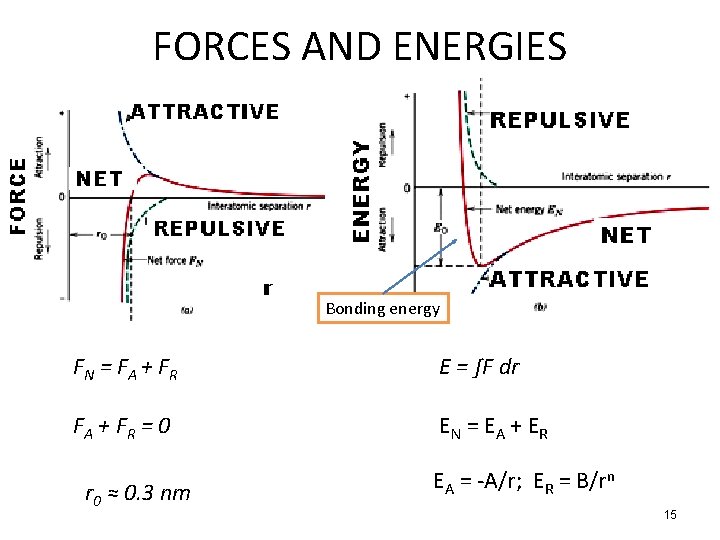
FORCES AND ENERGIES Bonding energy FN = F A + FR E = ∫F dr FA + F R = 0 EN = EA + ER r 0 ≈ 0. 3 nm EA = -A/r; ER = B/rn 15
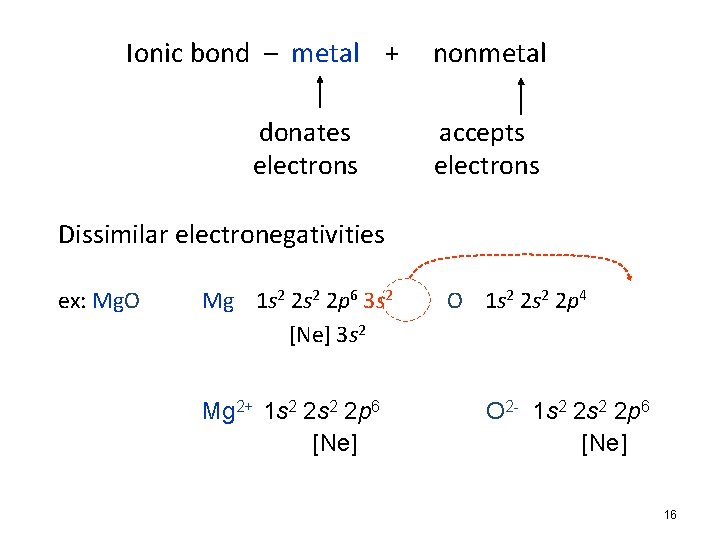
Ionic bond – metal + nonmetal donates accepts electrons Dissimilar electronegativities ex: Mg. O Mg 1 s 2 2 p 6 3 s 2 O 1 s 2 2 p 4 [Ne] 3 s 2 Mg 2+ 1 s 2 2 p 6 [Ne] O 2 - 1 s 2 2 p 6 [Ne] 16

Electrons in Sodium and Chlorine TABLE 2. 2 / P 25 3 s 1 3 s 2 3 p 5 17
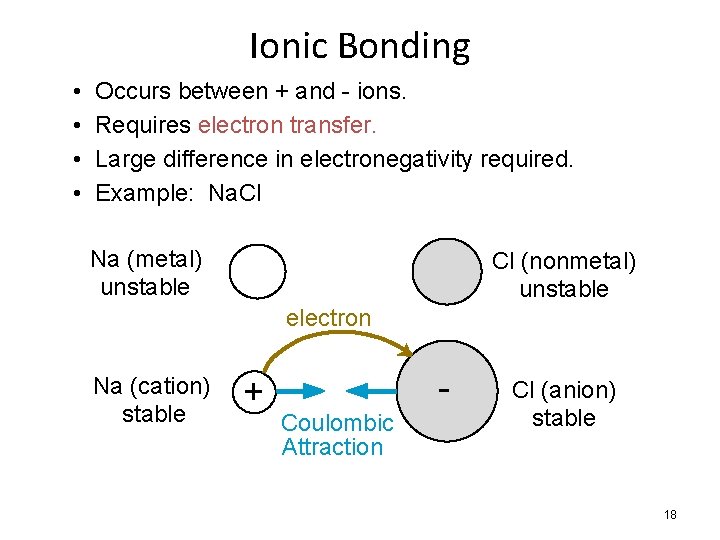
Ionic Bonding • • Occurs between + and - ions. Requires electron transfer. Large difference in electronegativity required. Example: Na. Cl Na (metal) unstable Cl (nonmetal) unstable electron Na (cation) stable + Coulombic Attraction Cl (anion) stable 18
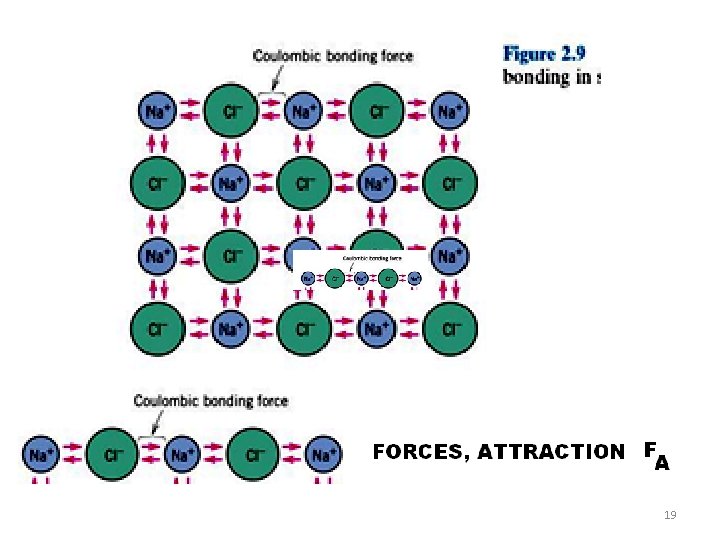
19

Bonding Forces and Energies 2. 13 Calculate the force of attraction between a K+ and an O 2 - ion the centers of which are separated by a distance of r 0 =1. 5 nm. Solution The attractive force between two ions FA is just the derivative with respect to the interatomic separation of the attractive energy expression, Equation 2. 8, which is just 20

The constant A in this expression is defined in footnote 3. Since the valences of the K+ and O 2 - ions (Z 1 and Z 2) are +1 and -2, respectively, Z 1 = 1 and Z 2 = 2, then 21

=2. 05 10 -10 N 22

IONIC FORCE / P 31 FOOT-NOTE F= (Z 1 *Z 2 * e^2)/(4*π*ε 0*r^2); e= 1. 602 *10^(-19) COULOMBS ; ε 0 = 8. 85 * 10^(-12 ) Z 1, Z 2 = VALENCIES OF IONS 23
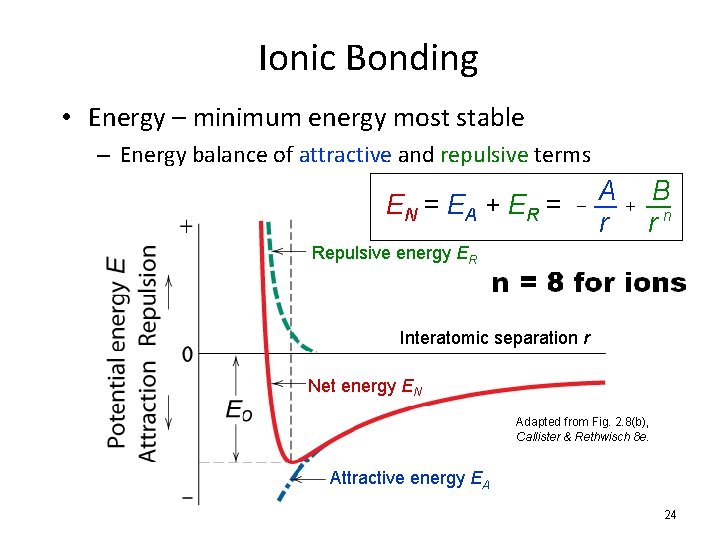
Ionic Bonding • Energy – minimum energy most stable – Energy balance of attractive and repulsive terms EN = EA + ER = - A r + B rn Repulsive energy ER Interatomic separation r Net energy EN Adapted from Fig. 2. 8(b), Callister & Rethwisch 8 e. Attractive energy EA 24

• Examples: Ionic Bonding Predominant bonding in Ceramics Na. Cl Mg. O Ca. F 2 Cs. Cl Give up electrons Acquire electrons Adapted from Fig. 2. 7, Callister & Rethwisch 8 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. 25
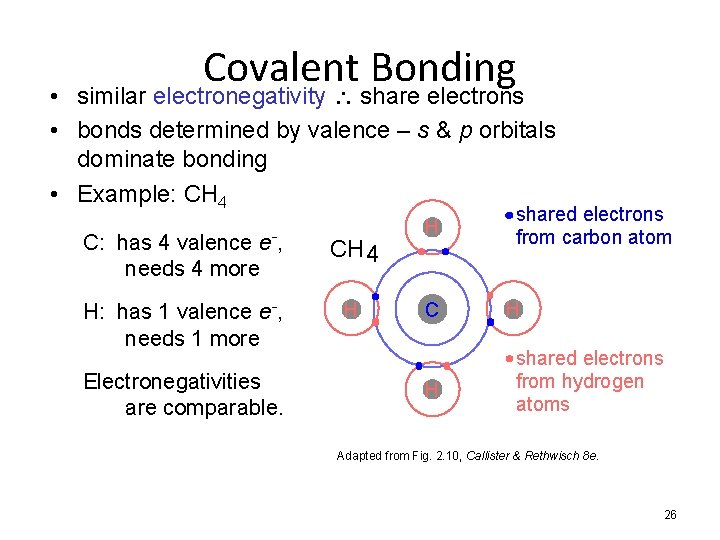
Covalent Bonding • similar electronegativity share electrons • bonds determined by valence – s & p orbitals dominate bonding • Example: CH 4 C: has 4 valence e-, needs 4 more CH 4 H: has 1 valence e-, needs 1 more H Electronegativities are comparable. H C H shared electrons from carbon atom H shared electrons from hydrogen atoms Adapted from Fig. 2. 10, Callister & Rethwisch 8 e. 26
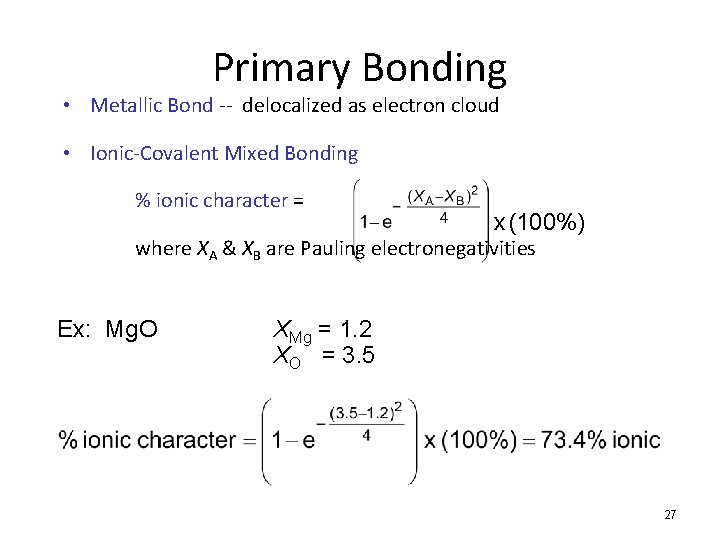
Primary Bonding • Metallic Bond -- delocalized as electron cloud • Ionic-Covalent Mixed Bonding % ionic character = x (100%) where XA & XB are Pauling electronegativities Ex: Mg. O XMg = 1. 2 XO = 3. 5 27
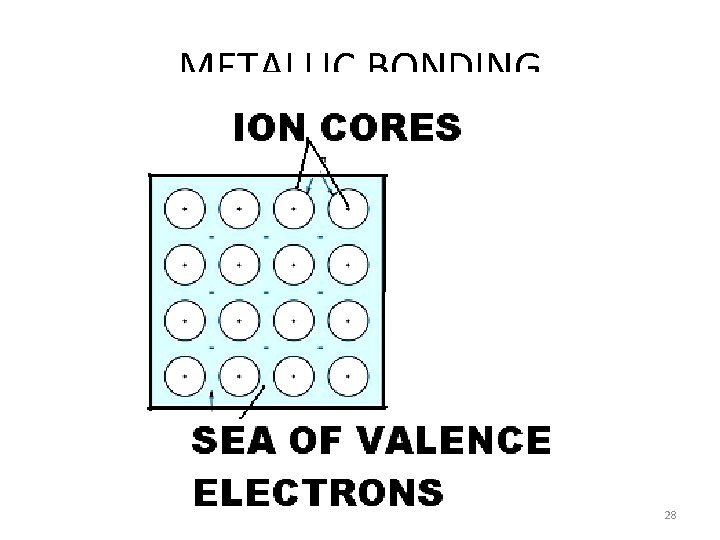
METALLIC BONDING 28
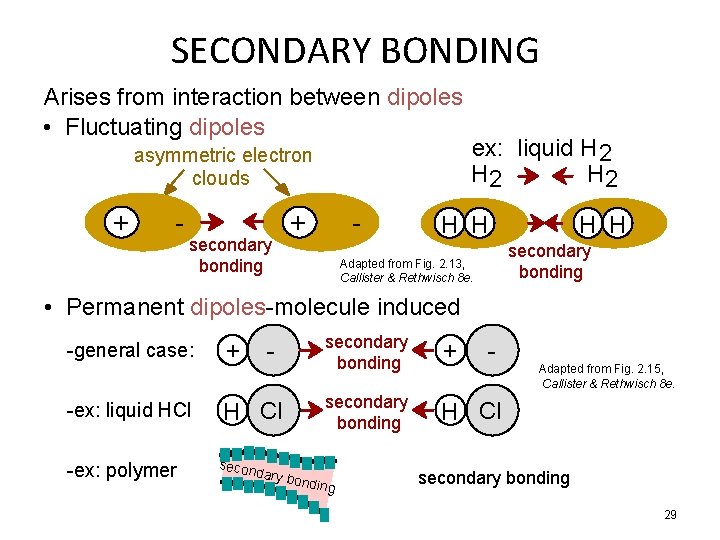
SECONDARY BONDING Arises from interaction between dipoles • Fluctuating dipoles asymmetric electron clouds + - secondary bonding + - ex: liquid H 2 H 2 H H secondary bonding Adapted from Fig. 2. 13, Callister & Rethwisch 8 e. • Permanent dipoles-molecule induced -general case: -ex: liquid HCl -ex: polymer + - H Cl secon dary b secondary bonding + secondary bonding H Cl ondin g - Adapted from Fig. 2. 15, Callister & Rethwisch 8 e. secondary bonding 29
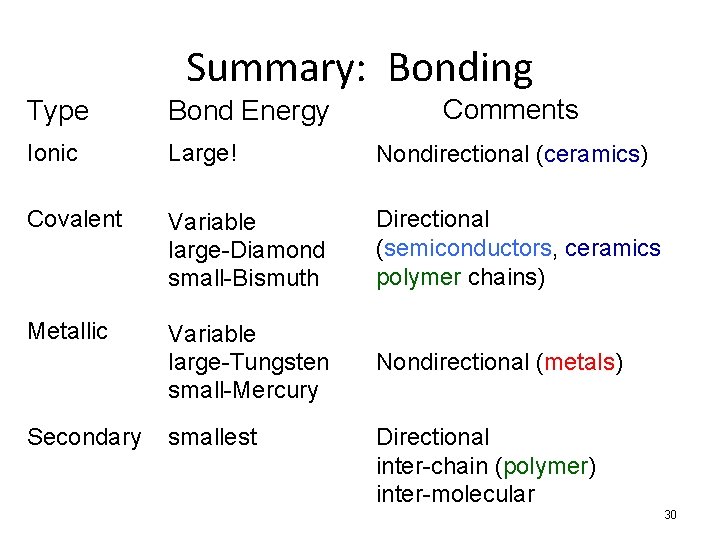
Summary: Bonding Comments Type Bond Energy Ionic Large! Nondirectional (ceramics) Covalent Variable large-Diamond small-Bismuth Directional (semiconductors, ceramics polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) Secondary smallest Directional inter-chain (polymer) inter-molecular 30
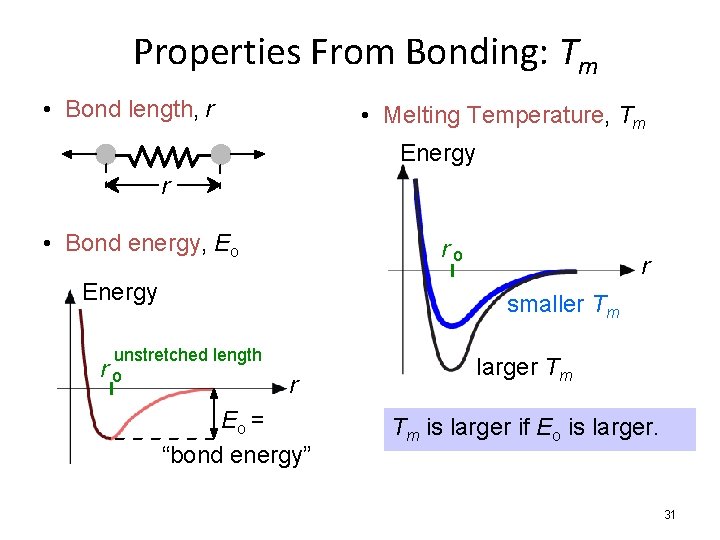
Properties From Bonding: Tm • Bond length, r • Melting Temperature, Tm Energy r • Bond energy, Eo ro Energy r smaller Tm unstretched length ro r Eo = “bond energy” larger Tm Tm is larger if Eo is larger. 31
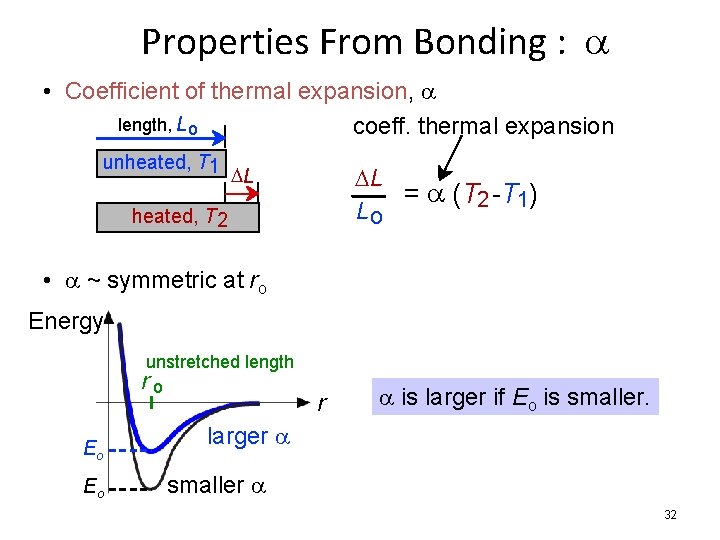
Properties From Bonding : a • Coefficient of thermal expansion, a length, L o coeff. thermal expansion unheated, T 1 DL = a (T 2 -T 1) Lo DL heated, T 2 • a ~ symmetric at ro Energy unstretched length ro Eo Eo r a is larger if Eo is smaller. larger a smaller a 32
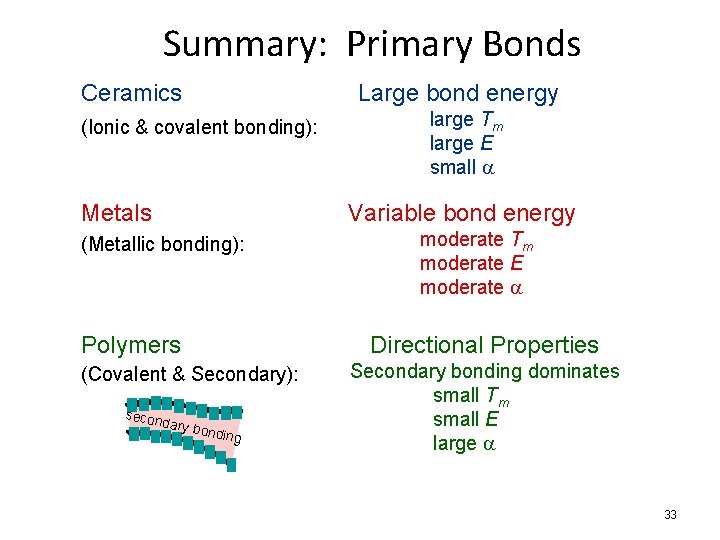
Summary: Primary Bonds Ceramics Large bond energy (Ionic & covalent bonding): Metals large Tm large E small a Variable bond energy (Metallic bonding): Polymers Directional Properties (Covalent & Secondary): secon moderate Tm moderate E moderate a dary b o nding Secondary bonding dominates small Tm small E large a 33
- Slides: 33