Chapter 2 Atomic structure and atomic bonding ISSUES





Chapter 2 Atomic structure and atomic bonding

ISSUES TO ADDRESS. . . • What promotes bonding? • What types of bonds are there? • What properties are inferred from bonding?
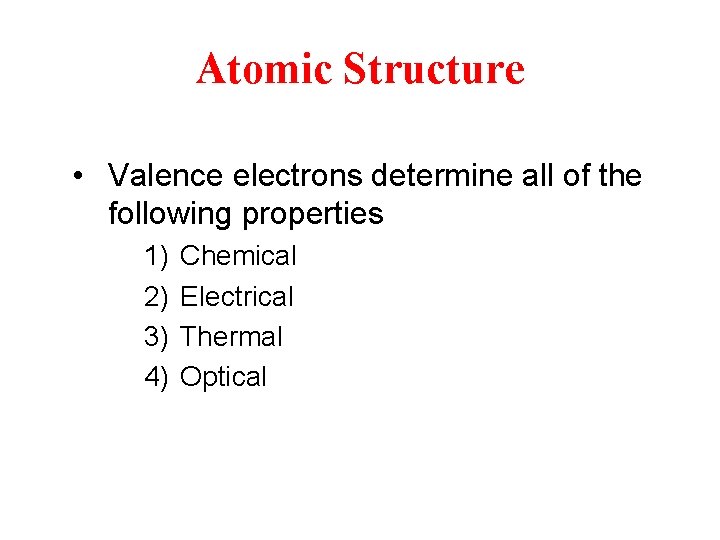
Atomic Structure • Valence electrons determine all of the following properties 1) 2) 3) 4) Chemical Electrical Thermal Optical
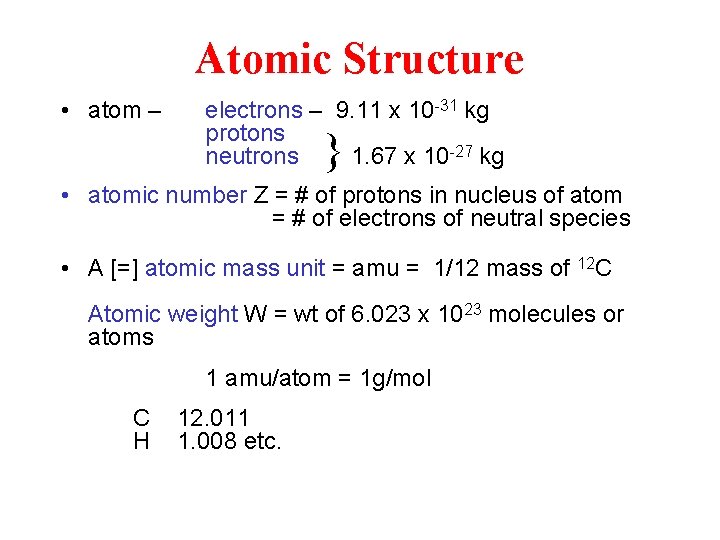
Atomic Structure • atom – electrons – 9. 11 x 10 -31 kg protons 1. 67 x 10 -27 kg neutrons } • atomic number Z = # of protons in nucleus of atom = # of electrons of neutral species • A [=] atomic mass unit = amu = 1/12 mass of 12 C Atomic weight W = wt of 6. 023 x 1023 molecules or atoms 1 amu/atom = 1 g/mol C H 12. 011 1. 008 etc.
Atomic structure • • • Atomic number Z Atomic mass A and atomic weight W Neutron number N A=Z+N Isotopes (hydrogen氫A=Z, deuterium氘 A=Z+1 N, tritium氚A=Z+2 N) • 1 amu/atom = 1 g/mol
p. 21
p. 21
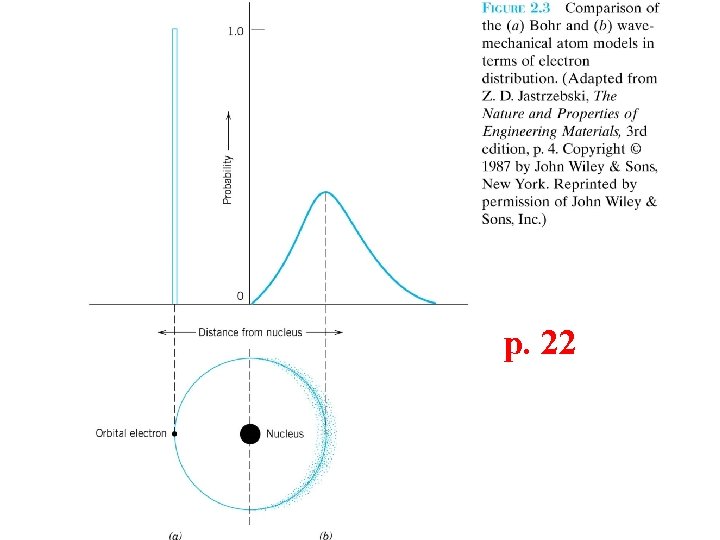
p. 22
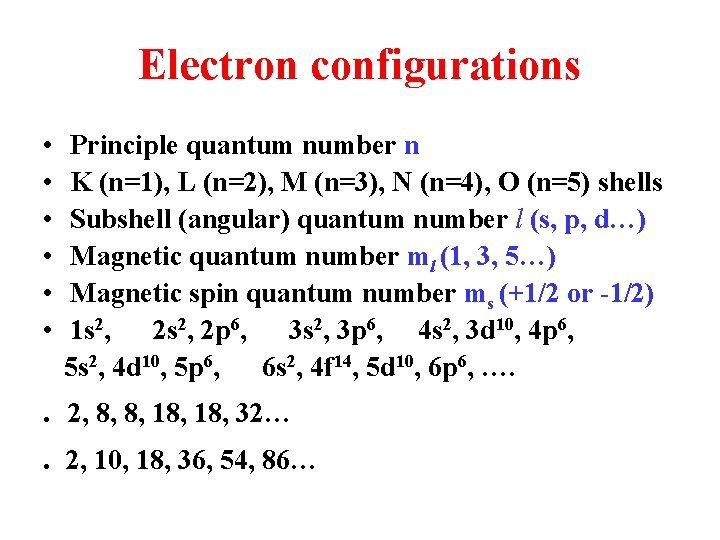
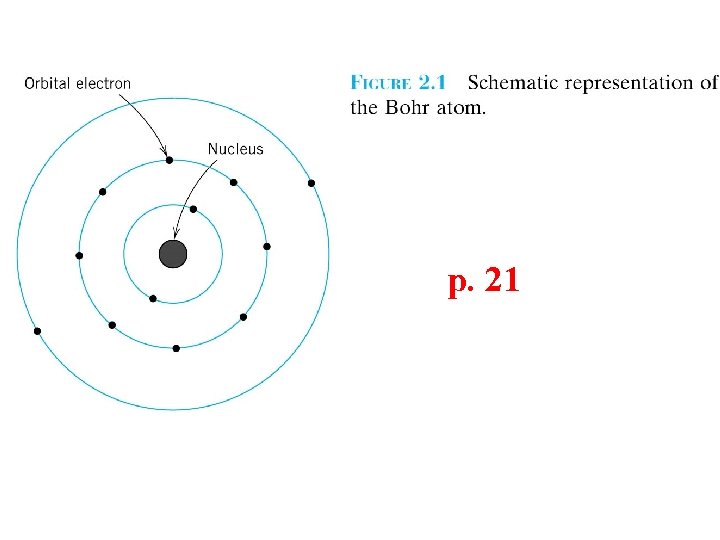
Electron configurations • • • Principle quantum number n K (n=1), L (n=2), M (n=3), N (n=4), O (n=5) shells Subshell (angular) quantum number l (s, p, d…) Magnetic quantum number ml (1, 3, 5…) Magnetic spin quantum number ms (+1/2 or -1/2) 1 s 2, 2 p 6, 3 s 2, 3 p 6, 4 s 2, 3 d 10, 4 p 6, 5 s 2, 4 d 10, 5 p 6, 6 s 2, 4 f 14, 5 d 10, 6 p 6, …. . . 2, 8, 8, 18, 32… 2, 10, 18, 36, 54, 86…
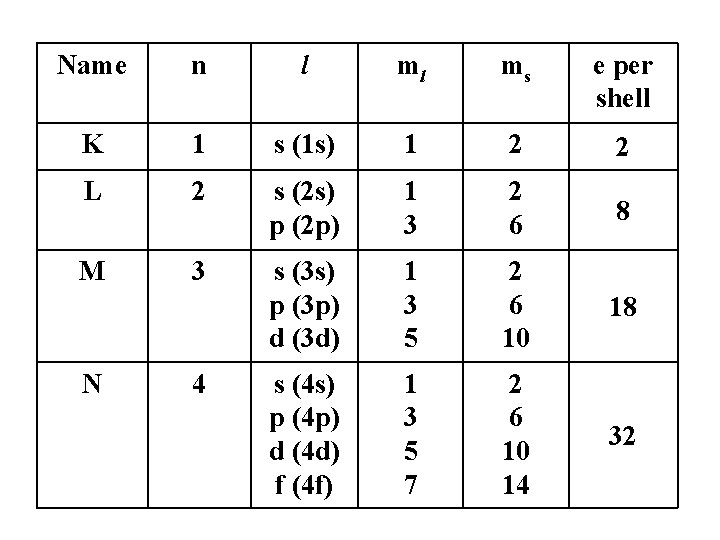
Name n l ml ms e per shell K 1 s (1 s) 1 2 2 L 2 s (2 s) p (2 p) 1 3 2 6 8 M 3 s (3 s) p (3 p) d (3 d) 1 3 5 2 6 10 18 s (4 s) p (4 p) d (4 d) f (4 f) 1 3 5 7 2 6 10 14 32 N 4
p. 24
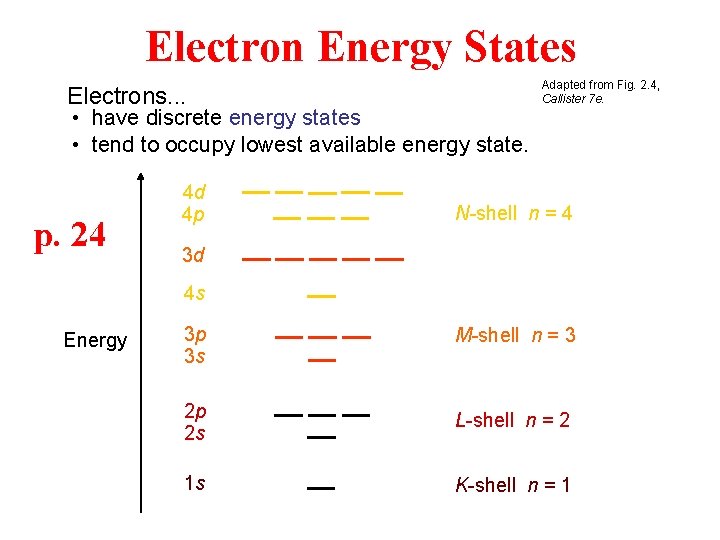
Electron Energy States Electrons. . . • have discrete energy states • tend to occupy lowest available energy state. p. 24 4 d 4 p Adapted from Fig. 2. 4, Callister 7 e. N-shell n = 4 3 d 4 s Energy 3 p 3 s M-shell n = 3 2 p 2 s L-shell n = 2 1 s K-shell n = 1
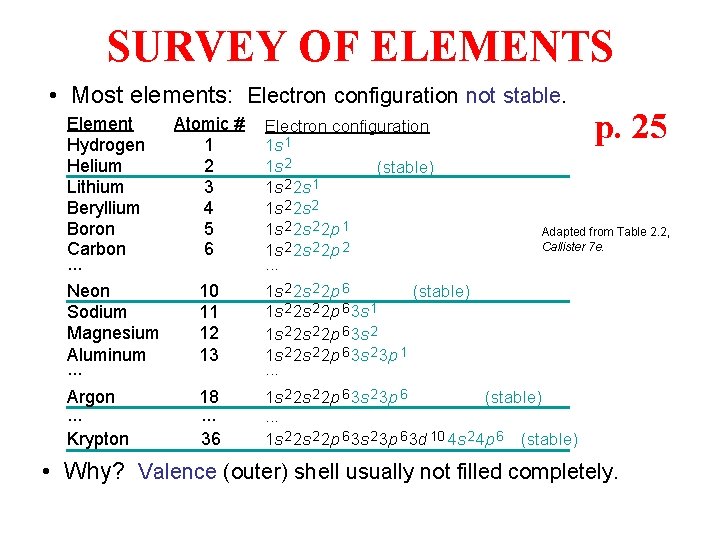
SURVEY OF ELEMENTS • Most elements: Electron configuration not stable. Element Atomic # Hydrogen 1 Helium 2 Lithium 3 Beryllium 4 Boron 5 Carbon 6. . . Neon 10 Sodium 11 Magnesium 12 Aluminum 13. . . Electron configuration 1 s 1 1 s 2 (stable) 1 s 2 2 s 1 1 s 2 2 s 2 2 p 1 1 s 2 2 p 2. . . Argon. . . Krypton 1 s 2 2 p 6 3 s 2 3 p 6 (stable). . . 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 (stable) 18. . . 36 p. 25 Adapted from Table 2. 2, Callister 7 e. 1 s 2 2 p 6 (stable) 1 s 2 2 p 6 3 s 1 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1. . . • Why? Valence (outer) shell usually not filled completely.
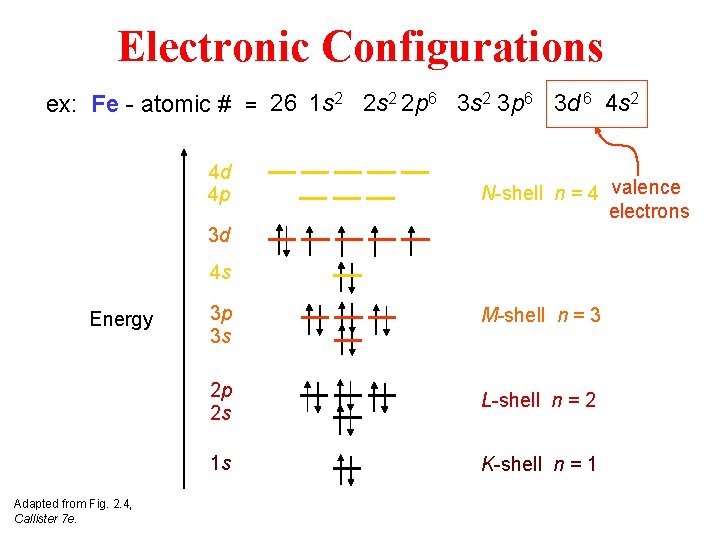
Electronic Configurations ex: Fe - atomic # = 26 1 s 2 2 p 6 3 s 2 3 p 6 3 d 6 4 s 2 4 d 4 p N-shell n = 4 valence electrons 3 d 4 s Energy Adapted from Fig. 2. 4, Callister 7 e. 3 p 3 s M-shell n = 3 2 p 2 s L-shell n = 2 1 s K-shell n = 1
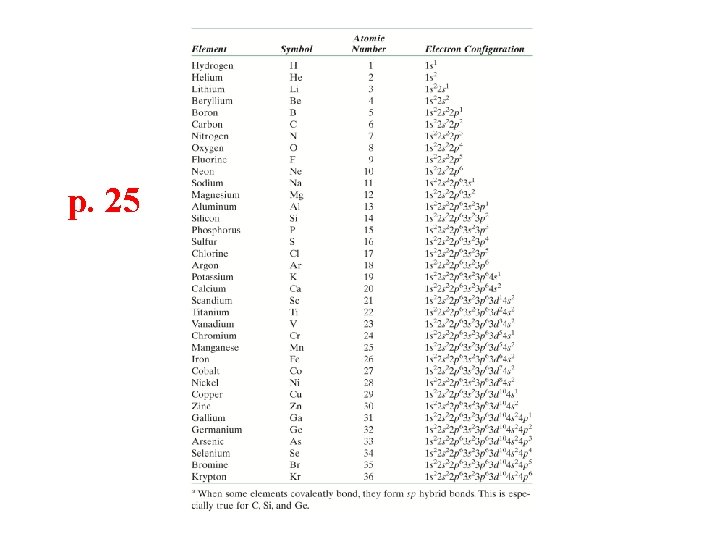
p. 25
p. 27
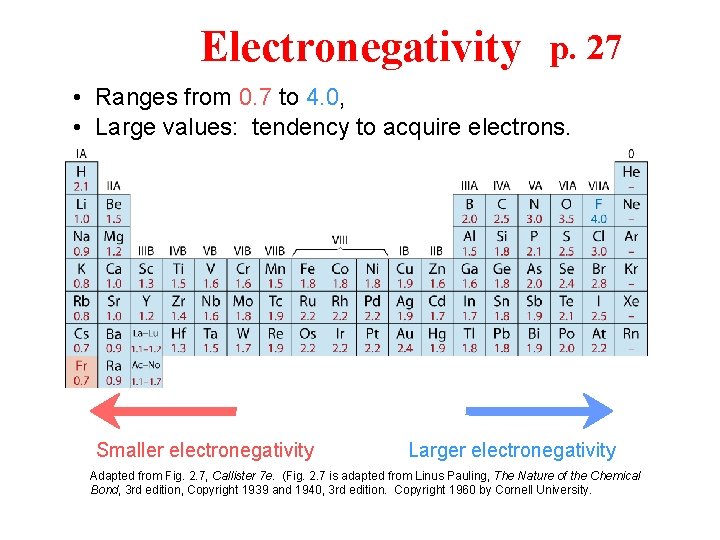
Electronegativity p. 27 • Ranges from 0. 7 to 4. 0, • Large values: tendency to acquire electrons. Smaller electronegativity Larger electronegativity Adapted from Fig. 2. 7, Callister 7 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University.
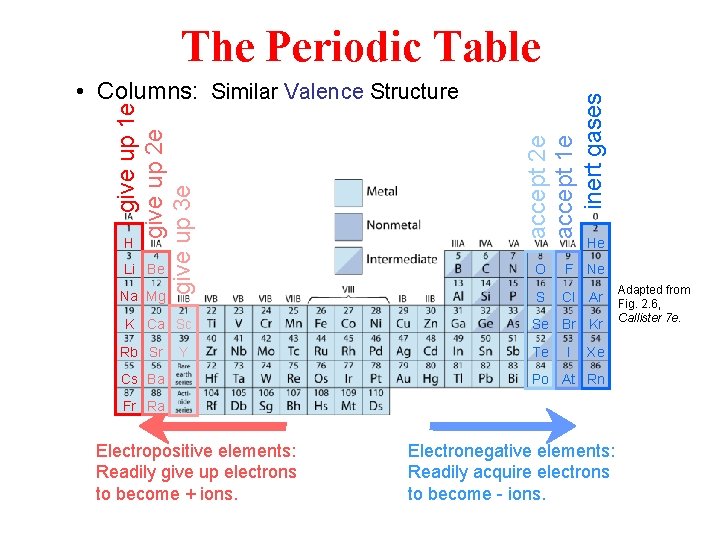
give up 1 e give up 2 e give up 3 e • Columns: Similar Valence Structure H accept 2 e accept 1 e inert gases The Periodic Table He Li Be O F Ne Na Mg S Cl Ar K Ca Sc Rb Sr Y Cs Ba Se Br Kr Te I Xe Po At Rn Fr Ra Electropositive elements: Readily give up electrons to become + ions. Electronegative elements: Readily acquire electrons to become - ions. Adapted from Fig. 2. 6, Callister 7 e.

Atomic bonding 原子鍵結 • Ionic bonding (ceramics) 離子鍵 • Covalent bonding (semiconductors, polymers) 共價鍵 • Metallic bonding (metals) 金屬鍵 • Van der Waals bonding (polymers) 凡 得瓦力 • Hydrogen bonding (polymers) 氫鍵
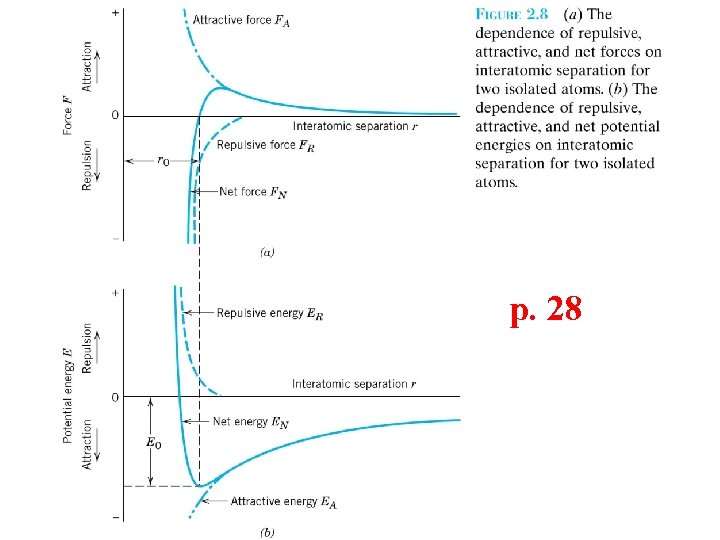
p. 28
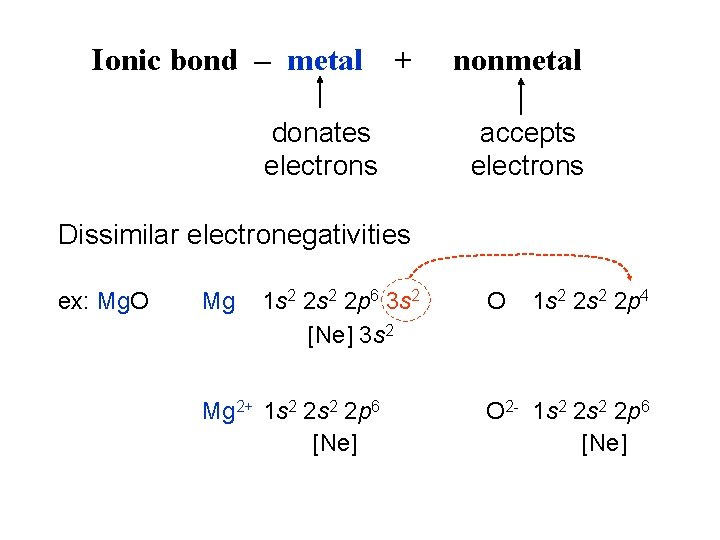
Ionic bond – metal + donates electrons nonmetal accepts electrons Dissimilar electronegativities ex: Mg. O Mg 1 s 2 2 p 6 3 s 2 [Ne] 3 s 2 Mg 2+ 1 s 2 2 p 6 [Ne] O 1 s 2 2 p 4 O 2 - 1 s 2 2 p 6 [Ne]
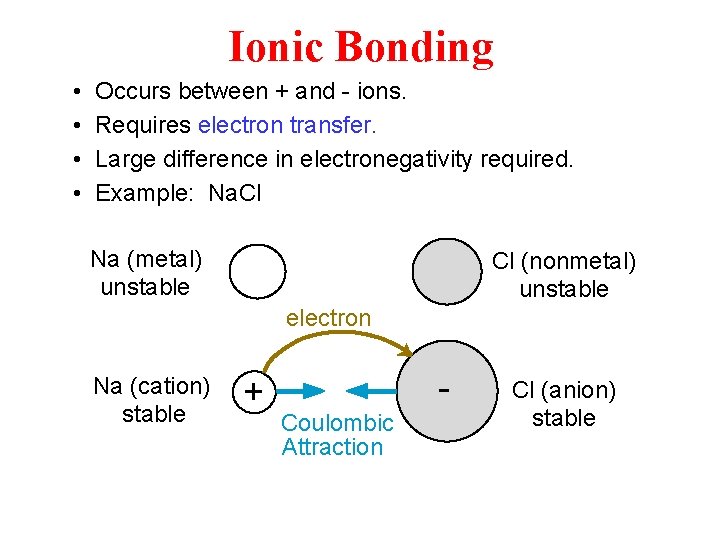
Ionic Bonding • • Occurs between + and - ions. Requires electron transfer. Large difference in electronegativity required. Example: Na. Cl Na (metal) unstable Cl (nonmetal) unstable electron Na (cation) stable + Coulombic Attraction Cl (anion) stable
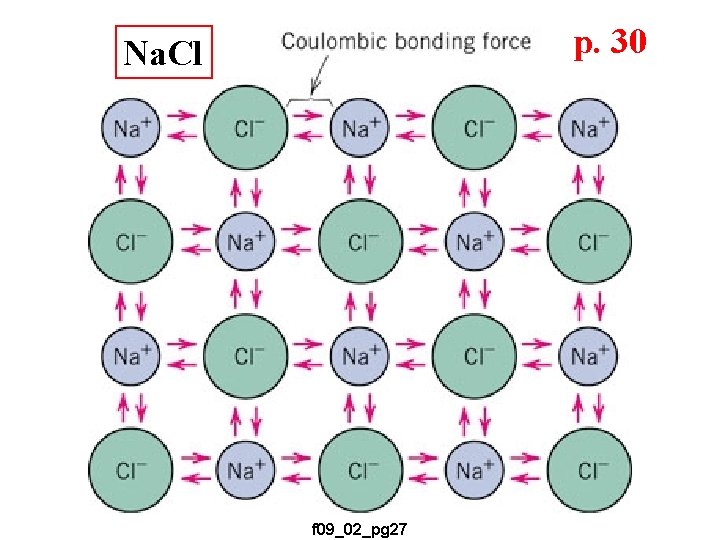
p. 30 Na. Cl f 09_02_pg 27
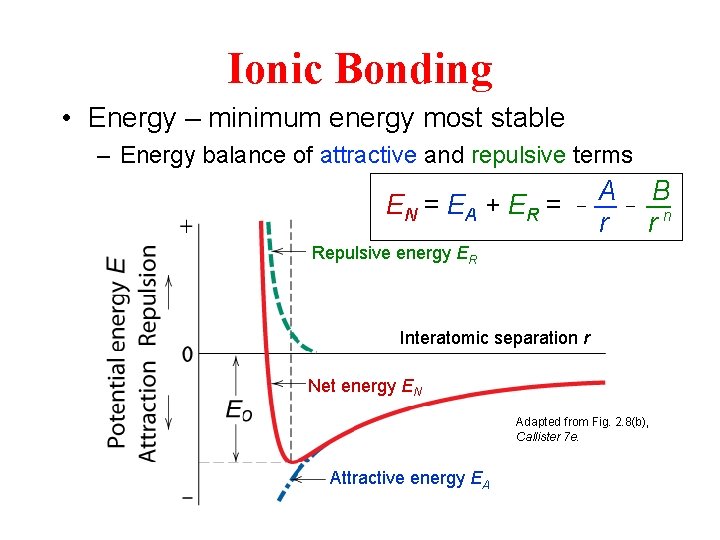
Ionic Bonding • Energy – minimum energy most stable – Energy balance of attractive and repulsive terms EN = EA + ER = - A r - B rn Repulsive energy ER Interatomic separation r Net energy EN Adapted from Fig. 2. 8(b), Callister 7 e. Attractive energy EA
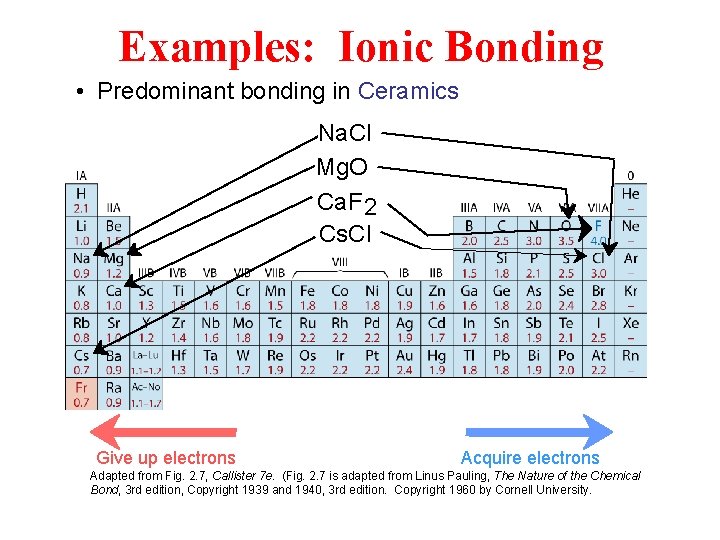
Examples: Ionic Bonding • Predominant bonding in Ceramics Na. Cl Mg. O Ca. F 2 Cs. Cl Give up electrons Acquire electrons Adapted from Fig. 2. 7, Callister 7 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University.
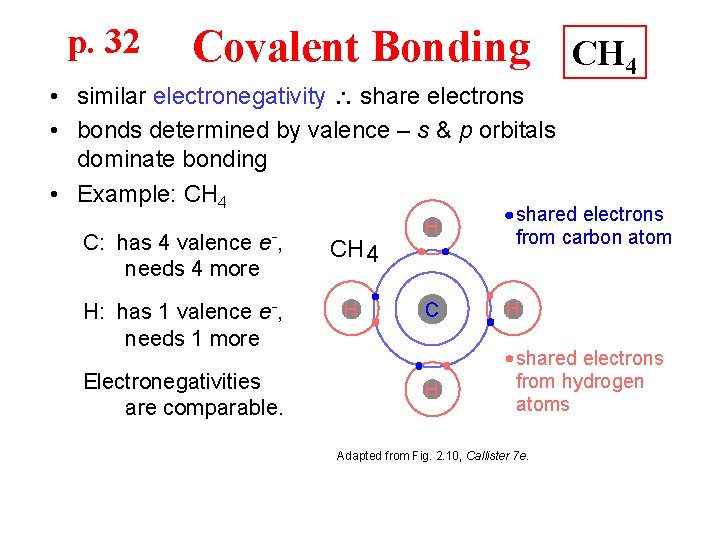
p. 32 Covalent Bonding CH 4 • similar electronegativity share electrons • bonds determined by valence – s & p orbitals dominate bonding • Example: CH 4 C: has 4 valence e-, needs 4 more CH 4 H: has 1 valence e-, needs 1 more H Electronegativities are comparable. H C H shared electrons from carbon atom H shared electrons from hydrogen atoms Adapted from Fig. 2. 10, Callister 7 e.
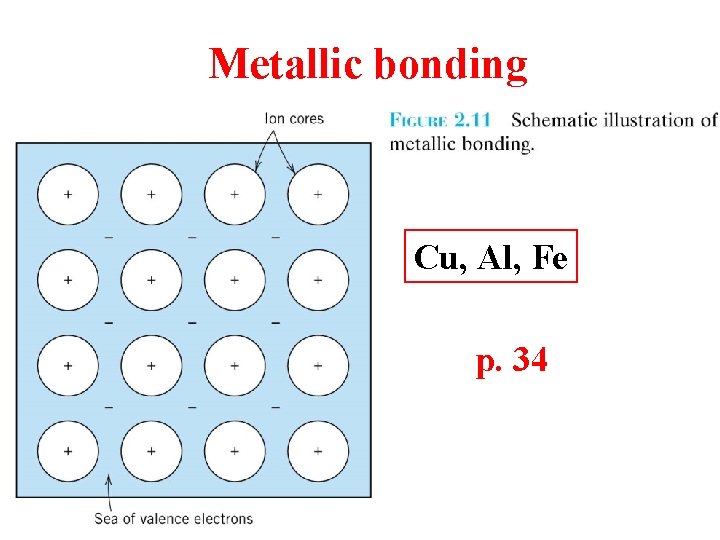
Metallic bonding Cu, Al, Fe p. 34
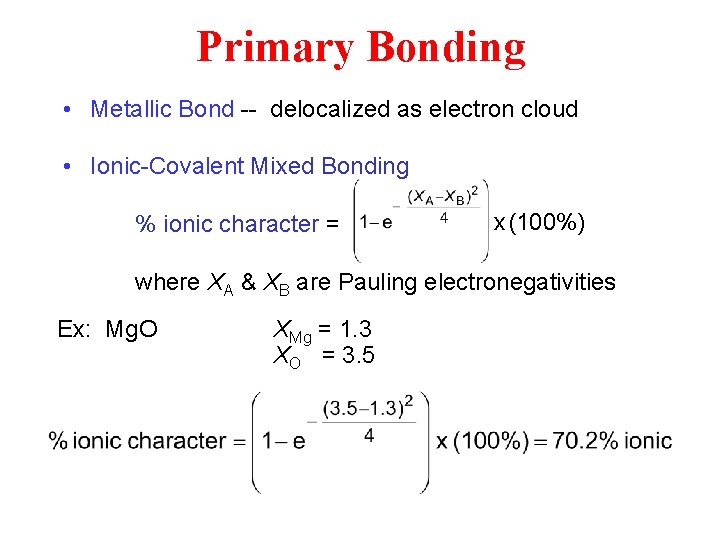
Primary Bonding • Metallic Bond -- delocalized as electron cloud • Ionic-Covalent Mixed Bonding % ionic character = x (100%) where XA & XB are Pauling electronegativities Ex: Mg. O XMg = 1. 3 XO = 3. 5
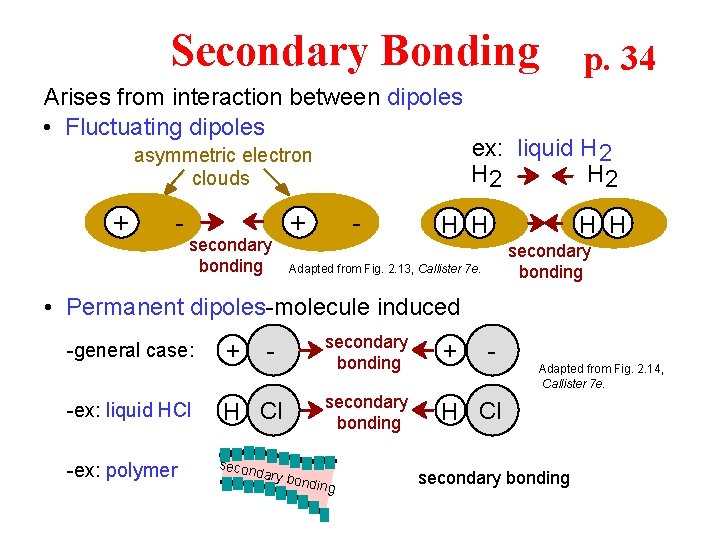
Secondary Bonding p. 34 Arises from interaction between dipoles • Fluctuating dipoles asymmetric electron clouds + - secondary bonding + - ex: liquid H 2 H 2 H H secondary bonding Adapted from Fig. 2. 13, Callister 7 e. • Permanent dipoles-molecule induced -general case: -ex: liquid HCl -ex: polymer + - H Cl secon dary b secondary bonding + secondary bonding H Cl ondin g - Adapted from Fig. 2. 14, Callister 7 e. secondary bonding
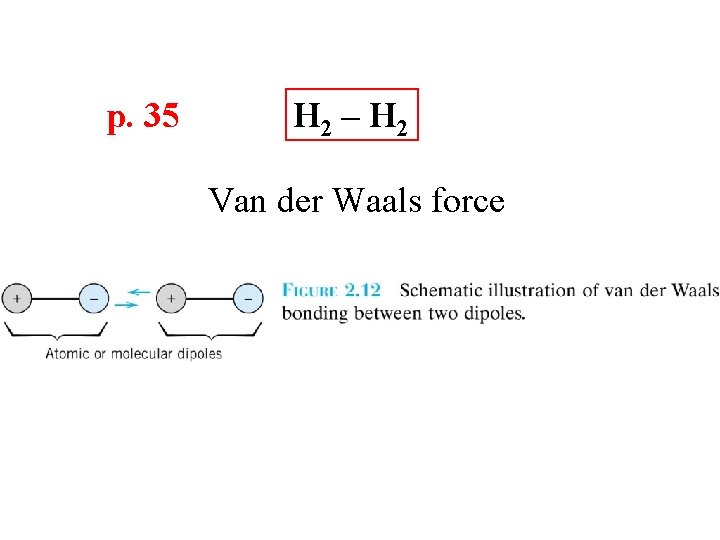
p. 35 H 2 – H 2 Van der Waals force
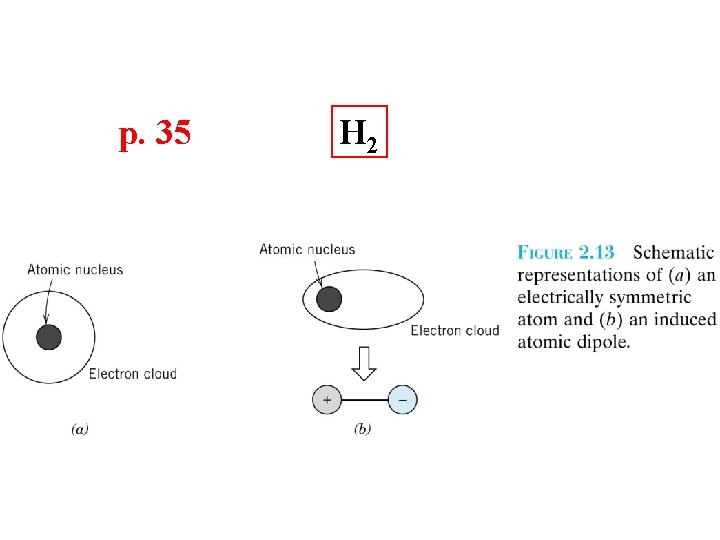
p. 35 H 2
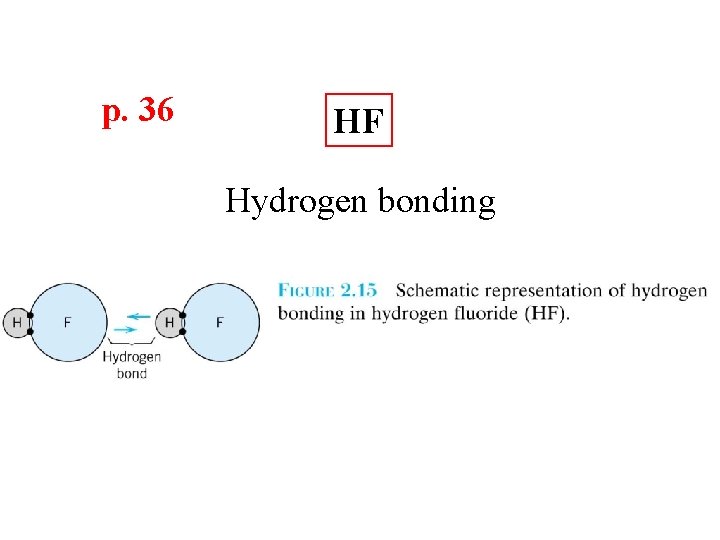
p. 36 HF Hydrogen bonding
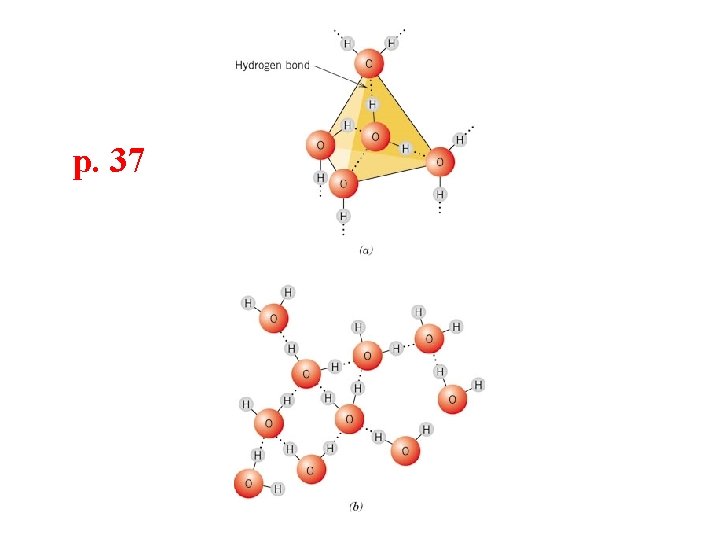
p. 37
Atom Molecule Macromolecule (Polymer)
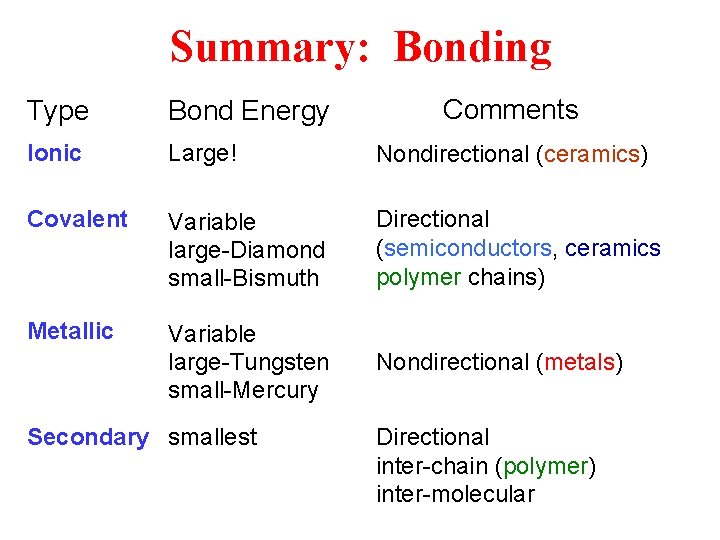
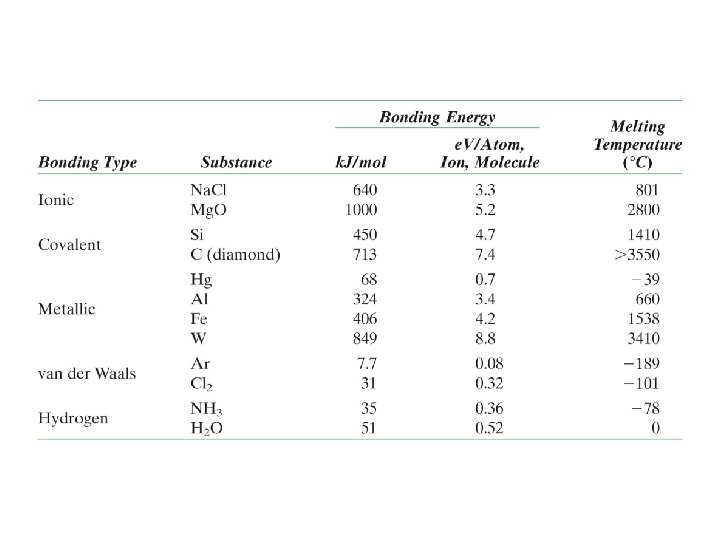
Summary: Bonding Comments Type Bond Energy Ionic Large! Nondirectional (ceramics) Covalent Variable large-Diamond small-Bismuth Directional (semiconductors, ceramics polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) Secondary smallest Directional inter-chain (polymer) inter-molecular
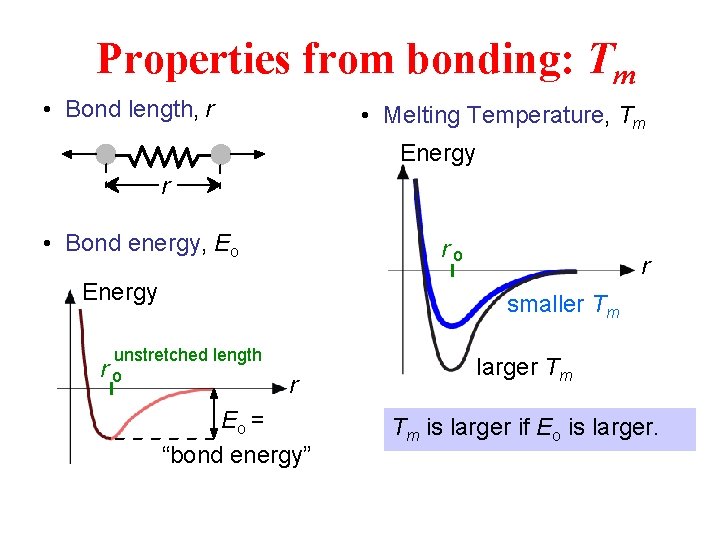
Properties from bonding: Tm • Bond length, r • Melting Temperature, Tm Energy r • Bond energy, Eo ro Energy r smaller Tm unstretched length ro r Eo = “bond energy” larger Tm Tm is larger if Eo is larger.
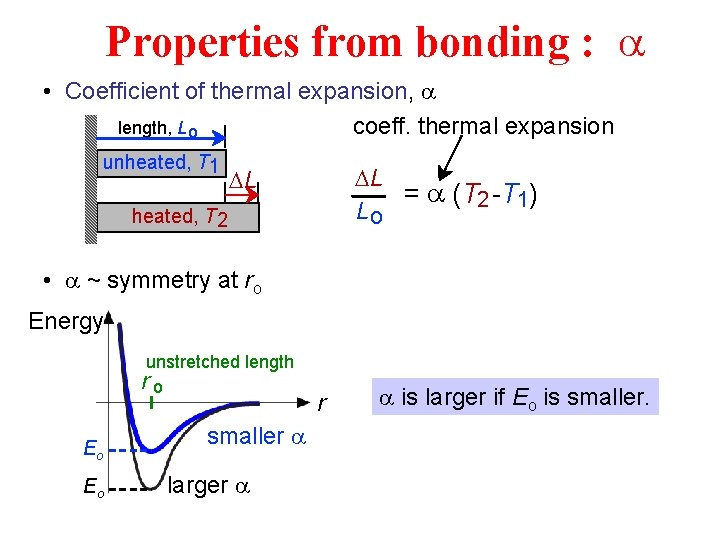
Properties from bonding : a • Coefficient of thermal expansion, a length, L o coeff. thermal expansion unheated, T 1 DL = a (T 2 -T 1) Lo DL heated, T 2 • a ~ symmetry at ro Energy unstretched length ro Eo Eo smaller a larger a is larger if Eo is smaller.
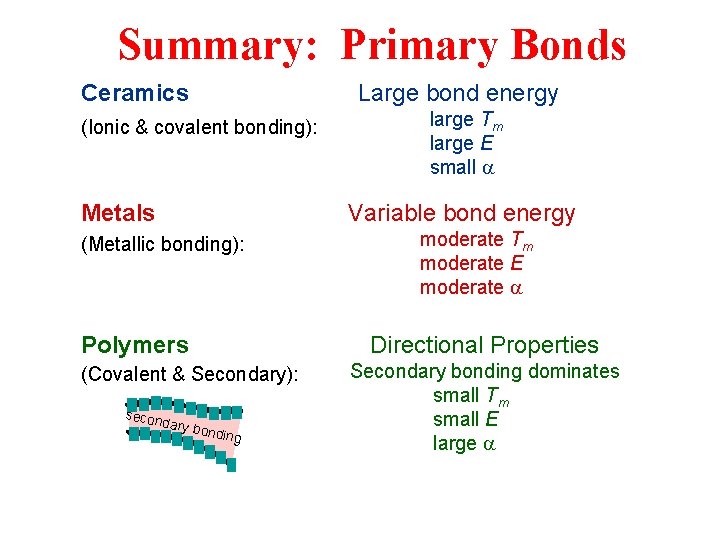
Summary: Primary Bonds Ceramics Large bond energy (Ionic & covalent bonding): Metals large Tm large E small a Variable bond energy (Metallic bonding): Polymers Directional Properties (Covalent & Secondary): secon moderate Tm moderate E moderate a dary b o nding Secondary bonding dominates small Tm small E large a
- Slides: 45