Chapter 2 Air Pollution Effects 1 Air Pollution




























- Slides: 58

Chapter 2 Air Pollution Effects 1

Air Pollution Effects OUTLINE: p 2. 1 Effects on Human Health p 2. 2 Effects on Property p 2. 3 Effects on Visibility

2. 1 Effects on Human Health Table 2. 1 in your textbook lists the air pollutants that are regulated in the United States because exposure to them is harmful to human health. Usually air pollution control efforts is devoted to the control of the pollutants on this list. p A. Criteria pollutants p B. Hazardous pollutants

Criteria Air Pollutants EPA uses six "criteria pollutants" as indicators of air quality. These pollutants are regulated by National Ambient Air Quality Standards ((NAAQS). 1. Sulfur Dioxide: SO 2 2. Nitrogen Dioxide: NO 2 3. Carbon monoxide: CO 4. Lead: Pb 5. Particulate Matter: PM 10 (PM 2. 5) 6. Ozone: ground level O 3

Hazardous Air Pollutants regulated by National Emission Standards for Hazardous Air Pollutants (NESHAP). § Asbestos § Benzene § Beryllium § Coke oven emissions § Inorganic arsenic § Mercury § Radionuclides § Vinyl chloride

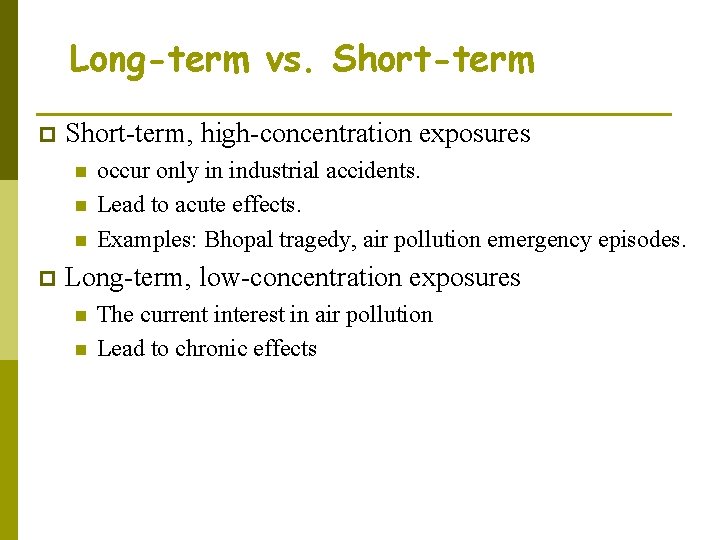
Long-term vs. Short-term p Short-term, high-concentration exposures n n n p occur only in industrial accidents. Lead to acute effects. Examples: Bhopal tragedy, air pollution emergency episodes. Long-term, low-concentration exposures n n The current interest in air pollution Lead to chronic effects

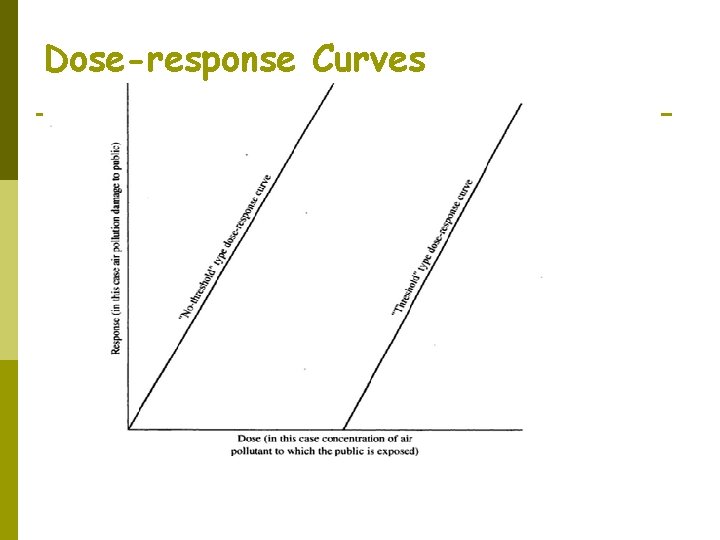
How do we know the harmful dose? p Dosage = ∫(concentration in air breathed). d(time) n n p High concentration in a small time may lead to acute effects Low concentration in a large time may lead to chronic effects To determine what dosage is harmful, a dose-response curve is needed. n n n Such a curve can be plotted only for individual pollutants, not for “air pollution in general”. Figure 2. 1 No-threshold type curve vs. threshold type curve.

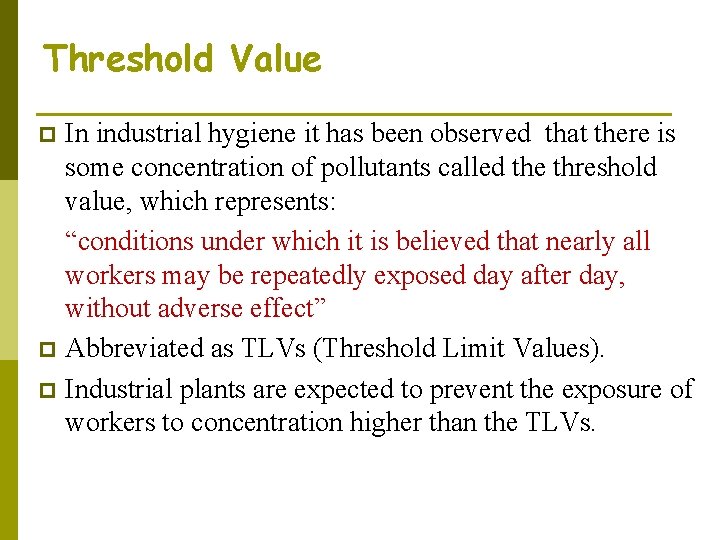
Threshold Value In industrial hygiene it has been observed that there is some concentration of pollutants called the threshold value, which represents: “conditions under which it is believed that nearly all workers may be repeatedly exposed day after day, without adverse effect” p Abbreviated as TLVs (Threshold Limit Values). p Industrial plants are expected to prevent the exposure of workers to concentration higher than the TLVs. p

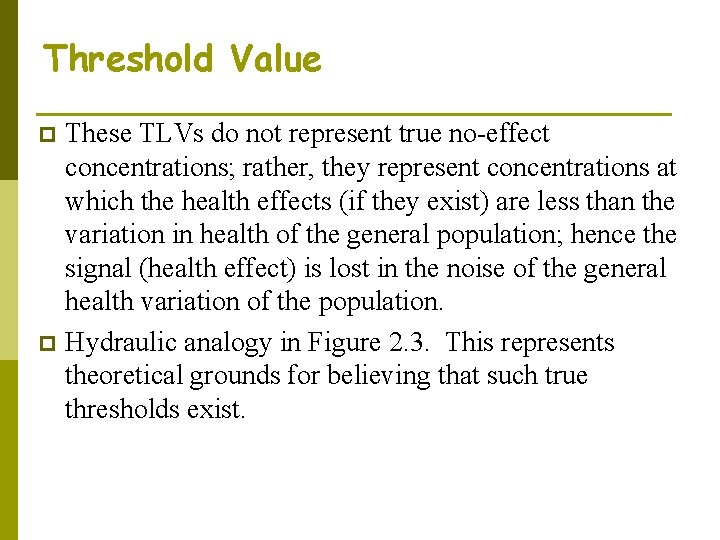
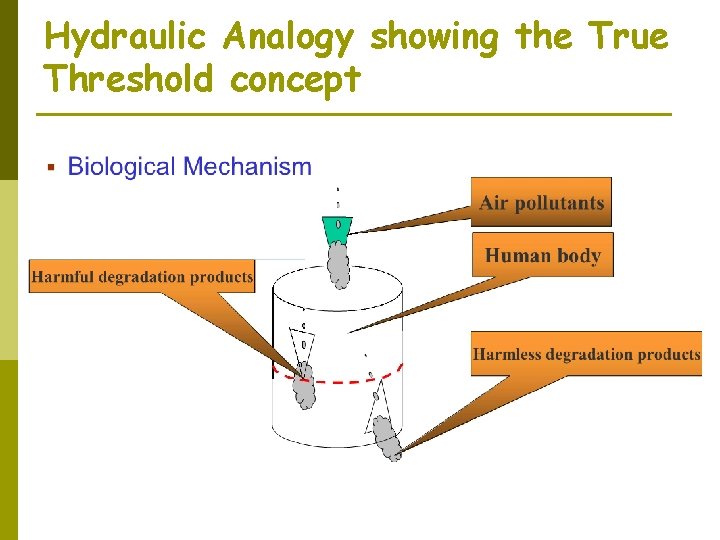
Threshold Value These TLVs do not represent true no-effect concentrations; rather, they represent concentrations at which the health effects (if they exist) are less than the variation in health of the general population; hence the signal (health effect) is lost in the noise of the general health variation of the population. p Hydraulic analogy in Figure 2. 3. This represents theoretical grounds for believing that such true thresholds exist. p

Dose-response Curves

Hydraulic Analogy showing the True Threshold concept

How to establish the dose-response curve for a pollutant? 1) 2) 3) Animal experiments Laboratory experiments with humans. Epidemiological studies of human populations.


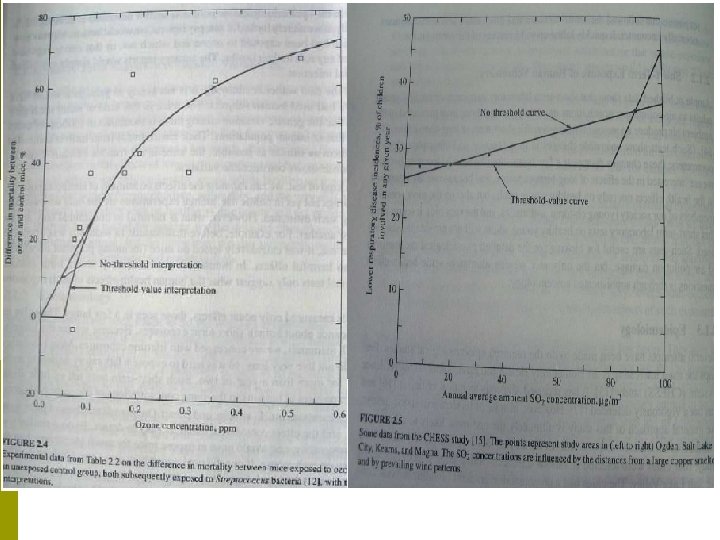
Description of the mice experiment in Figure 2. 4 p Exposing two groups of mice to aerosol containing Streptococcus C bacteria. n Control group vs. ozone group. The ozone group had previously been exposed for three hours to various concentrations of ozone, while the control group had not been exposed to ozone p The difference in mortality is plotted against the ozone concentration p

Lessons learnt from the mice experiment in Figure 2. 4 Ozone exposure produces a significant effect on mortality at concentrations above about 0. 1 ppm, the effect increases with increasing ozone concentration. p The air pollution effect was indirect. p n p No mice died as a result of ozone exposure alone. Rather, the ozone, which is a respiratory irritant, presumable irritated the lungs of the exposed mice, making it easier for lethal numbers of bacteria to enter the bloodstream. If we didn’t know the history of the test, we could conclude that exposure to high concentrations of ozone lead to increased mortality.

…Lessons learnt from the mice experiment in Figure 2. 4 These tests measured only acute effects. i. e. they give us some guidance about human short-term exposure. p However, we are actually concerned with lifetime exposures since humans are one of the longest-lived of all mammals. It is hard to expose a laboratory animal to some pollutant for more than a year. p

…Lessons learnt from the mice experiment in Figure 2. 4 Threshold vs. no-threshold interpretation At High Concentrations The effect is clear At Low Concentrations The uncertainty and scatter introduced by the variability make it impossible to determine the true shape of the curve. If one wished to settle completely the threshold or nothreshold question for one substance suspected of being a carcinogen using mice as the experimental animal, then an experimental program involving at least a million mice would be needed. p The concentration at which significant effects are seen is near the currently permitted value (NAAQS) of 0. 08 ppm in the United States. p

Regulations to Protect Human Health Difficult to obtain unambiguous dose-response curves. p Therefore, there is controversy over how clean people want the air to be. p The U. S. Environmental Protection Agency (U. S. EPA), acting under the Clean Air Act, concluded that: p n n p the six criteria pollutants have thresholds. The last eight do not have demonstrable thresholds. The Clean Air Act requires the EPA to establish National Ambient Air Quality Standards (NAAQS); i. e. maximum allowable levels of contamination in order to protect the public health with an adequate margin of safety.

…Regulations to Protect Human Health p For pollutants for which there does not appear to be a demonstrable safe threshold value, such a standard cannot be set. n n Instead, the Clean Air Act regulates the eight no-threshold pollutants via the National Emission Standard for Hazardous Air Pollutants (NESHAP) regulations. Congress listed 189 chemicals as hazardous air pollutants.

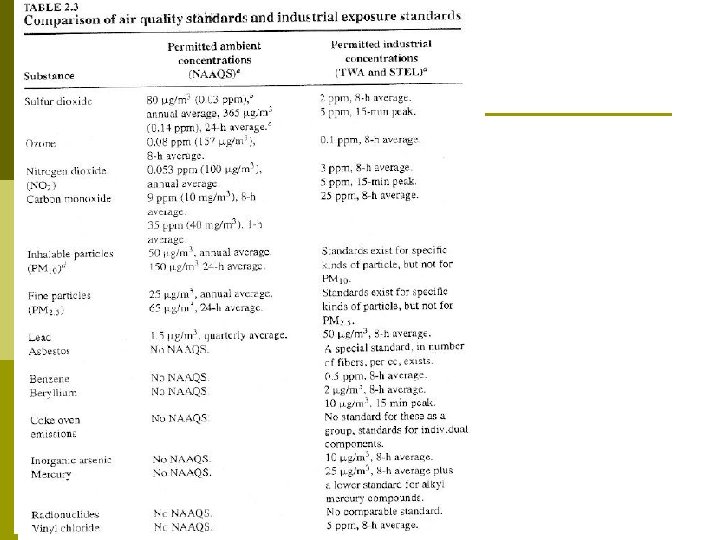
Permitted Industrial Concentrations vs. Permitted Ambient Air Concentrations Table 2. 3 p The permitted industrial concentrations are generally much higher than the permitted ambient air concentrations. This difference reflects two facts: p 1. 2. We are exposed to ambient air 168 hours a week but are on the job only 40 hours a week. The working population does not contain the most susceptible members of the population (infants, asthmatics, and very old people). i. e. industrial standards are not intended to protect everyone.



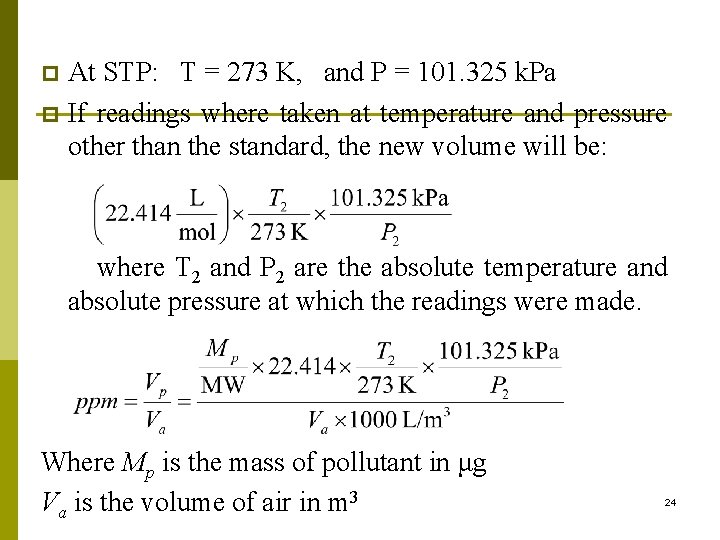
Conversion from ppm to μg/m 3 Can you express the 1130 ppm in μg/m 3: n The conversion is based on two things: ppm is a volume to volume ratio (while μg/m 3 is a wt to vol ratio) p One mole of ideal gas occupies 22. 414 L p p Therefore we need to write an equation that relates the mass of the pollutant to its equivalent volume Vp at standard temperature and pressure (STP). p If readings were made at temperatures and pressures other than standard conditions, then corrections must be applied to the 22. 414 L/mol since one mole of gas will no longer occupy the 22. 414 L volume. 23

At STP: T = 273 K, and P = 101. 325 k. Pa p If readings where taken at temperature and pressure other than the standard, the new volume will be: p where T 2 and P 2 are the absolute temperature and absolute pressure at which the readings were made. Where Mp is the mass of pollutant in μg Va is the volume of air in m 3 24

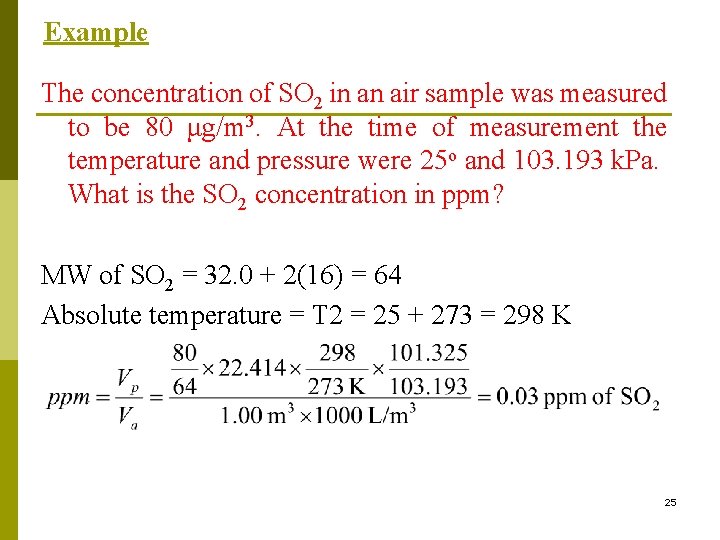
Example The concentration of SO 2 in an air sample was measured to be 80 μg/m 3. At the time of measurement the temperature and pressure were 25 o and 103. 193 k. Pa. What is the SO 2 concentration in ppm? MW of SO 2 = 32. 0 + 2(16) = 64 Absolute temperature = T 2 = 25 + 273 = 298 K 25

2. 2 Effects on Property Costs of property damages are substantial. However, our concern with them is not comparable to our concern with human health. p 50 years ago, a great deal of attention was paid to air pollution damage to property. Today a little attention is paid. p Why? p n At that time pollutants caused visible damage to plants and animals. Emitters were sued and strict controls were imposed on emitters to protect human health.

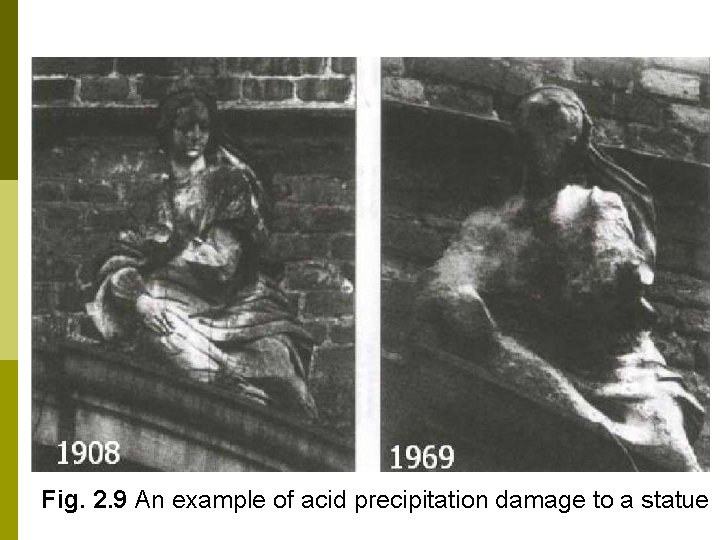
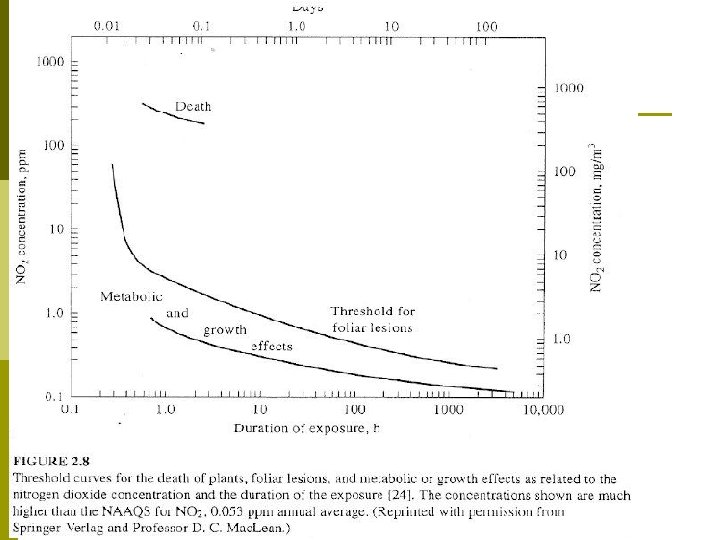
… Effects on Property Metals corrode faster p Paints do not last as long p Tires and other rubber goods fail due to ozone cracking p Some green plants are harmed (Fig. 2. 8) p Damage to historical monuments (Fig. 2. 9) p

Damage to historical monuments Acid precipitation is damaging the sandstone and marble statues and monuments of Europe and the US. p Such damage is not easily replaced p Statues at the Parthenon have been moved into an airconditioned museum; fiberglass and epoxy replicas now stand outdoor in their place. p

Fig. 2. 9 An example of acid precipitation damage to a statue


Example on the Effect of human health regulations

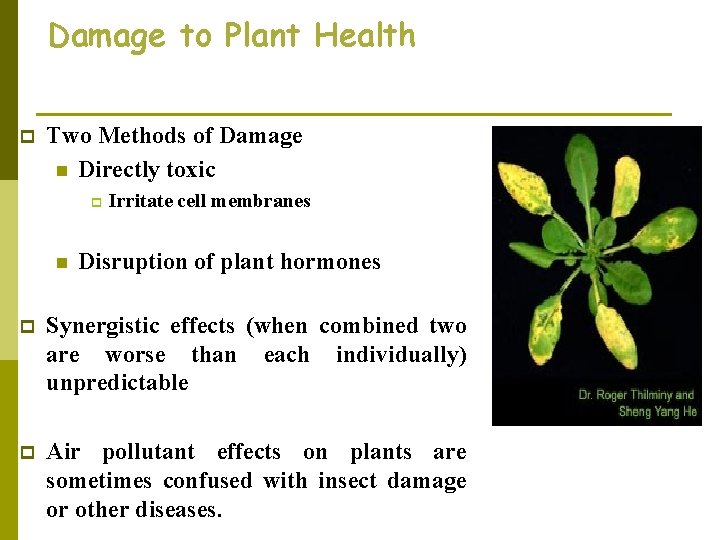
Damage to Plant Health p Two Methods of Damage n Directly toxic p n Irritate cell membranes Disruption of plant hormones p Synergistic effects (when combined two are worse than each individually) unpredictable p Air pollutant effects on plants are sometimes confused with insect damage or other diseases.

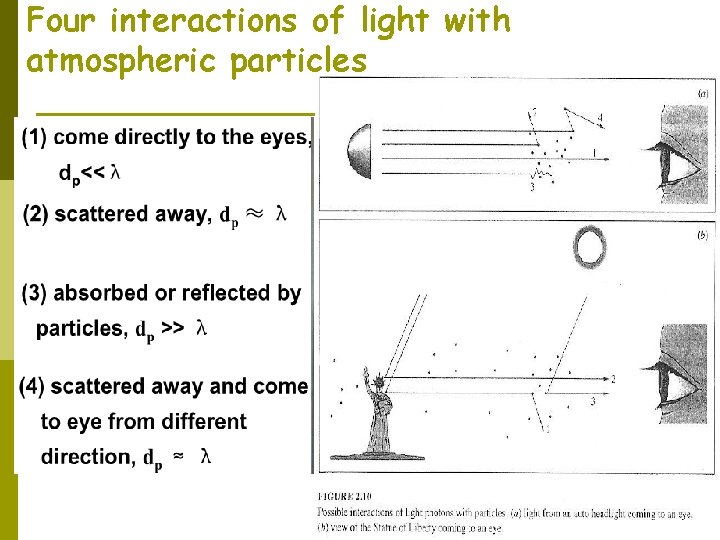
3. Effects on Visibility When we speak of air being hazy, we normally mean that it contains particles that scatter sunlight (or moonlight or streetlight) toward us, which prevents us from seeing distant scenes clearly. Most visible effects of air pollution are caused by the interaction of light with suspended particles. Fig. 2. 10 shows the possible interactions of a light photon with atmospheric particles. Most gaseous air pollutants are totally transparent. The only common exception is NO 2, which is brown. Some urban smogs appear brown because of the NO 2 they contain.

Four interactions of light with atmospheric particles

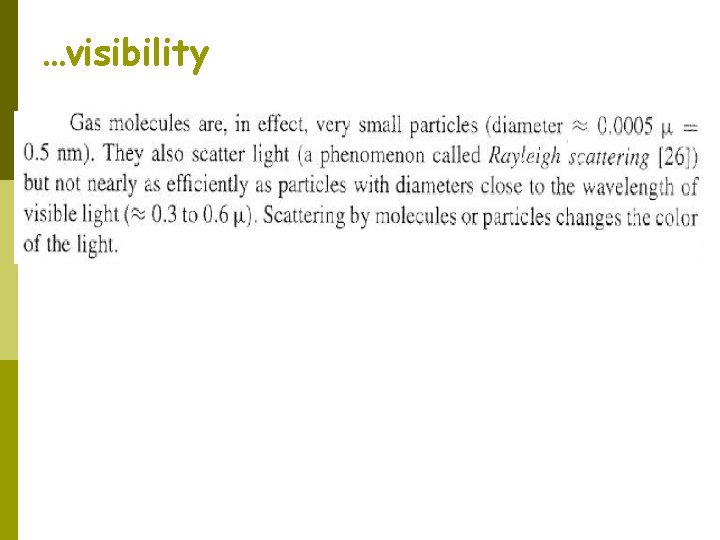
…visibility

…visibility – Figure 2. 10 b

…visibility

…visibility

…visibility

…visibility

Applications Why are bright white clouds unlikely to produce rain? Why does the sky appear look blue when we look away from the sun? Why is visibility normally much better in dry climate than in moist one?

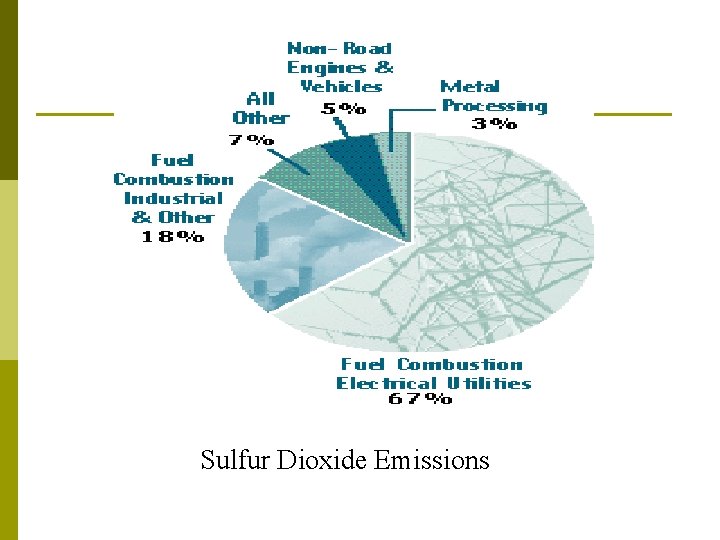
Sulfur Dioxide (SO 2) Effects: produces acid rain (H 2 SO 4), breathing difficulties, eutrophication due to sulfate formation. Sources: burning high sulfur coal or oil in power plants, smelting or metals, paper manufacture. EPA Standard: 0. 3 ppm (annual mean) 2 nd largest cause of air pollution-related health damage. (1 st is smoking). Sulfate particles reduce visibility in the U. S. as much as 80%

Sulfur Dioxide Emissions

Nitrogen Dioxide (NO 2) p Effects: acid rain, lung and heart problems, decreased visibility (yellow haze), suppresses plant growth p Sources: fossil fuels combustion, power plants, forest fires, volcanoes, bacteria in soil, fertilizers p EPA Standard: 0. 053 ppm p Excess nitrogen is causing fertilization & eutrophication of inland waters & seas

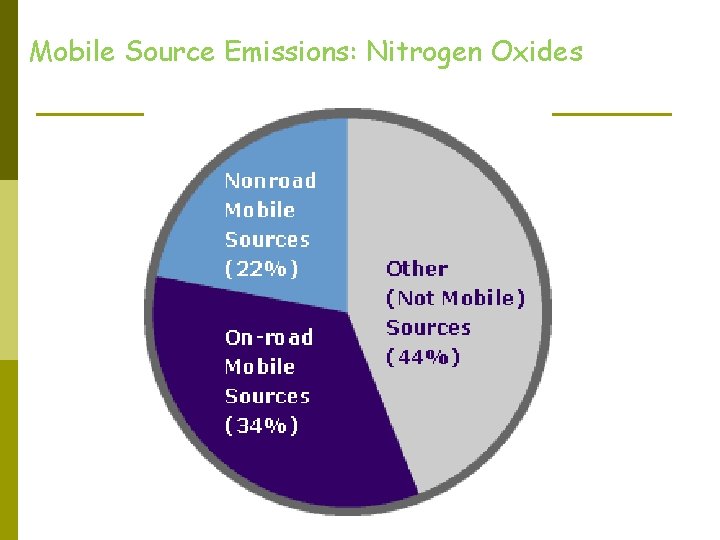
Mobile Source Emissions: Nitrogen Oxides

Carbon Monoxide (CO) p Effects : binds tighter to Hemoglobin (Hb) than O 2, so organs do not get O 2 needed, makes you sleepy, weakens mental functions and visual acuity, even at low levels p Sources: incomplete combustion of fossil fuels 60 - 95% from auto exhaust p EPA Standard: 9 ppm p 1 billion tons enter atmosphere/year

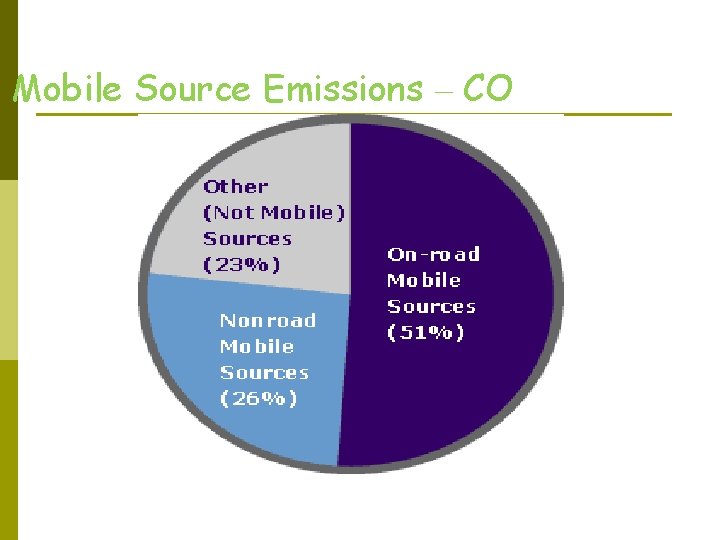
Mobile Source Emissions – CO

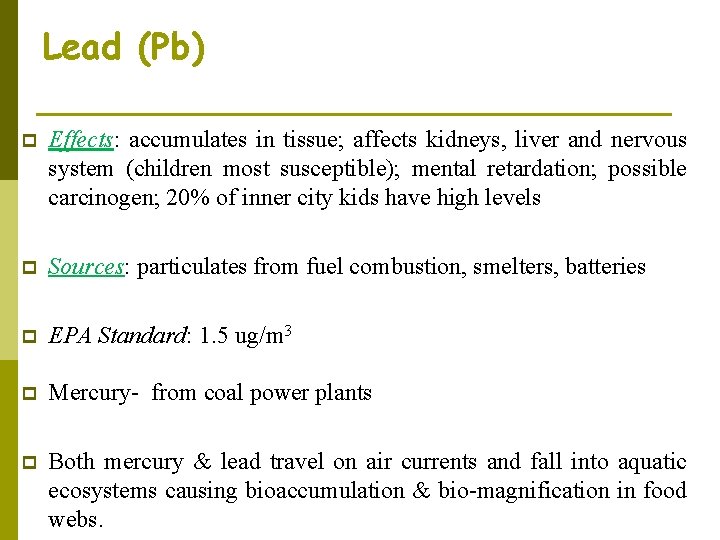
Lead (Pb) p Effects: accumulates in tissue; affects kidneys, liver and nervous system (children most susceptible); mental retardation; possible carcinogen; 20% of inner city kids have high levels p Sources: particulates from fuel combustion, smelters, batteries p EPA Standard: 1. 5 ug/m 3 p Mercury- from coal power plants p Both mercury & lead travel on air currents and fall into aquatic ecosystems causing bioaccumulation & bio-magnification in food webs.

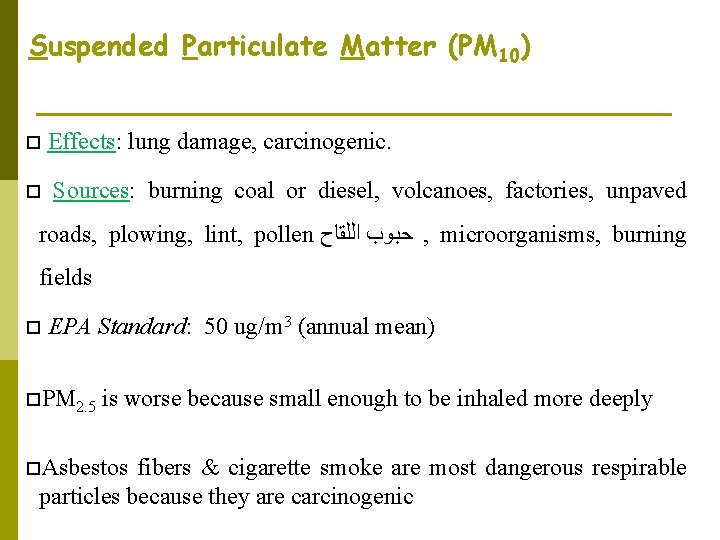
Suspended Particulate Matter (PM 10) Effects: lung damage, carcinogenic. Sources: burning coal or diesel, volcanoes, factories, unpaved roads, plowing, lint, pollen ﺣﺒﻮﺏ ﺍﻟﻠﻘﺎﺡ , microorganisms, burning fields EPA Standard: 50 ug/m 3 (annual mean) PM 2. 5 is worse because small enough to be inhaled more deeply Asbestos fibers & cigarette smoke are most dangerous respirable particles because they are carcinogenic

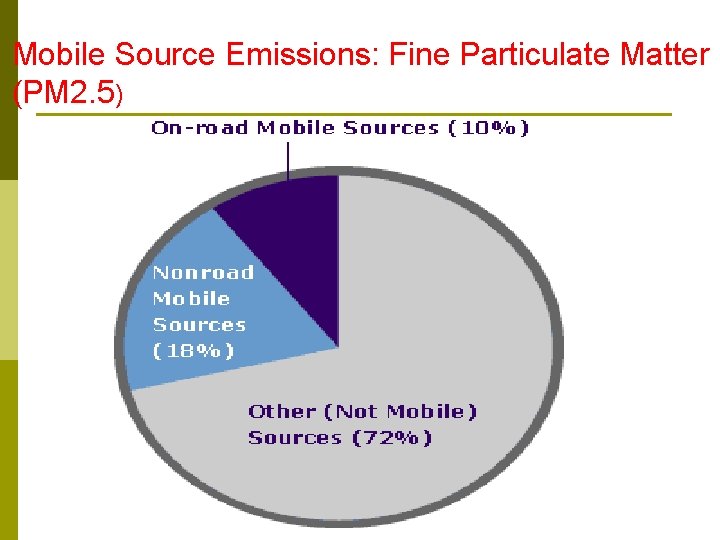
Mobile Source Emissions: Fine Particulate Matter (PM 2. 5)

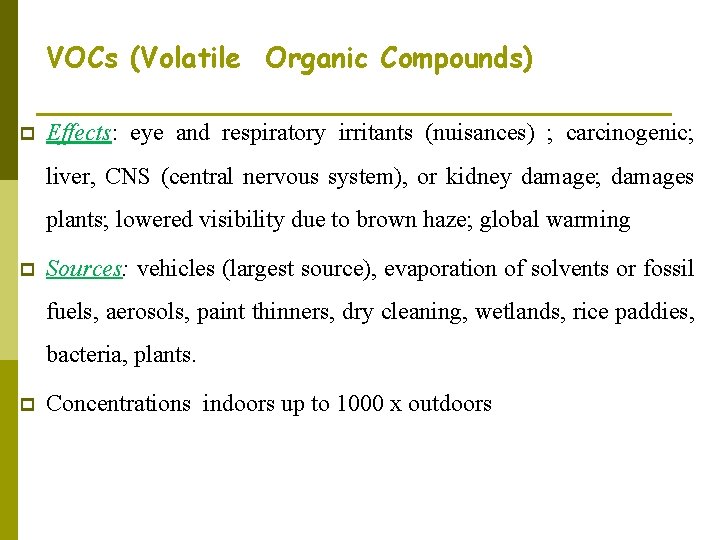
VOCs (Volatile Organic Compounds) p Effects: eye and respiratory irritants (nuisances) ; carcinogenic; liver, CNS (central nervous system), or kidney damage; damages plants; lowered visibility due to brown haze; global warming p Sources: vehicles (largest source), evaporation of solvents or fossil fuels, aerosols, paint thinners, dry cleaning, wetlands, rice paddies, bacteria, plants. p Concentrations indoors up to 1000 x outdoors

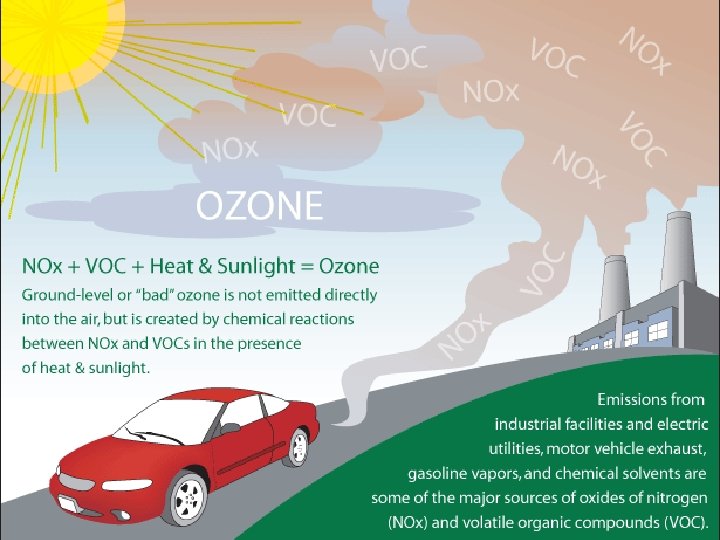
Ozone (O 3) p Effects: lung irritant, damages plants, rubber, fabric, eyes p Sources: Created by sunlight acting on NOx and VOC , photocopiers, cars, industry, gas vapors, chemical solvents, incomplete fuel combustion products p Good ozone vs. bad ozone- good is in stratosphere and bad is at ground level (from cars)


Other Air Pollutants Carbon dioxide- natural source from breathing; human caused from fossil fuels & deforestation Chloro. Fluoro. Carbons (CFC’s)- from refrigerants, Styrofoam (a ( kind of expanded polystyrene) Formaldehyde- building materials & household products Benzene- paint Asbestos- car brakes, building materials Dioxins- pesticides Cadmium- batteries. aerosols,

Formation & Intensity of Pollutant is influenced by… Local climate (inversions, air pressure, temperature, humidity) Topography (hills and mountains) Population density Amount of industry Fuels used by population and industry for heating, manufacturing, transportation, power Weather: rain, snow, wind Buildings (slow wind speed)

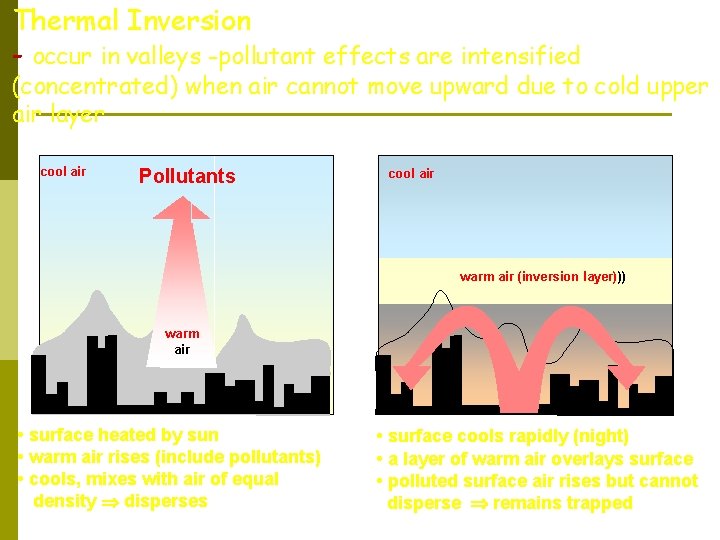
Thermal Inversion - occur in valleys -pollutant effects are intensified (concentrated) when air cannot move upward due to cold upper air layer cool air Pollutants cool air warm air (inversion layer))) warm air • surface heated by sun • warm air rises (include pollutants) • cools, mixes with air of equal density disperses • surface cools rapidly (night) • a layer of warm air overlays surface • polluted surface air rises but cannot disperse remains trapped

Smog Forms (report). . . when polluted air is stagnant (inactive) (weather conditions, geographic location)

Urban Heat Islands (report) Cities are generally 3 -5ºC warmer than rural areas Caused by: Lack of vegetation to absorb heat Dark buildings & roads trap heat Buildings create windbreaks Dust Dome- trapping of dirt & particulates over city (example? )