Chapter 2 A Measurements Calculations West Valley High






- Slides: 18

Chapter 2 A: Measurements & Calculations West Valley High School General Chemistry Mr. Mata Cartoon courtesy of Nearing. Zero. net

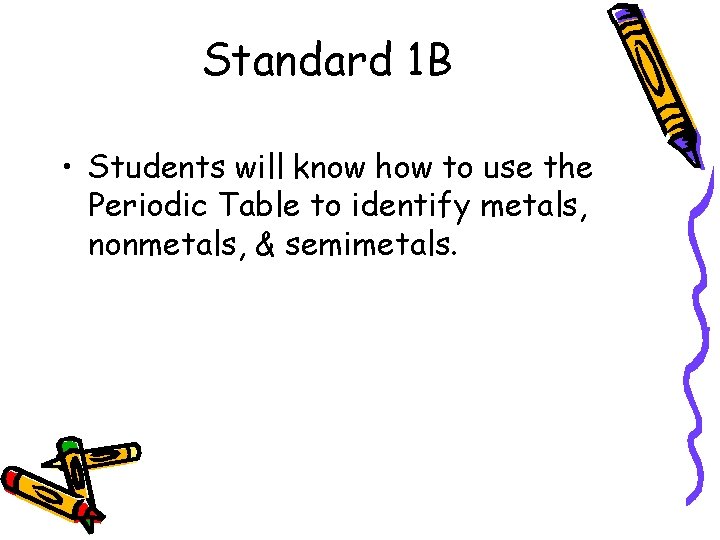
Standard 1 B • Students will know how to use the Periodic Table to identify metals, nonmetals, & semimetals.

Essential Question • How are measurements and units used in chemistry?

Section 2 -1 Steps in the Scientific Method 1. Observations quantitative (numbers) ex: 25 g qualitative (descriptive) ex: red 2. Formulating hypotheses - possible explanation for observation. 3. Performing experiments - gathering new information to decide whether the hypothesis is valid.

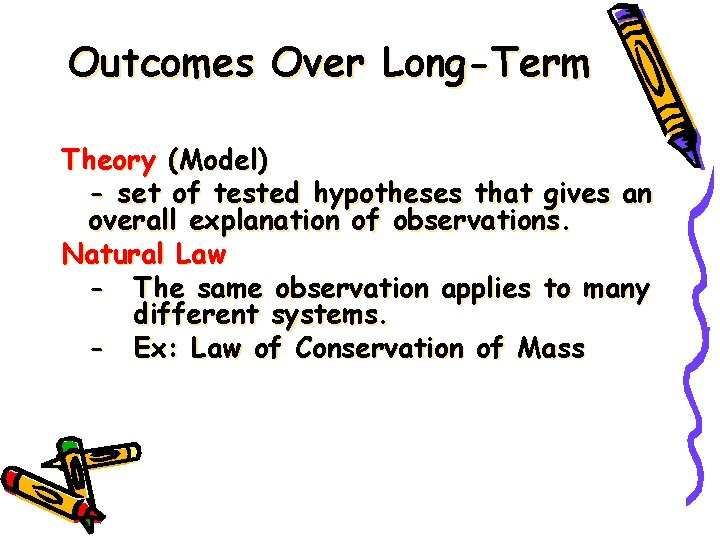
Outcomes Over Long-Term Theory (Model) - set of tested hypotheses that gives an overall explanation of observations. Natural Law - The same observation applies to many different systems. - Ex: Law of Conservation of Mass

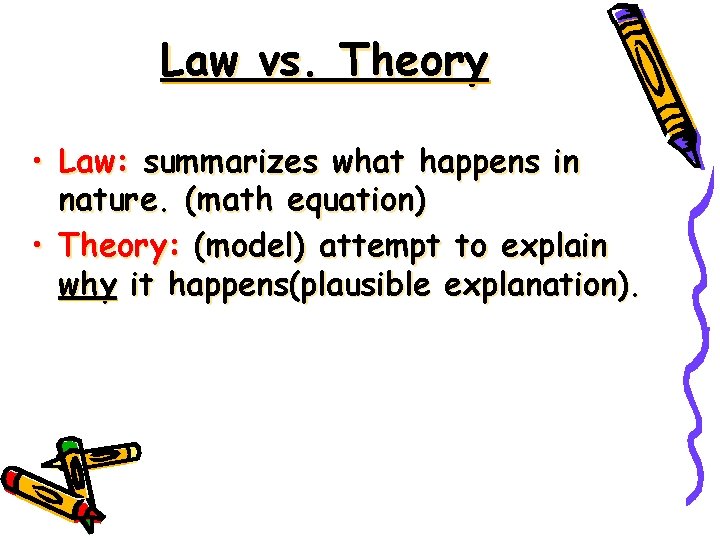
Law vs. Theory • Law: summarizes what happens in nature. (math equation) • Theory: (model) attempt to explain why it happens(plausible explanation).

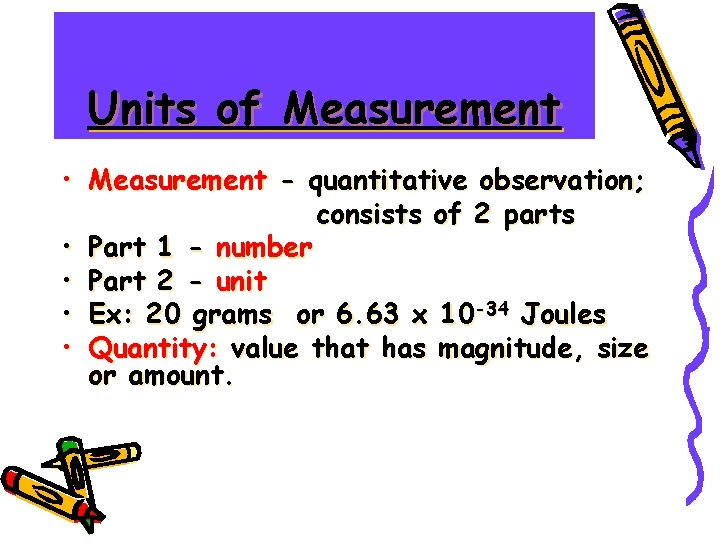
Units of Measurement • Measurement - quantitative observation; consists of 2 parts • Part 1 - number • Part 2 - unit • Ex: 20 grams or 6. 63 x 10 -34 Joules • Quantity: value that has magnitude, size or amount.

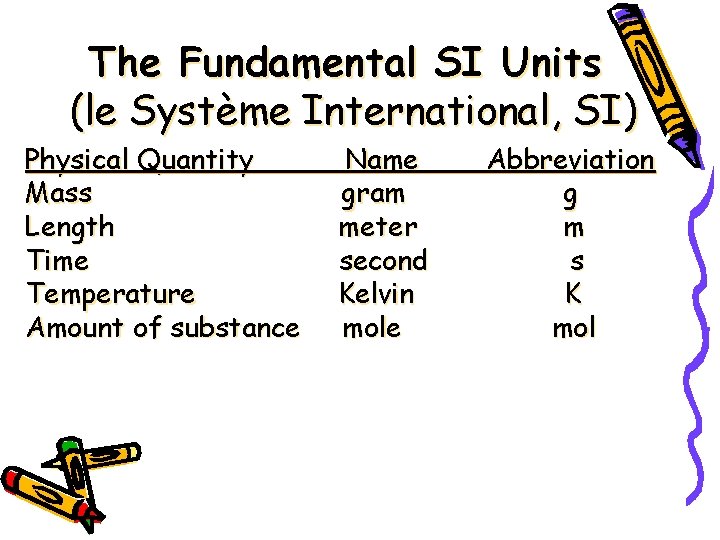
The Fundamental SI Units (le Système International, SI) Physical Quantity Mass Length Time Temperature Amount of substance Name gram meter second Kelvin mole Abbreviation g m s K mol

Derived SI units • Derived unit - obtained from combinations of fundamental units. Ex: volume, density. • Volume - amount of space occupied by an object; units in m. L or cm 3 • Density= mass/Volume • Density: quantity of matter per unit volume; g/m. L or g/cm 3

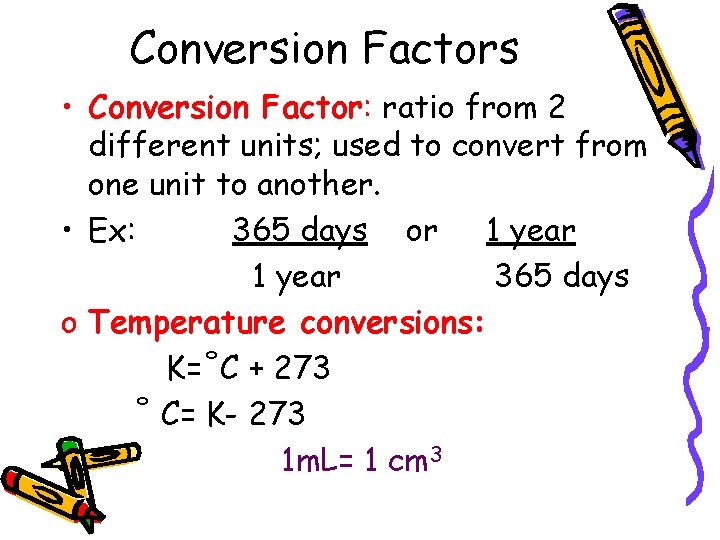
Conversion Factors • Conversion Factor: ratio from 2 different units; used to convert from one unit to another. • Ex: 365 days or 1 year 365 days o Temperature conversions: K=˚C + 273 ˚ C= K- 273 1 m. L= 1 cm 3

Precision and Accuracy • Accuracy: closeness to true value. • Precision: agreement in several trials.

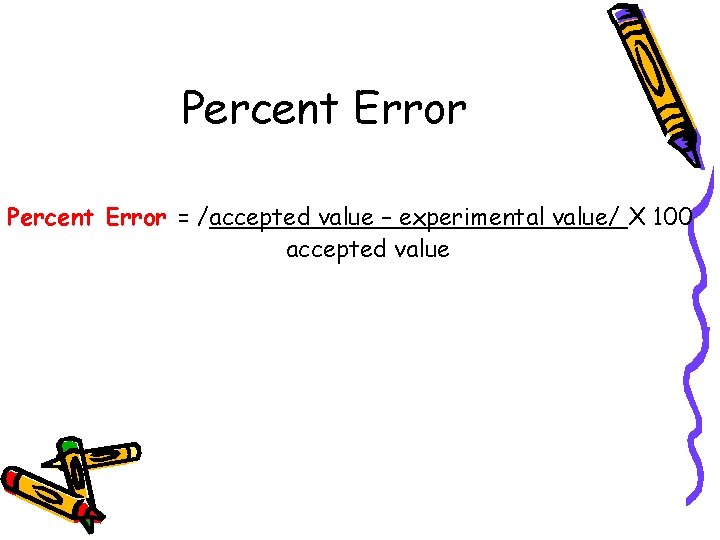
Percent Error = /accepted value – experimental value/ X 100 accepted value

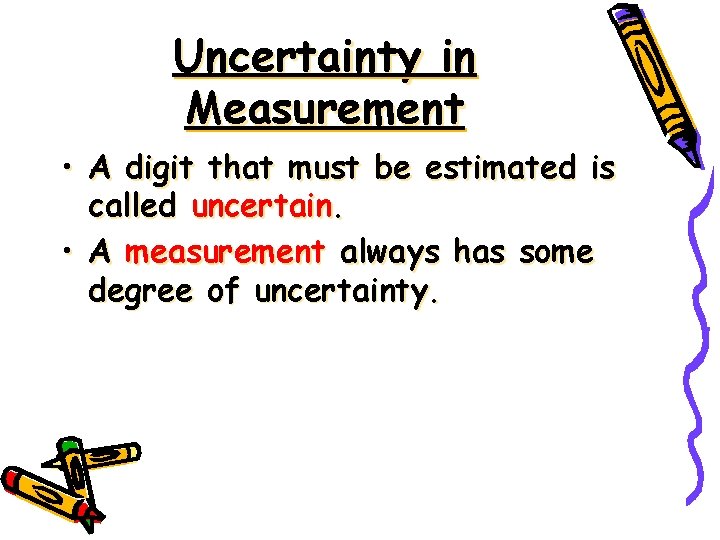
Uncertainty in Measurement • A digit that must be estimated is called uncertain. • A measurement always has some degree of uncertainty.

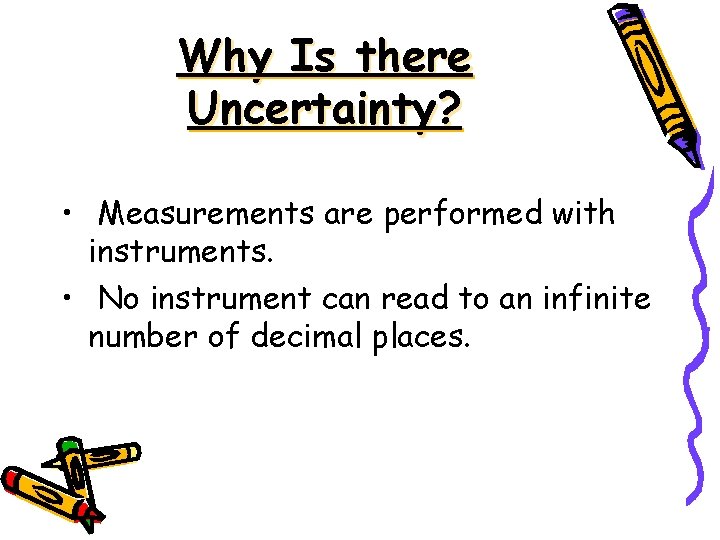
Why Is there Uncertainty? • Measurements are performed with instruments. • No instrument can read to an infinite number of decimal places.

Rules for Counting Significant Figures • Nonzero integers always count as significant figures (sig figs). • 3456 has 4 sig figs. • Zeros -Leading zeros do not count as sig figs. • 0. 0486 has only 3 sig figs.

• Zeros –”captive” zeros always count as sig figs. • 16. 07 has 4 sig figs. • Zeros -Trailing zeros are sig only if the number contains a decimal point. • 9. 300 has 4 sig figs. • Exact numbers have an infinite number of sig figs. • 1 inch = 2. 54 cm, exactly

Sig Fig Practice #1 • How many significant figures in each of the following? 1. 0070 m 5 sig figs 17. 10 kg 4 sig figs 100 890 L 5 sig figs 3. 29 x 103 s 3 sig figs 0. 0054 cm 2 sig figs 3 200 000 2 sig figs

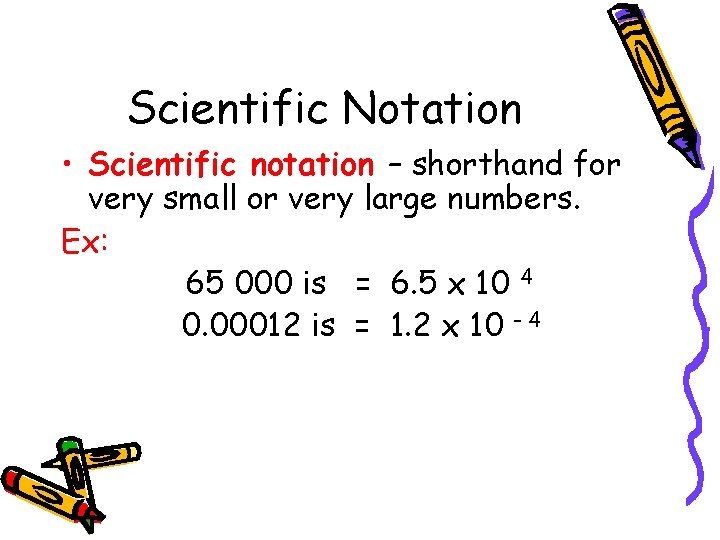
Scientific Notation • Scientific notation – shorthand for very small or very large numbers. Ex: 65 000 is = 6. 5 x 10 4 0. 00012 is = 1. 2 x 10 - 4