CHAPTER 2 A 2 BTHE PARTICLE MODEL OF

CHAPTER 2 A & 2 BTHE PARTICLE MODEL OF MATTER & CLASSIFICATION OF MATTER

WHAT IS MATTER? • Matter- anything that takes up space and has mass • Made of atoms • EVERYTHING IS MATTER • Seriously • Everything • Anything you can see, touch, smell and/or taste is made of matter.

WHAT MAKES UP MATTER? • Greek philosophers realized everything was made of particles, which they called atomos (indivisible) • This is called the particle theory • Most common particle: atom • Atoms are the building blocks of matter • Anything made with matter is made of atoms

HOW DO WE KNOW THE PARTICLE MODEL IS TRUE? • Two things were observed: • Diffusion of sugar/ salt/ food coloring/ tea flavor & color through water • Brownian motion of cells seen in a microscope in 1827 by Robert Brown

DIFFUSION

BROWNIAN MOTION • https: //www. youtube. com/watch? v=4 m 5 Jn. JBq 2 AU
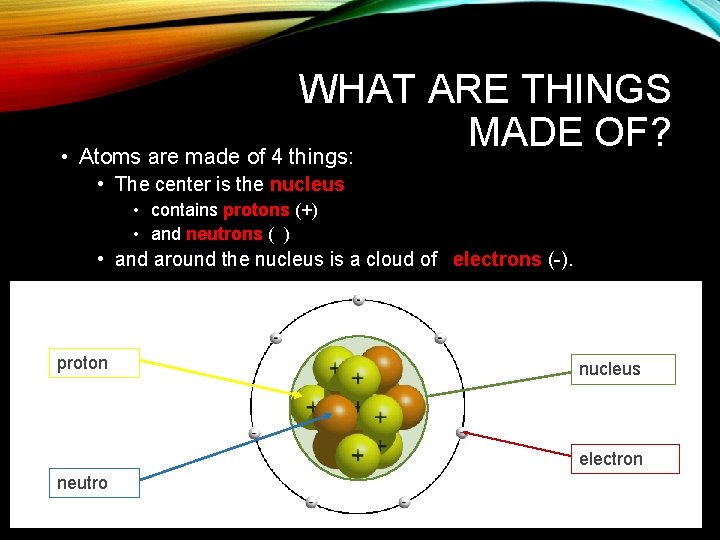
WHAT ARE THINGS MADE OF? • Atoms are made of 4 things: • The center is the nucleus • contains protons (+) • and neutrons ( ) • and around the nucleus is a cloud of electrons (-). proton nucleus electrons neutron
• Magic happens! WHEN ATOMS GET TOGETHER… • We get molecules • Formed with bonds • Also- atoms can gain or lose electrons. Then they become ions • anions (gained, so now (-)) • cations (lost, so now (+)) • Example: NH 4 +=ammonium, HSO 4 -= hydrogen sulfate
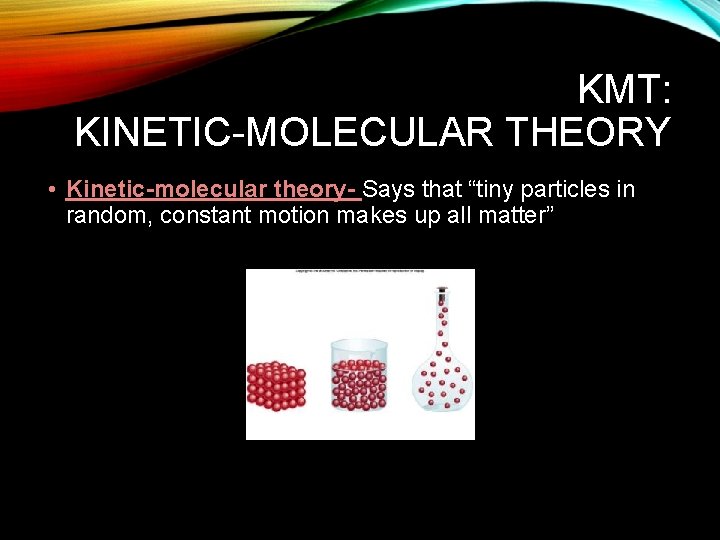
KMT: KINETIC-MOLECULAR THEORY • Kinetic-molecular theory- Says that “tiny particles in random, constant motion makes up all matter”
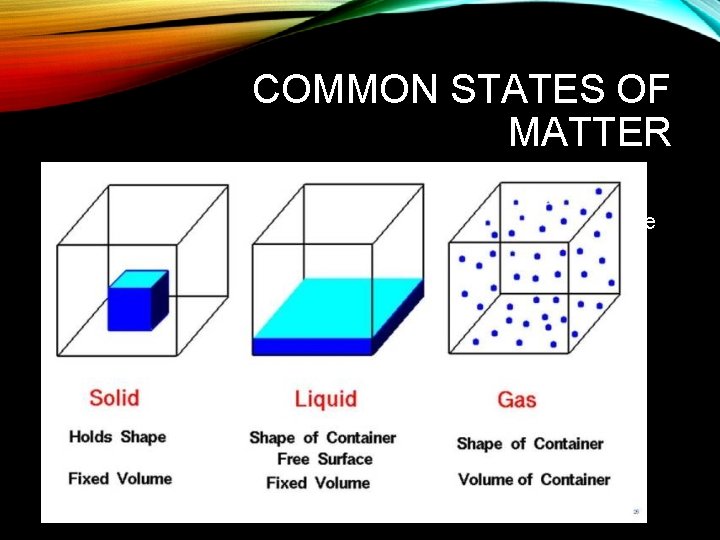
COMMON STATES OF MATTER • Solid- state in which particles occupy fixed positions • Liquid- state in which particles are close together but mobile • Gas- state in which particles are far apart and have large kinetic energies
STATES OF MATTER • 3 states of matter • Solid • Resist change in shape, have a definite volume, low compressibility • Also- particles close together & in a fixed position • Liquid • No definite shape, definite volume, low compressibility (not as low as solid) • Particles move, but stay close together • Gas • No definite shape, no definite volume, highly (easily) compressible • Particles move quickly and are far apart • Also, Gas and Liquids are called fluids (because they flow)
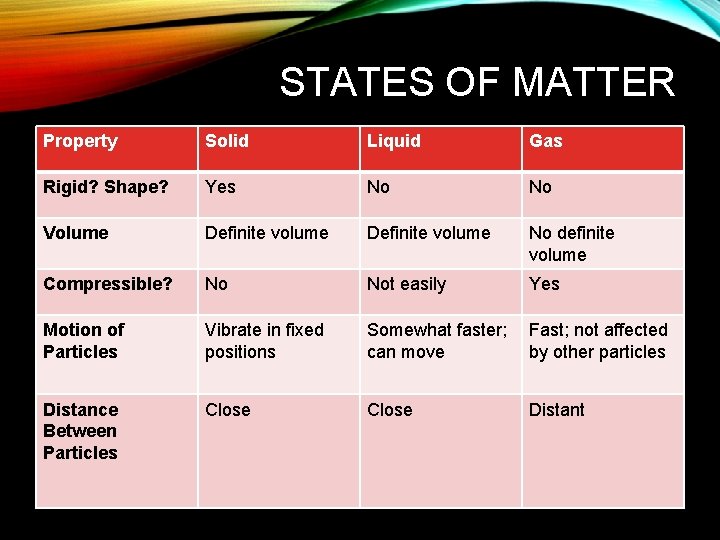
STATES OF MATTER Property Solid Liquid Gas Rigid? Shape? Yes No No Volume Definite volume No definite volume Compressible? No Not easily Yes Motion of Particles Vibrate in fixed positions Somewhat faster; can move Fast; not affected by other particles Distance Between Particles Close Distant
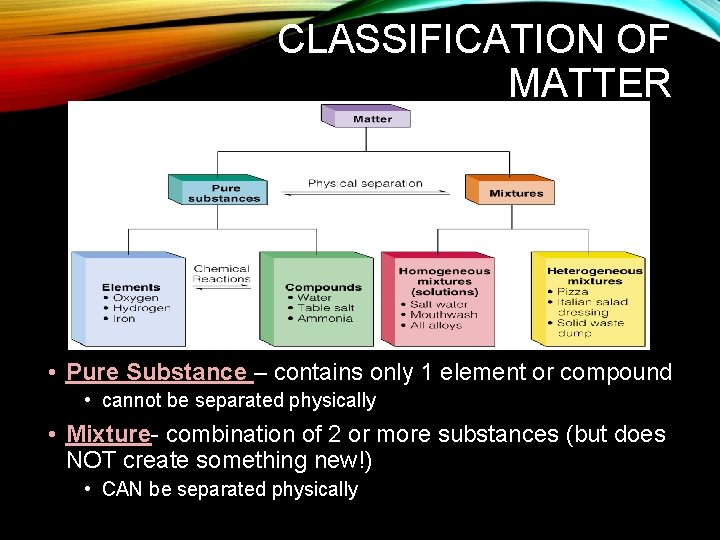
CLASSIFICATION OF MATTER • Pure Substance – contains only 1 element or compound • cannot be separated physically • Mixture- combination of 2 or more substances (but does NOT create something new!) • CAN be separated physically

VOCABULARY WORDS • Matter • Anything that takes up space and has mass • Particle theory • The concept that all matter is made of small particles • Atom • Basic particle of matter which everything is made of. Made of protons, electrons, neutrons. • Diffusion • Process of spreading out and mixing due to particle motion • Brownian Motion • Microscopic, random motion of matter due to collisions of gas or liquid particles • Bond • Connection between atoms to make bigger structures (molecules, ions, etc)
VOCABULARY WORDS • Nucleus • The center of an atom made of protons and usually neutrons • Proton • Nuclear particle in an atom that carries a single positive charge • Neutron • Neutral nuclear particle in an atom • Electron • Particle that carries a single negative charge and moves around the nucleus of an atom • Molecule • Particle formed when 2 or more atoms bond together • Ion • Atom or molecule that has gained or lost electrons, producing an imbalance between protons and electrons
- Slides: 15