Chapter 2 4 Enzymes CHEMICAL REACTIONS Chemical reaction








- Slides: 17

Chapter 2. 4 Enzymes

CHEMICAL REACTIONS Chemical reaction- process that changes one set of chemicals into another set of chemicals. Chemical reactions always involve the breaking of bonds in reactants and the formation of new bonds in products. reactant + reactant -> product (+ another product) A + B -> C + D Reactant- element or compound that enters into a chemical reaction Product- element or compound that by a chemical reaction

Example of a chemical reaction: When CO 2 is produced in the cells as a byproduct of cellular respiration, CO 2 can’t be dissolved by blood cells; therefore, it reacts with water to form a soluble compound called carbonic acid- H 2 CO 3. CO 2 + H 2 O-> H 2 CO 3 Blood carries H 2 CO 3 to lungs where is changes back to CO 2. H 2 CO 3 ->CO 2 + H 2 O

ENERGY IN REACTIONS Releases energy Spontaneous Absorbs energy Not spontaneous Chemical reactions that release energy often occur spontaneously. Chemical reactions that absorb energy will not occur without a source of energy. Every organism must have a source of energy to carry out chemical reactions. Plants get energy from the sunlight, and animals get energy from plants or other animals.

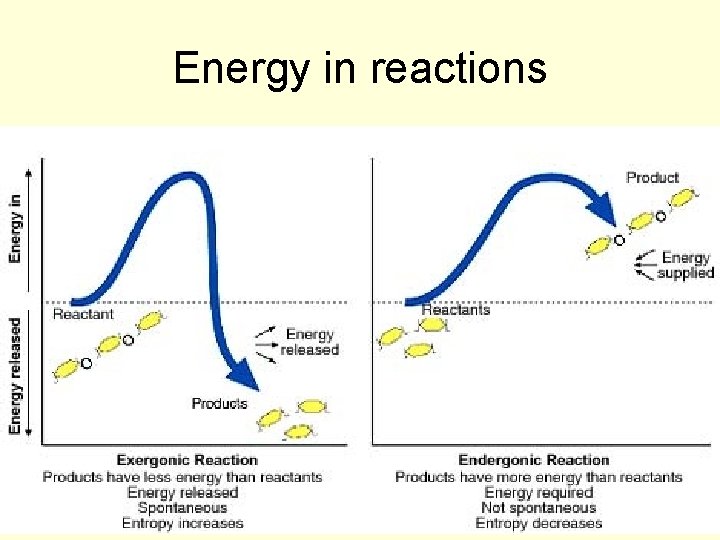
Energy in reactions

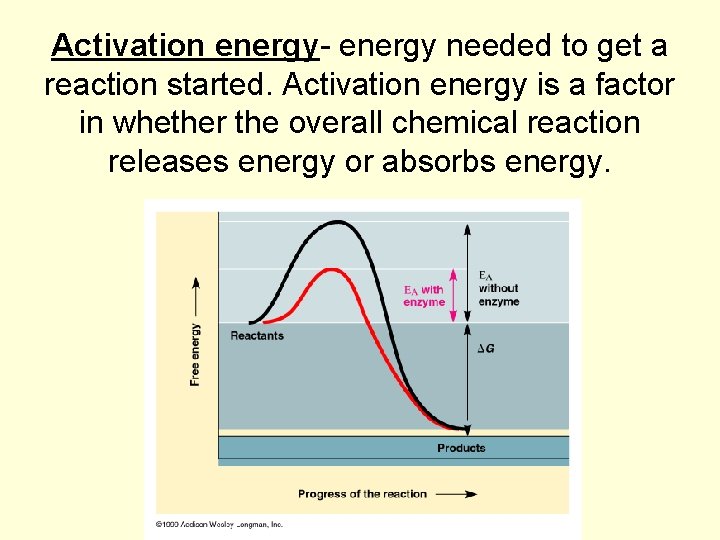
Activation energy- energy needed to get a reaction started. Activation energy is a factor in whether the overall chemical reaction releases energy or absorbs energy.

ENZYMES Catalyst - substance that speeds up the rate of a chemical reaction Enzyme - is a protein that acts as a biological catalyst. Enzymes act by lowering the activation energy. Enzymes play essential roles in regulating chemical pathways, making materials that cells need, releasing energy and transferring information. Cells use enzymes to speed up chemical reactions that take place in cells. Enzymes are very specific, generally catalyzing only one chemical reaction.

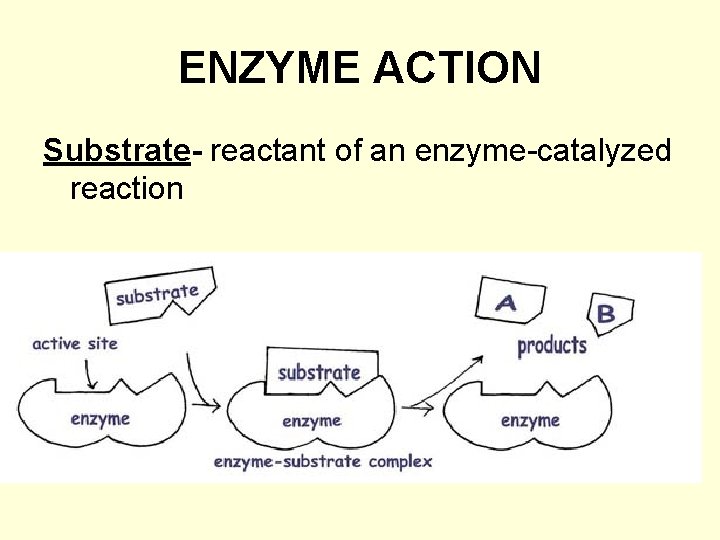
ENZYME ACTION Substrate- reactant of an enzyme-catalyzed reaction

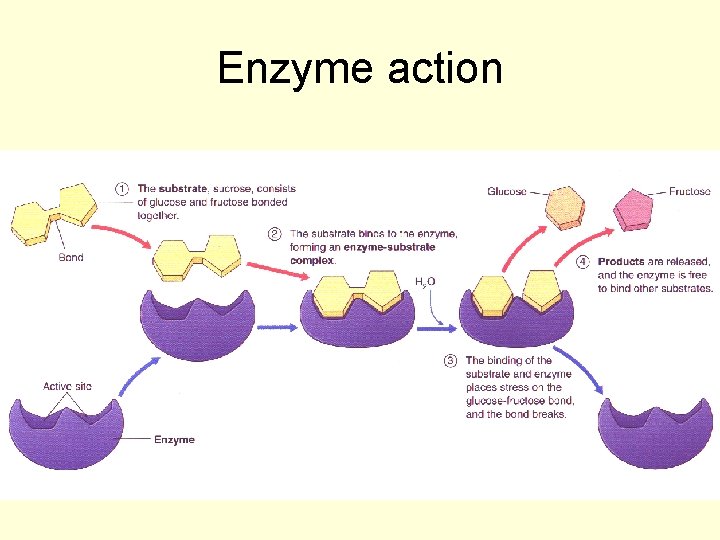
Enzyme action

Enzyme animation • http: //highered. mcgrawhill. com/sites/0072495855/student_view 0/ chapter 2/animation__how_enzymes_work. html

Regulation of Enzyme Activity: -work best at certain p. H levels (mostly neutral which is p. H=7) -best temperature is near 370 C (body temperature) NOTE: cells can regulate the activities of enzymes. Cells have proteins that help to turn key enzymes “on” and “off” at critical stages in the life of cell.

2 -4 Section Assessment: What happens to chemical bonds during chemical reactions?

How do energy changes affect whether a chemical reaction will occur?

Why are enzymes important to living things?

Describe how enzymes work, including the role of enzyme-substrate complex.

In what conditions does an enzyme work the best?

Critical Thinking: A change in p. H can change the shape of a protein. How might a change in p. H affect the function of an enzyme such as hexokinase (page 52)?