Chapter 2 4 Chemical Compounds of Life Concepts















- Slides: 24

Chapter 2. 4 Chemical Compounds of Life Concepts: Organic Compounds- compounds that contain carbon…. . What is their function and how do we test for them? ? ?

Organic Chemistry • Organic compounds contain the element carbon and are essential to life.

Organic means… • the element carbon is present (along with H, O, and N frequently) CH 4 = organic H 2 O = inorganic (no C)

Water It is INORGANIC, but very important!!!! Your body weight is 65% water It is so important because many biological processes can only take place in water. If there is no water, none of these processes can occur.

Other Water Info: • Polar Molecule- has areas that are positively charged and areas that are negatively charged • Cohesion- the force of attraction between two water molecules

• Adhesion- the force of attraction between water molecules and another substance (like glass) • Capillary Action- the moving of water against the force of gravity using adhesion

4 Organic Compounds • 4 main types of organic compounds essential to your life: • 1) Carbohydrates 2) Lipids • 3) Proteins 4) Nucleic Acids

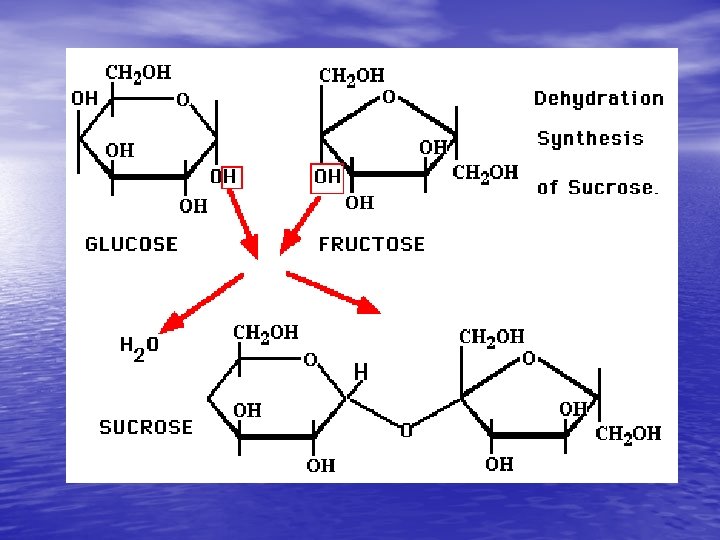
Carbohydrates (AKASaccharides) • Monosaccharides- BUILDING BLOCKS (small) • or they are also called “Simple sugars” • ex. Glucose (Blood sugar) C 6 H 12 O 6 v C 1: H 2: O 1 • Polysaccsaride- MACROMOLECULES (large) • ex. Complex Starches • USES- ENERGY, Cell Wall in plants (cellulose)

INDICATOR- IODINE • Tests for Starch- Iodine is rust/amber • Positive- blue-ish/black

INDICATOR- BENEDICTS • TEST FOR GLUCOSE/ simple sugars - BENEDICTS is light Blue • POSITIVE TEST- ORANGE

Making and Breaking Carbs • Carbs can be made by a process called dehydration synthesis. – Dehydration – removing water – Synthesis – putting together - Broken down by a process called hydrolysis (water-splitting).


Too Many Carbs? • Plants store extra sugar as STARCH • Animals store extra sugar as GLYCOGEN in the liver…this can turn to fat if it is not used.

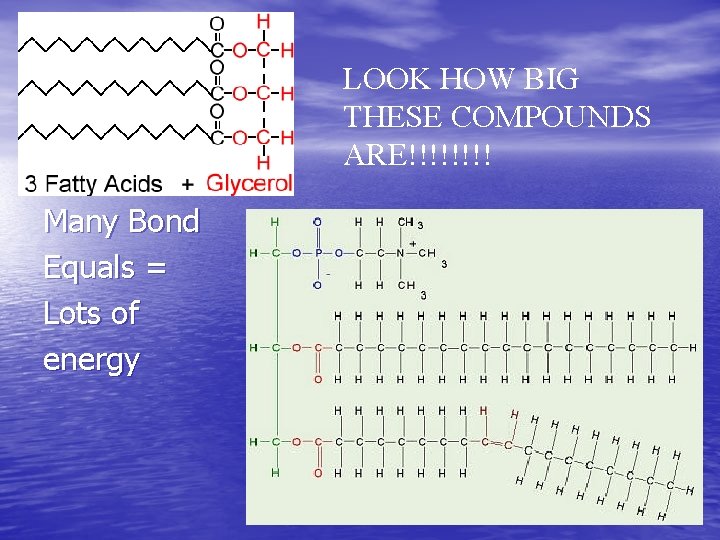
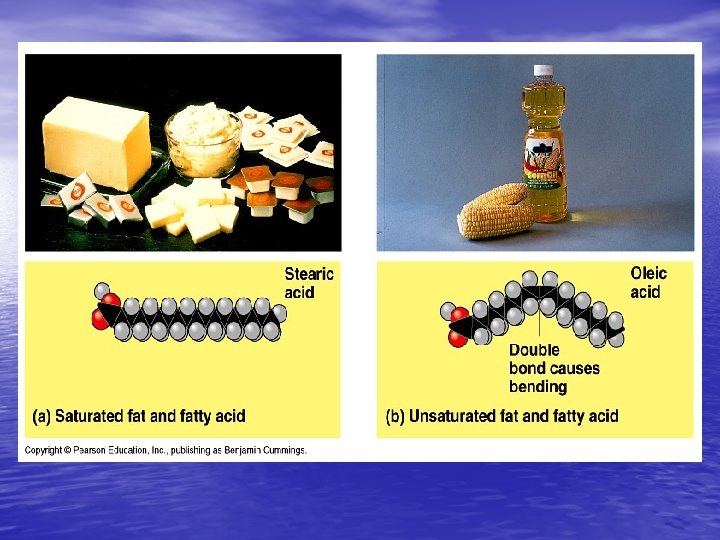
Lipids (Fats, Oils, and Waxes) Characteristics: - Long chains of carbon and hydrogen atoms BUILDING BLOCKS: 3 Fatty acids and 1 Glycerol Two kinds: – Saturated (usually solids - like butter) – Unsaturated (usually liquids- like olive oil) • USES: STORED ENERGY, Organ Protection

LOOK HOW BIG THESE COMPOUNDS ARE!!!! Many Bond Equals = Lots of energy



INDICATOR- PAPER BAG • Bag looks wet- Almost See-through

Proteins • Made of smaller units called amino acids. There are 20! • LARGER VERSION(macros) =PEPTIDE & POLYPEPTIDE • Used to build muscle, as well as enzymes and hormones • DNA carries the “recipe” to make proteins (protein synthesis)

Why are proteins so important? Enzymes, cell structures, hormones, parts of the blood, etc. are made out of proteins! Wow! That’s Important!

INDICATOR- BIURETS • TESTS FOR PROTEIN. Biurets is Blue. • POSITIVE TESTTurns purple/pink

Nucleic Acids • CONTROLS HEREDITY and CELL FUNCTIONS • BUILDING BLOCK is a NUCLEOTIDE!!!! • Two Kinds of MACROS: – DNA and RNA • Found in the NUCLEUS of a CELL

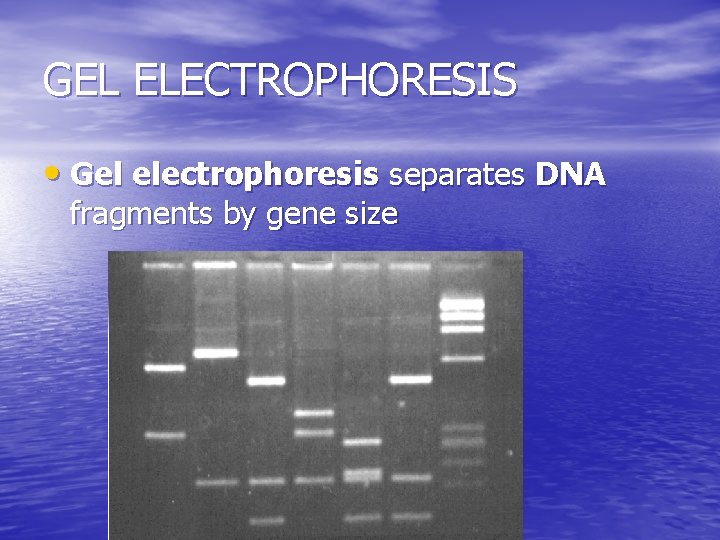
GEL ELECTROPHORESIS • Gel electrophoresis separates DNA fragments by gene size

THE END