CHAPTER 2 3 MATTER COMPOSITION OF MATTER TYPES






















- Slides: 35

CHAPTER 2 & 3 MATTER COMPOSITION OF MATTER TYPES OF SUBSTANCES DESCRIBING MATTER STATES OF MATTER CHANGE IN MATTER FLUIDS

MATTER Anything that has mass and takes up space

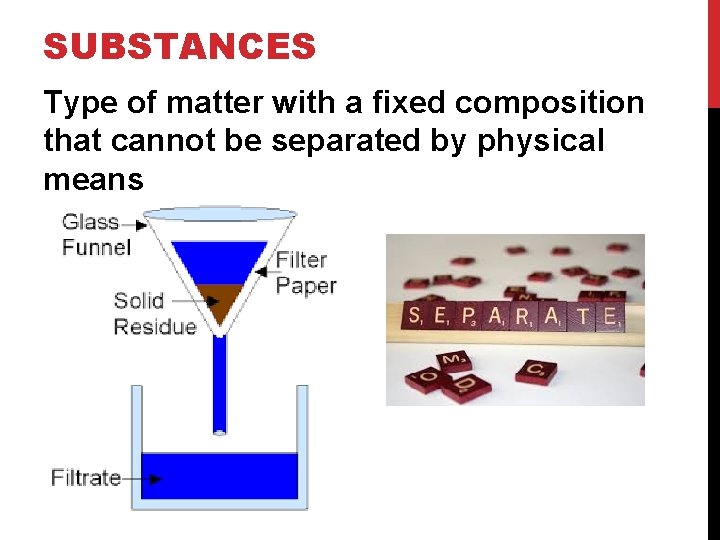
SUBSTANCES Type of matter with a fixed composition that cannot be separated by physical means

PHYSICAL PROPERTY Characteristic of a material that you can observe without changing the substance Physical Change A change in size, shape or state of matter; substance DOES NOT change identity Can be reversed easily

PHYSICAL PROPERTY - Examples Change in phase: state of matter change (solid, liquid or gas) Change in shape Malleable - bend or hammer without breaking Ductile - stretching without breaking Dissolving - making items smaller

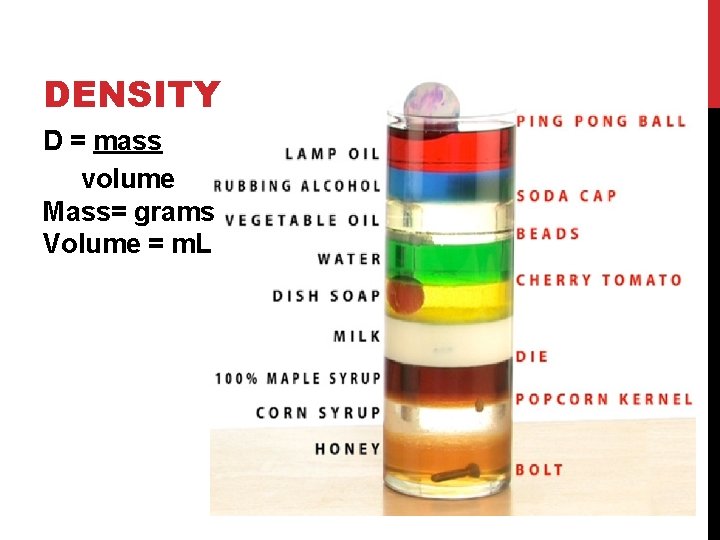
DENSITY D = mass volume Mass= grams Volume = m. L

BOILING POINT The temperature at which a liquid changes to a gas. For water: 100 o. C

FREEZING POINT The temperature at which a liquid changes to a solid. For water: 0 o. C

ODOR

COLOR

SHAPE

CHEMICAL PROPERTY Characteristic of a substance that indicates whether it can undergo a certain chemical change Chemical Change A change in one substance to another substance

CHEMICAL PROPERTY - Evidence 1. Gas produced 2. Bubbles form 3. A change in temperature 4. Color change 5. Burning

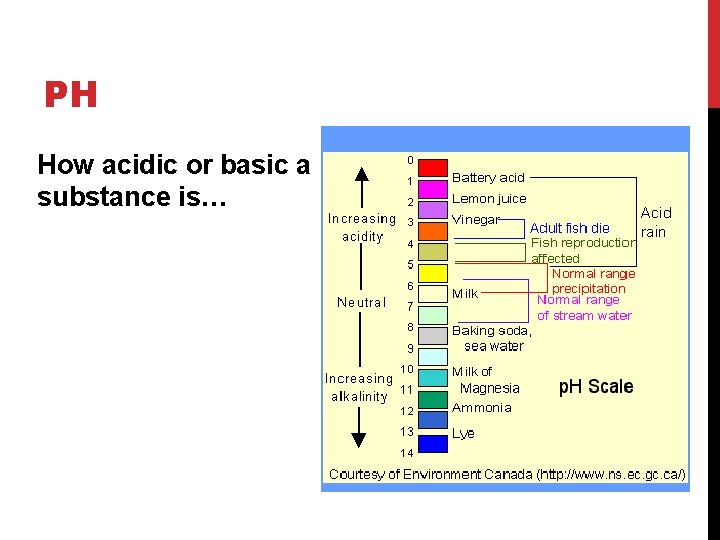
PH How acidic or basic a substance is…

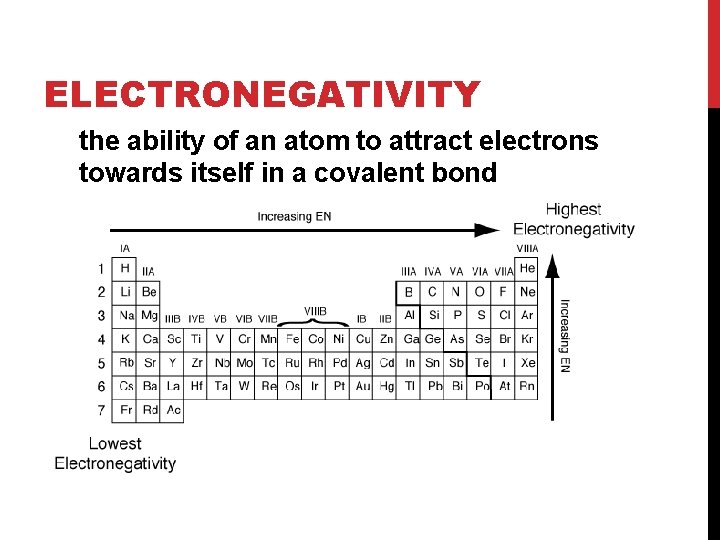
ELECTRONEGATIVITY the ability of an atom to attract electrons towards itself in a covalent bond

REACTIVITY WITH OTHER SUBSTANCES

FLAMMABILITY – PRESENCE OF OXYGEN

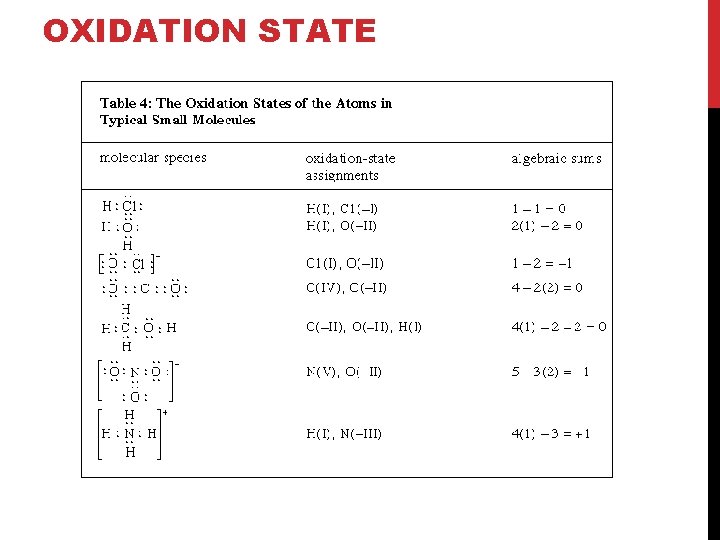
OXIDATION STATE

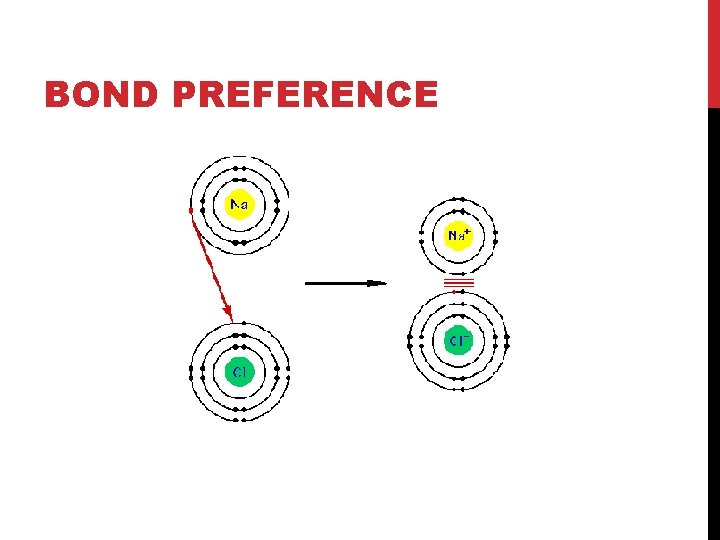
BOND PREFERENCE

MIXTURES Material made up of two or more substances that can be separated by physical means

Kinetic Theory Explains how particles in matter behave • All matter is composed of particles • Particles are in constant, random motion • Particles collide with each other and walls of the container

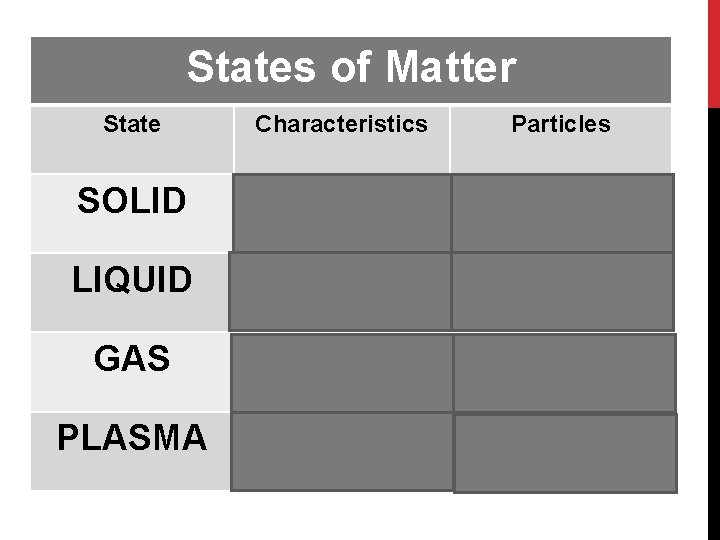
States of Matter State Characteristics Particles SOLID Definite shape AND definite volume LIQUID Indefinite shape AND More space, particles definite volume slide pass each other GAS Indefinite shape AND Space to spread out Indefinite volume evenly in a container PLASMA High temperature gas Closely packed in a geometric arrangement Positively and negatively charged

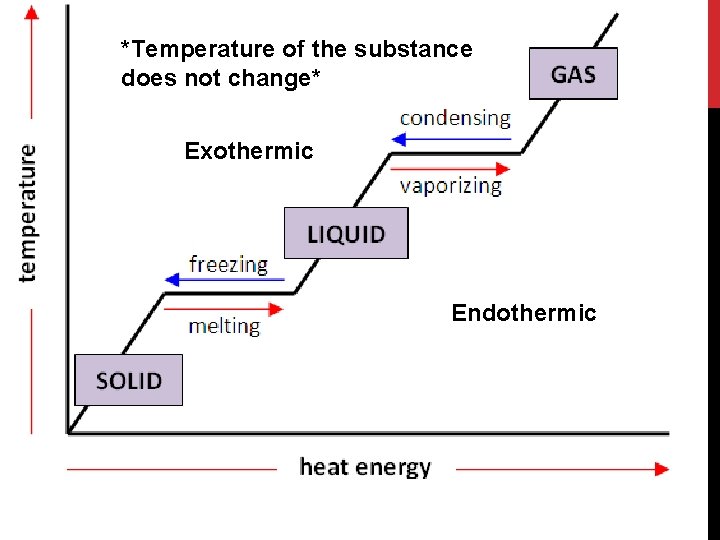
*Temperature of the substance does not change* Exothermic Endothermic

HTTP: //WWW. DAILYMOTI ON. COM/VIDEO/X 2 NCM 5 I

Mixtures & SOLUTIONS

ELEMENT Substance made up of atoms with the same identity

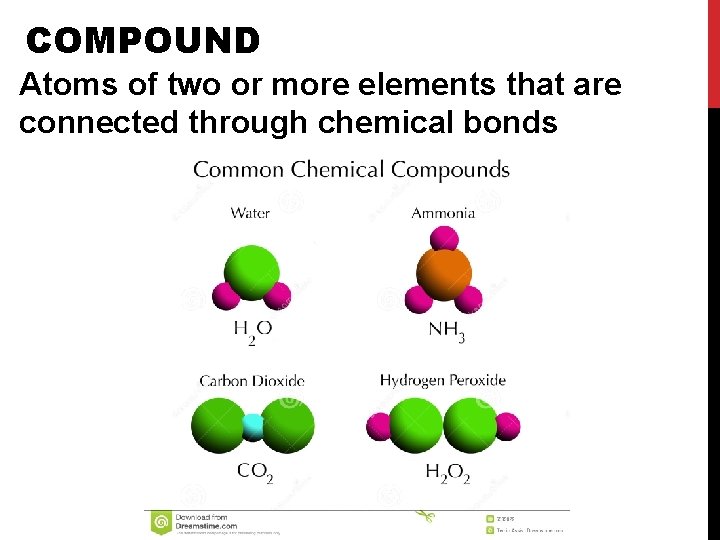
COMPOUND Atoms of two or more elements that are connected through chemical bonds

HOMOGENEOUS Mixture in which two or more substances are uniformly spread out

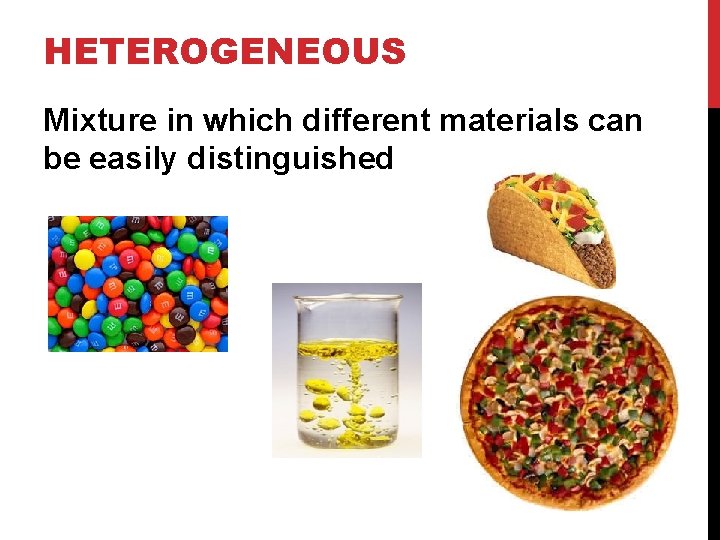
HETEROGENEOUS Mixture in which different materials can be easily distinguished

SIGNS THAT A CHEMICAL CHANGE HAS OCCURRED Bubbles of gas appear A precipitate(solid) appears where there was only liquid before Change in color Change in Temperature

SOLUTION Homogeneous mixture of particles so small that they cannot even be seen with a microscope and will never settle Example: Sugar water

COLLOID Type of mixture with particles that are larger than those in a solution, but still not able to settle out Examples: Shaving cream Lotion Ink

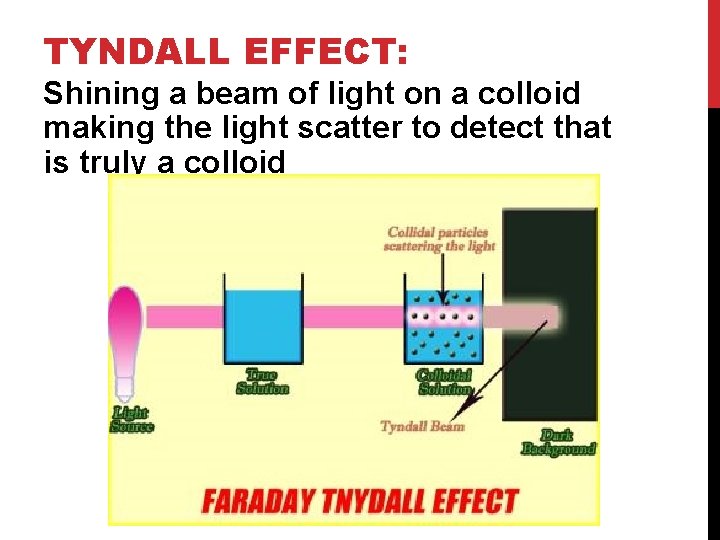
TYNDALL EFFECT: Shining a beam of light on a colloid making the light scatter to detect that is truly a colloid

SUSPENSION Heterogeneous mixture containing liquid in which visible particles settle Examples: Sand in water Salad dressing Apple cider *HINT: If it has a label “Shake Well” then it has suspended particles making it a suspension

ACTIVITY - LABEL THESE EXAMPLES 1. Solution 1. Kool-Aid 2. Colloid 2. Toothpaste 3. Colloid 3. Fog 4. Suspension 4. OJ 5. Solution 5. Gatorade 6. Suspension 6. Protein Shake 7. Colloid 7. Hair dye 8. Colloid 8. Whipped Cream