Chapter 19 Thermodynamics First Law of Thermodynamics energy








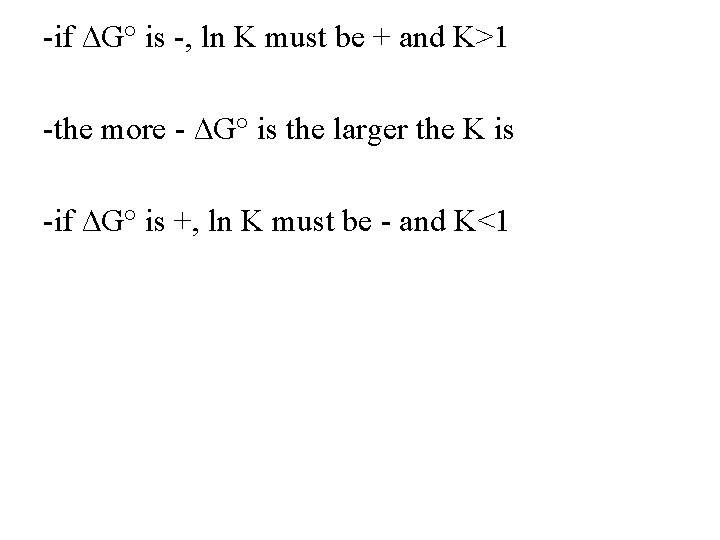
- Slides: 18

Chapter 19: Thermodynamics First Law of Thermodynamics: energy cannot be created or destroyed, energy is conserved *total energy of the universe cannot change *you can transfer energy *∆Euniverse = ∆Esystem + ∆Esurroundings = 0

-for an exo. reaction, “lost” energy from the system goes into the surroundings -two ways energy “lost” from a system *converted to heat, q *used to do work, w *∆E = q + w *∆E is a state function


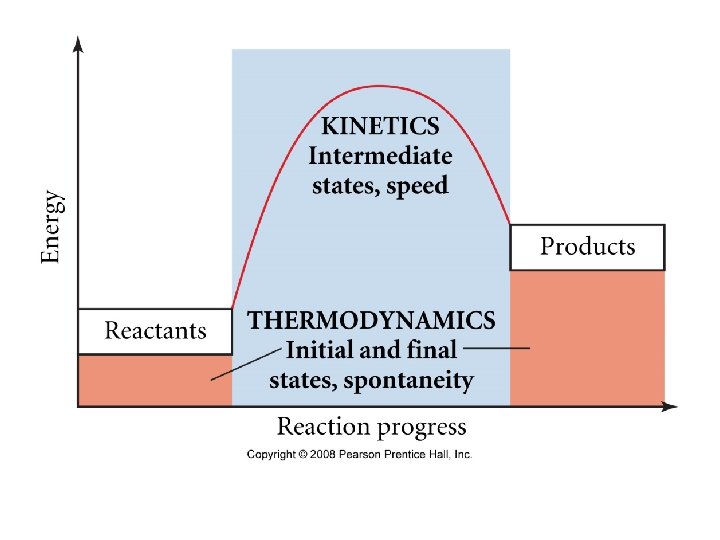
*-thermodynamics predicts whether a process will proceed under the given conditions spontaneous process- proceeds on its own without outside assistance regardless of speed (irreversible) ex- iron rusting -occur in one direction only ex: H 2 O forming from H 2 + O 2 is spon, but the reverse is not -reverse process is nonspontaneous requires energy input

-temp is important when determining if a reaction is spon. ex: ice melting above 0°C is spon, but the reverse process is not

-a reversible process will proceed back and forth between the two end conditions -at equilibrium -results in no change in free energy

Entropy (∆S) -relates to the randomness of a system -entropy change is favorable when the result is a more random system *∆S is + Factors that inc the entropy: 1. solid < liquid < gas 2. reactions with more moles gaseous product than reactant *equal # mol of gases have = entropy unless one is an atom and one a molecule (molec higher b/c they can rotate and vibrate and atoms cannot) 3. inc in temp

2 nd Law of Thermodynamics -entropy of the universe must be + to be spontaneous ∆Suniv = ∆ Ssys + ∆ Ssurr *for reversible ∆ Suniv= 0 *for irreversible/spontaneous ∆ Suniv > 0

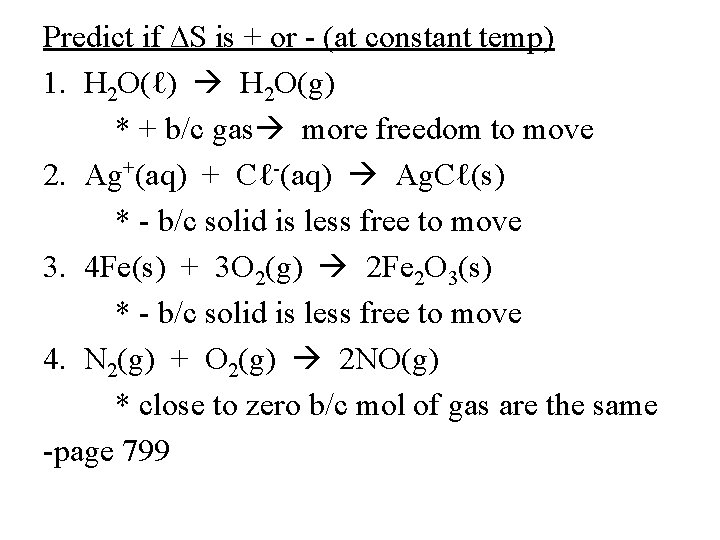
Predict if ∆S is + or - (at constant temp) 1. H 2 O(ℓ) H 2 O(g) * + b/c gas more freedom to move 2. Ag+(aq) + Cℓ-(aq) Ag. Cℓ(s) * - b/c solid is less free to move 3. 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) * - b/c solid is less free to move 4. N 2(g) + O 2(g) 2 NO(g) * close to zero b/c mol of gas are the same -page 799

3 rd Law of Thermodynamics -entropy of a pure crystalline substance at absolute zero (0 K) is zero -entropy will inc as temp inc

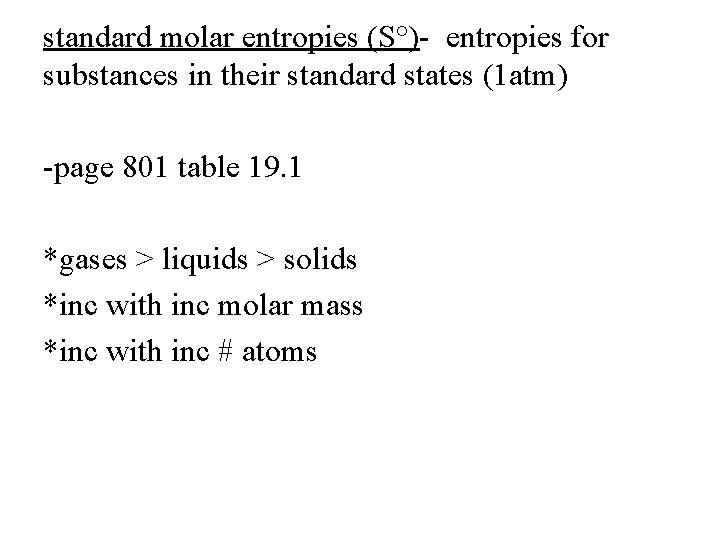
standard molar entropies (S°)- entropies for substances in their standard states (1 atm) -page 801 table 19. 1 *gases > liquids > solids *inc with inc molar mass *inc with inc # atoms

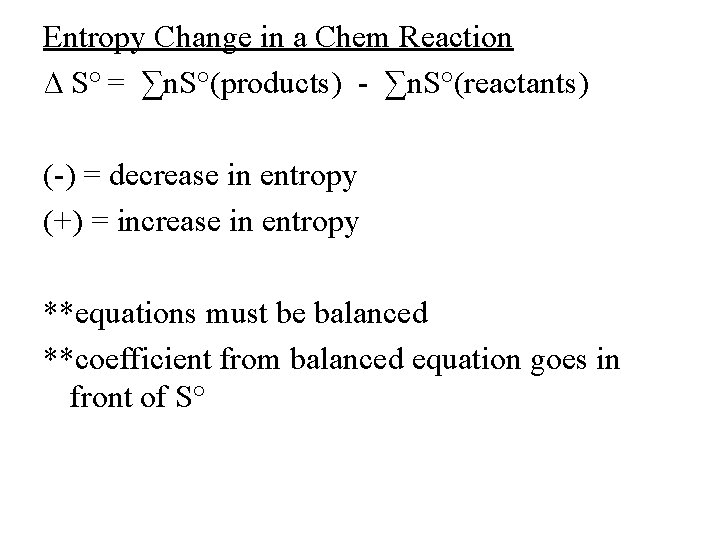
Entropy Change in a Chem Reaction ∆ S° = ∑n. S°(products) - ∑n. S°(reactants) (-) = decrease in entropy (+) = increase in entropy **equations must be balanced **coefficient from balanced equation goes in front of S°

Gibbs Free Energy (G) ∆G = ∆H - T∆S -using standard conditions ∆G° = ∆H° - T∆S° -if ∆G < 0, the reaction is spon. in the forward direction -if ∆G = 0, the reaction is at equilibrium -if ∆G > 0, the reaction in the forward direction is nonspon. , but reverse is spon.

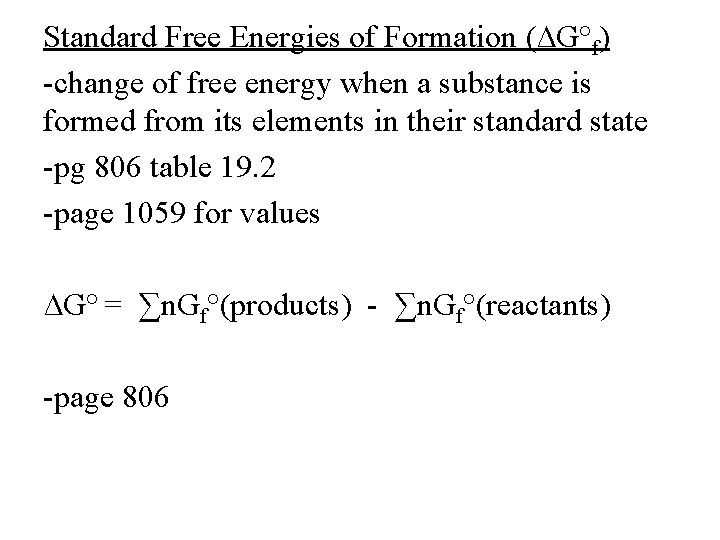
Standard Free Energies of Formation (∆G°f) -change of free energy when a substance is formed from its elements in their standard state -pg 806 table 19. 2 -page 1059 for values ∆G° = ∑n. Gf°(products) - ∑n. Gf°(reactants) -page 806

Free Energy and Temperature -may want to examine reactions not at 25°C -think about: ∆G = ∆H - T∆S -rewrite as: ∆G = ∆H + (-T∆S) -copy table 19. 3 page 809

Free Energy and the Equilibrium Constant Nonstandard Conditions -most reactions occur under nonstandard conditions ∆G = ∆G° + RT ln Q R= 8. 31 J/Kmol T= temp Q= reaction quotient (partial pressure in atm for gases and solutes conc in M) *under std. conditions, Q=1 so ln Q=0) and the equation becomes ∆G = ∆G°

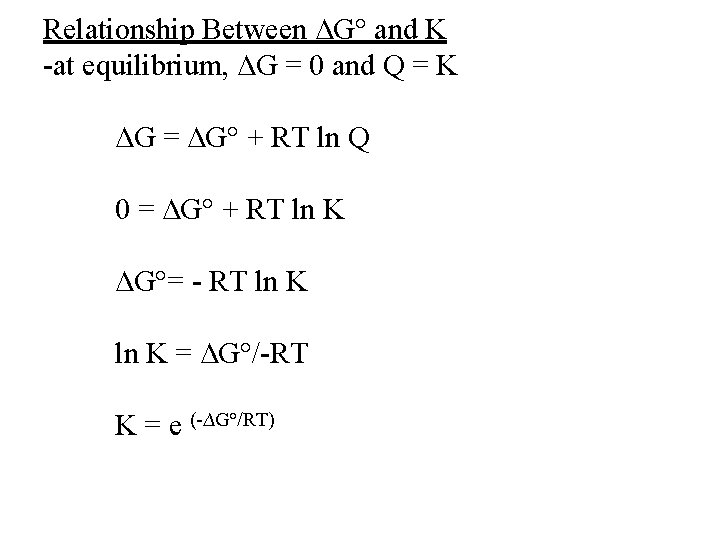
Relationship Between ∆G° and K -at equilibrium, ∆G = 0 and Q = K ∆G = ∆G° + RT ln Q 0 = ∆G° + RT ln K ∆G°= - RT ln K = ∆G°/-RT K = e (-∆G°/RT)
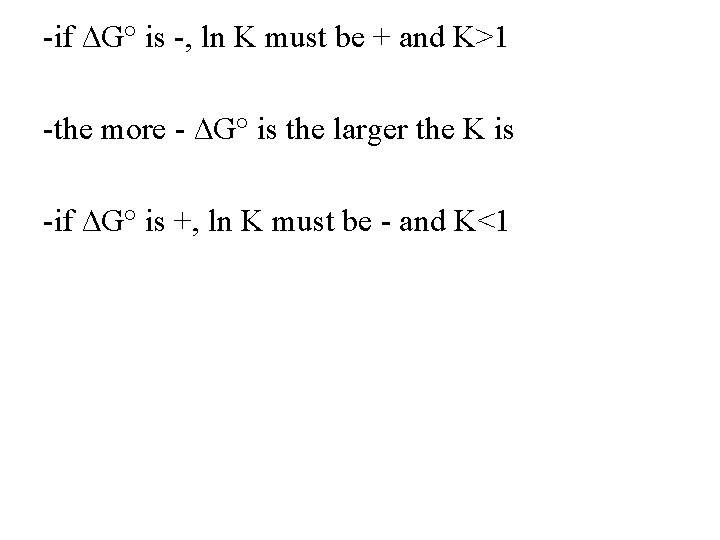
-if ∆G° is -, ln K must be + and K>1 -the more - ∆G° is the larger the K is -if ∆G° is +, ln K must be - and K<1