Chapter 19 The Ideal Gas Equation The Ideal





- Slides: 13

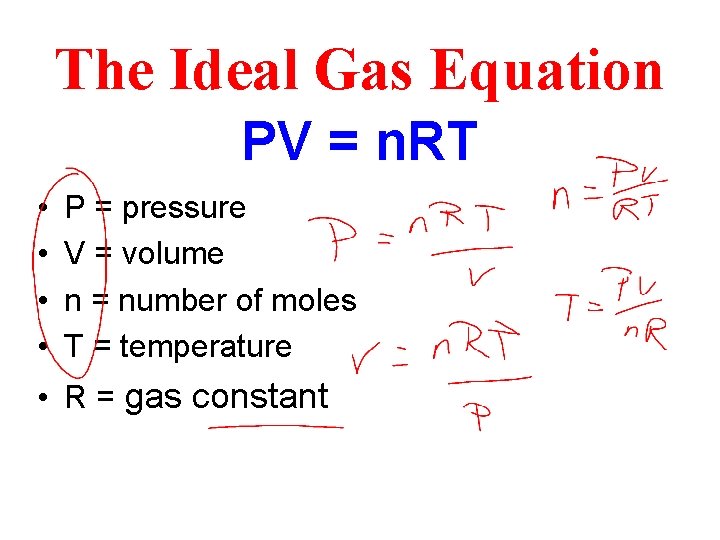
Chapter 19 The Ideal Gas Equation

The Ideal Gas Equation PV = n. RT • • P = pressure V = volume n = number of moles T = temperature • R = gas constant

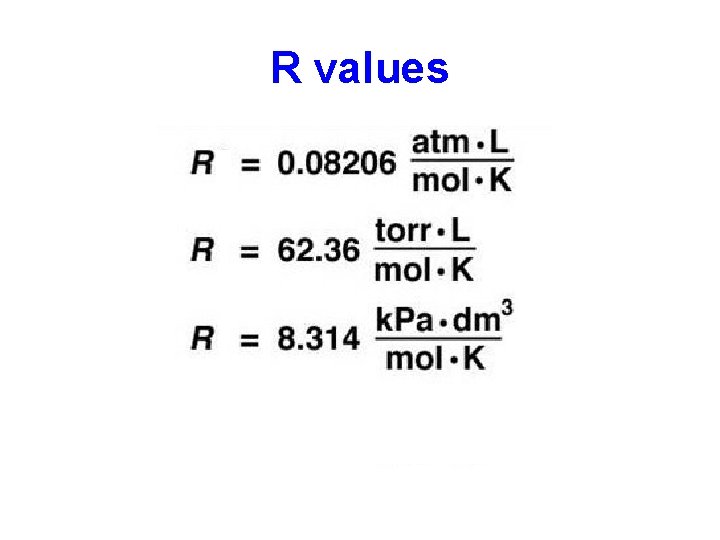
R values a A I

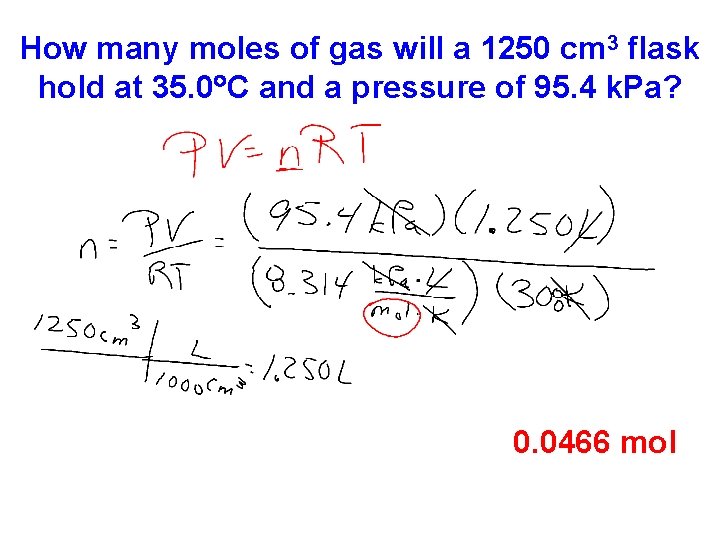
How many moles of gas will a 1250 cm 3 flask hold at 35. 0 C and a pressure of 95. 4 k. Pa? 0. 0466 mol

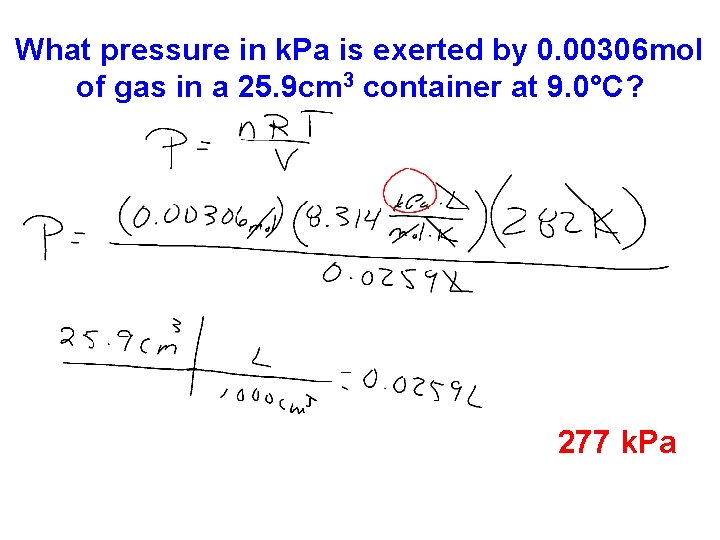
What pressure in k. Pa is exerted by 0. 00306 mol of gas in a 25. 9 cm 3 container at 9. 0°C? 277 k. Pa

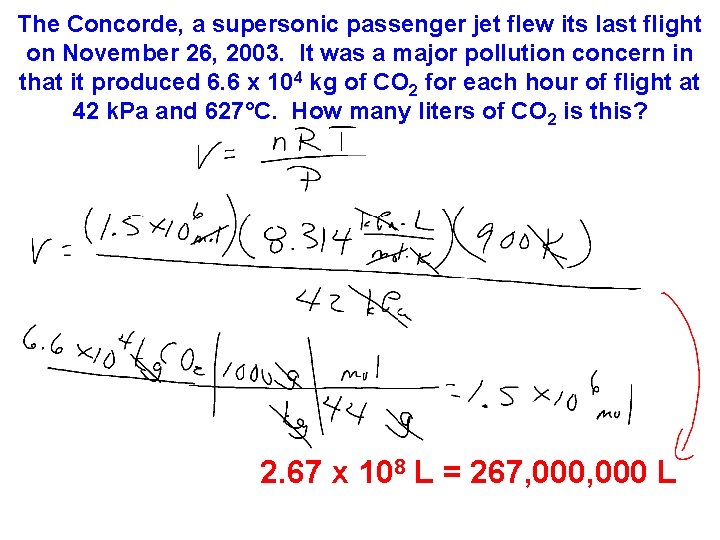
The Concorde, a supersonic passenger jet flew its last flight on November 26, 2003. It was a major pollution concern in that it produced 6. 6 x 104 kg of CO 2 for each hour of flight at 42 k. Pa and 627°C. How many liters of CO 2 is this?

The Concorde, a supersonic passenger jet flew its last flight on November 26, 2003. It was a major pollution concern in that it produced 6. 6 x 104 kg of CO 2 for each hour of flight at 42 k. Pa and 627°C. How many liters of CO 2 is this? 2. 67 x 108 L = 267, 000 L

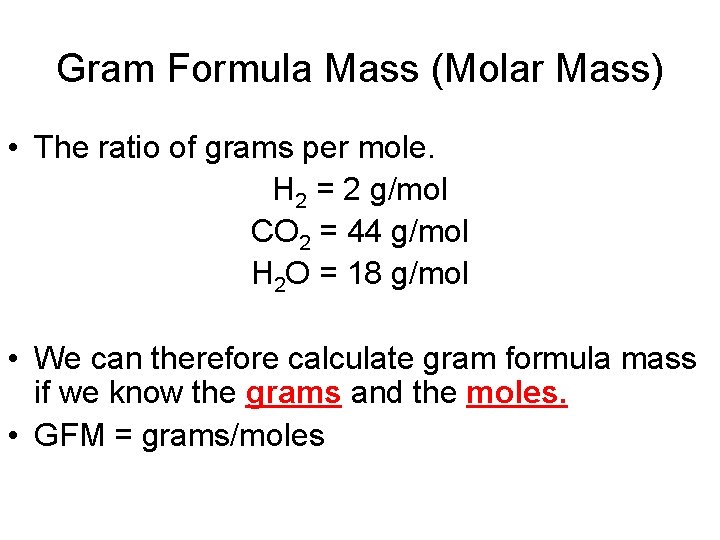
Gram Formula Mass (Molar Mass) • The ratio of grams per mole. H 2 = 2 g/mol CO 2 = 44 g/mol H 2 O = 18 g/mol • We can therefore calculate gram formula mass if we know the grams and the moles. • GFM = grams/moles

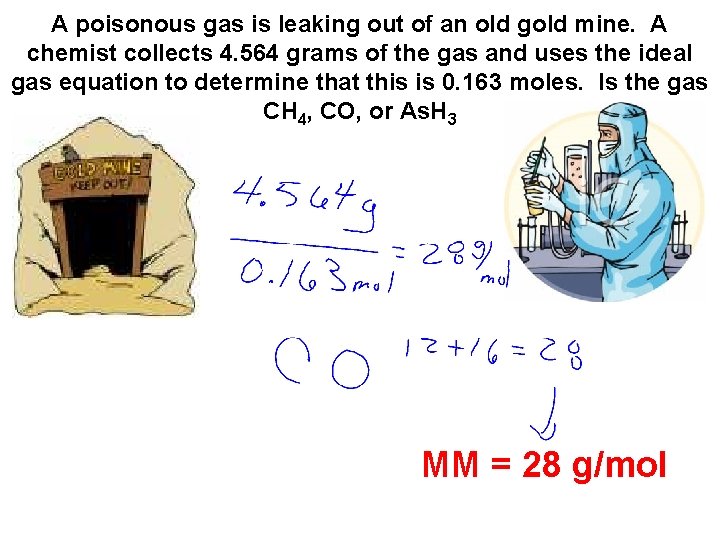
A poisonous gas is leaking out of an old gold mine. A chemist collects 4. 564 grams of the gas and uses the ideal gas equation to determine that this is 0. 163 moles. Is the gas CH 4, CO, or As. H 3 MM = 28 g/mol

Remember our first question today.

How many moles of gas will a 1250 cm 3 flask hold at 35. 0 C and a pressure of 95. 4 k. Pa? n = PV / RT = 0. 0466 mol • 0. 186 g of a gas at 35. 0 C and 95. 4 k. Pa occupies a volume of 1250 cm 3. Is the gas H 2, He, O 2, or CO 2? GFM = grams/mole = 0. 186 g/0. 0466 moles = 3. 99 g/mol ≈ 4 g/mol Therefore the gas is most likely He.

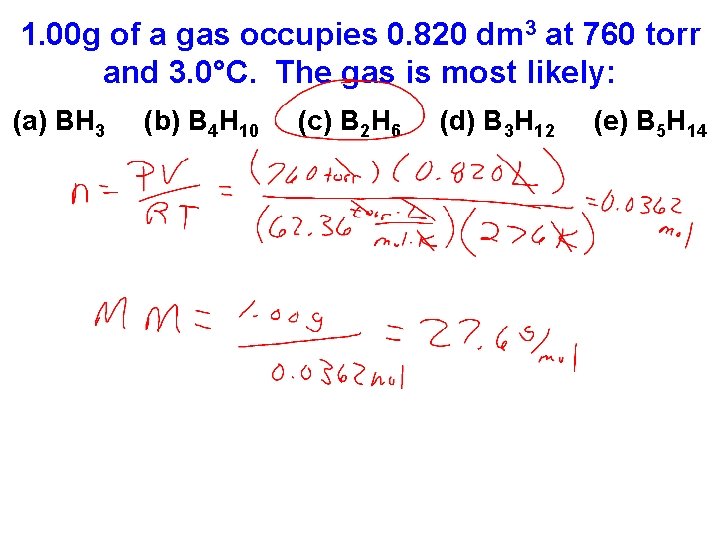
1. 00 g of a gas occupies 0. 820 dm 3 at 760 torr and 3. 0°C. The gas is most likely: (a) BH 3 (b) B 4 H 10 (c) B 2 H 6 (d) B 3 H 12 (e) B 5 H 14

Homework • Worksheet: The Ideal Gas Equation