Chapter 19 Section 1 Structure of the Atom
















- Slides: 48

Chapter 19 Section 1 Structure of the Atom Read Ch 19 from p 578

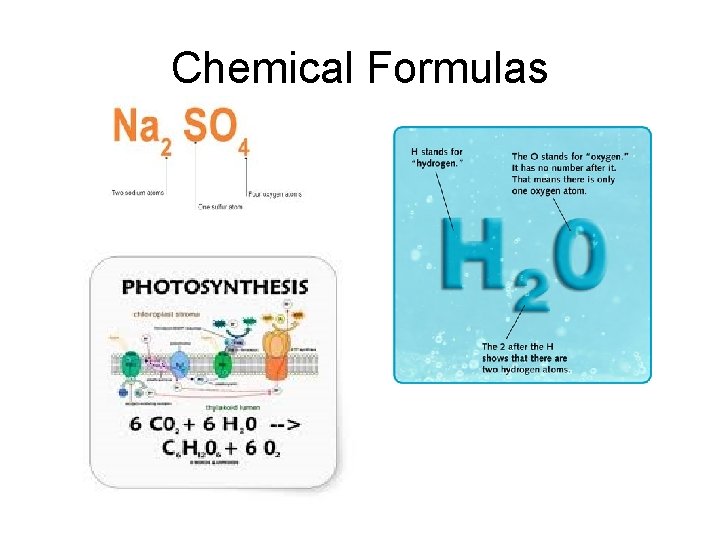
Scientific Shorthand • Scientists abbreviate the names of elements – Each element has a nickname • 1, 2 or sometimes 3 letters • First letter capitalized • Second and third letter would be lower case • Names of elements come from different sources – – – Latin names Honoring scientists Places Properties of the element Rules established by an international committee



Chemical Formulas

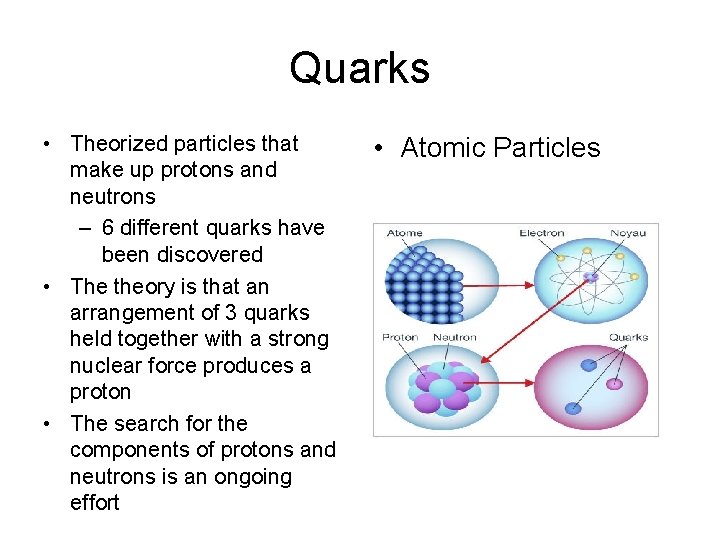
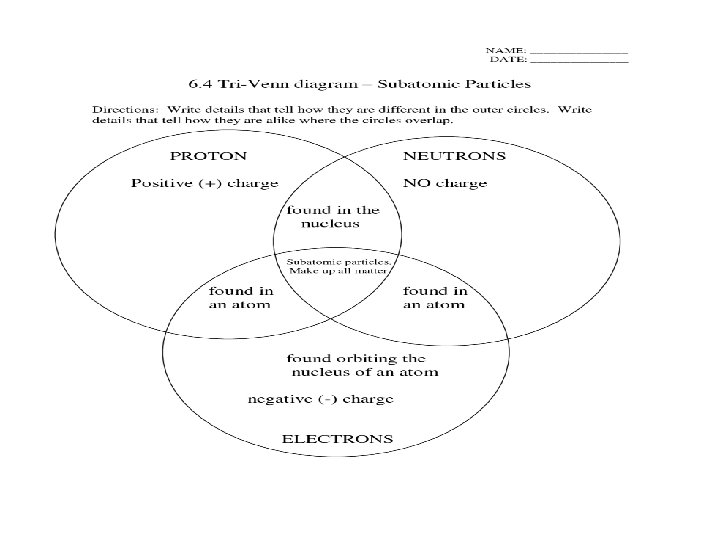
Atomic Components • The atom is made up of different parts – Protons • Large particles, found in nucleus • Positively charged – Neutrons • Large particles, found in nucleus • No charge – Electrons • Tiny particles, found outside of nucleus • Negatively charged

Quarks • Theorized particles that make up protons and neutrons – 6 different quarks have been discovered • The theory is that an arrangement of 3 quarks held together with a strong nuclear force produces a proton • The search for the components of protons and neutrons is an ongoing effort • Atomic Particles

Finding Quarks • To study quarks, scientists accelerate charged particles to tremendous speeds and then force them to smash into protons • Electric and magnetic fields are used to accelerate, focus, and smash the particles. • This causes the proton to break apart. • Particles from the collision are detected and reconstructed like crime scene data.

Models • There have been many changes to the model of the atom over history • It was not always known that all matter was composed of atoms – Aristotle disputed Democritus’ theory that matter is composed of smaller particles

Democritus Model • Atom could not be subdivided – Named it atomos, meaning uncuttable

John Dalton • Dalton offered proof that the atom existed • Dalton’s model was similar to Democritus • Dalton’s ideas gave a physical explanation for chemical reactions

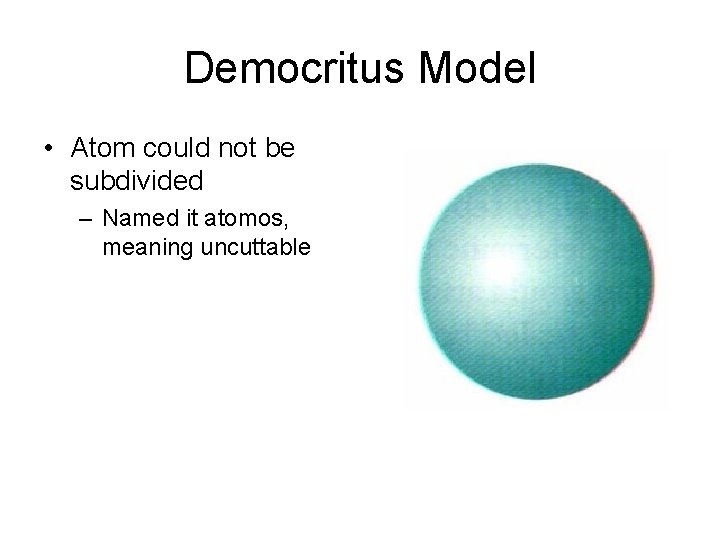
Thomson Model - 1904 • Electrons embedded in a positively charged sphere • Called the plum pudding model.

Rutherford Gold Foil Demonstration • Click on the following: • https: //www. youtube. com/watch? v=XBq. Hk raf 8 i. E

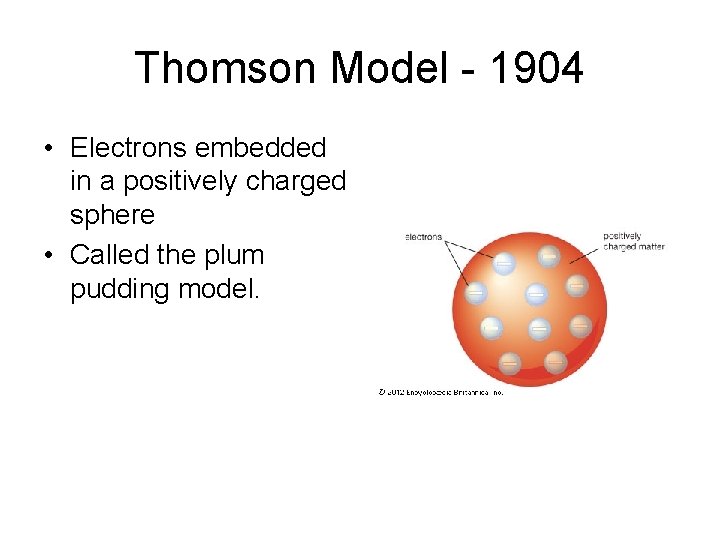
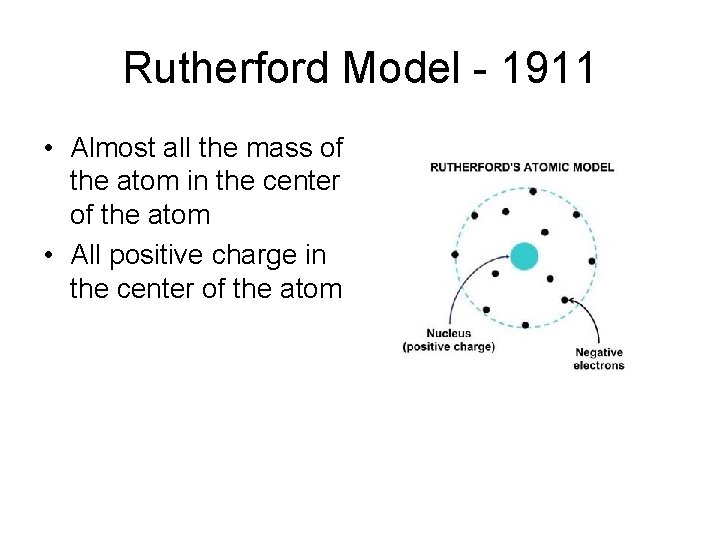
Rutherford Model - 1911 • Almost all the mass of the atom in the center of the atom • All positive charge in the center of the atom

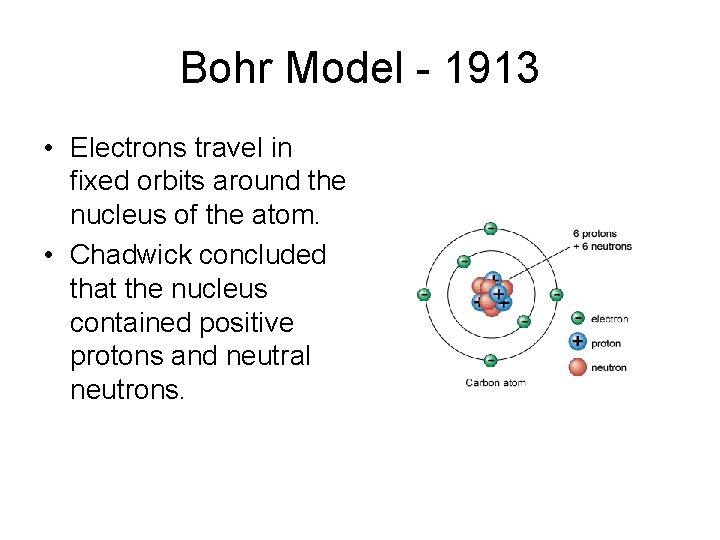
Bohr Model - 1913 • Electrons travel in fixed orbits around the nucleus of the atom. • Chadwick concluded that the nucleus contained positive protons and neutral neutrons.

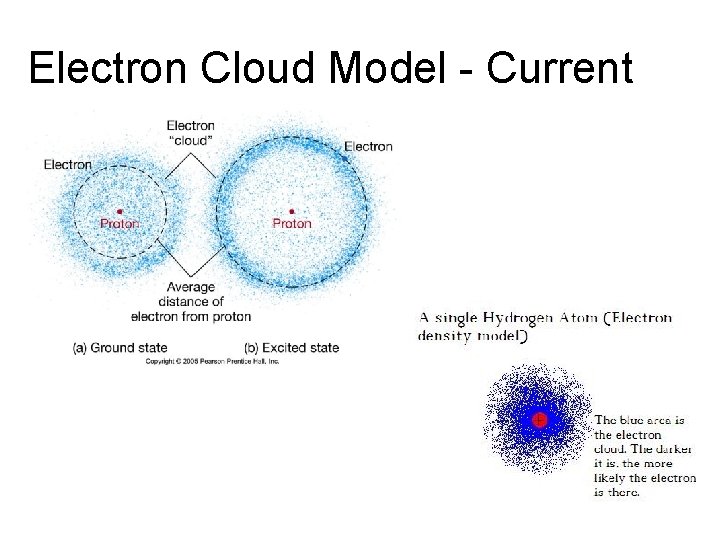
Electron Cloud Model - Current

Electron Cloud Model - 1926 • Electron Cloud is the area around the nucleus where electrons are likely found. • Electron Cloud is 100, 000 times larger than the diameter of the nucleus. Imagine a baseball field. An orange at the pitcher’s mound would be the nucleus and the rest of the field would be the electron cloud. • Electrons are moving so fast they are like spokes on a moving bicycle wheel.


Compare Protons and Neutrons Protons 1. 2. 3. 4. Large particles Notes 5. Compare Protons/Neutrons 1. 2. 3. 4. Large particles Notes

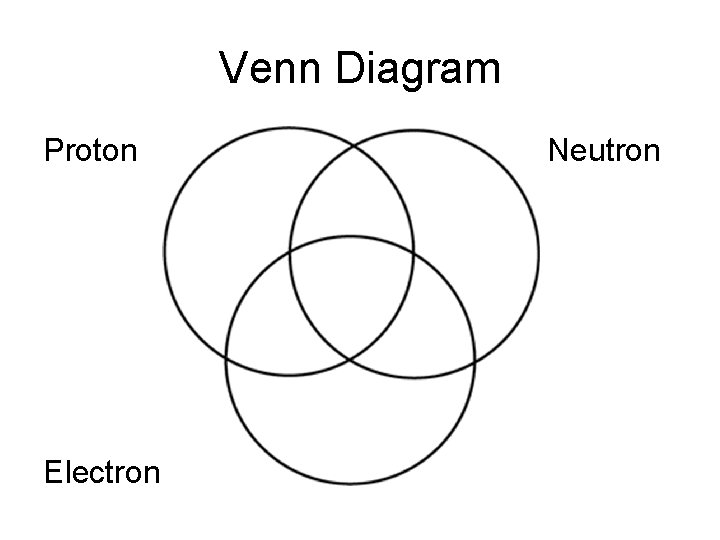
Venn Diagram Proton Electron Neutron


Ch 19. 1 Analysis: 1 paragraph 1. List chemical symbols for the elements carbon, aluminum, sodium, and sulfur. 2. Identify the names, charges, and location of the 3 main kinds of particles in an atom 3. Identify the smallest particle of matter. How were they discovered? 4. Describe the electron cloud model of the atom.

Chapter 19 Section 2 Masses of Atoms

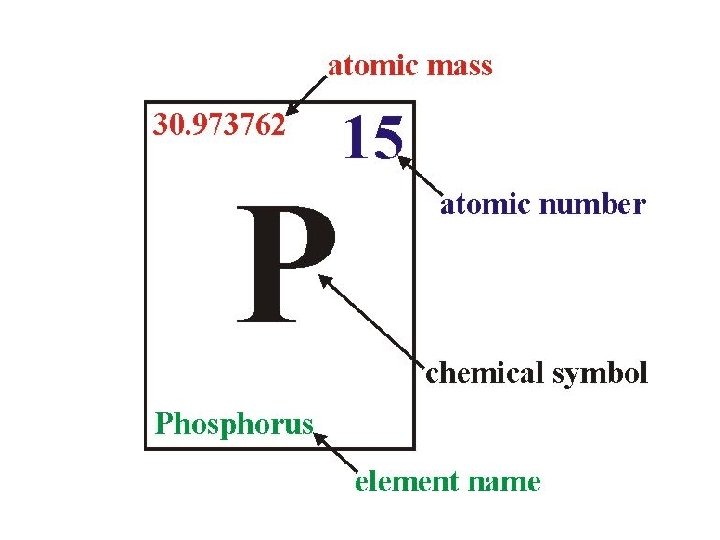
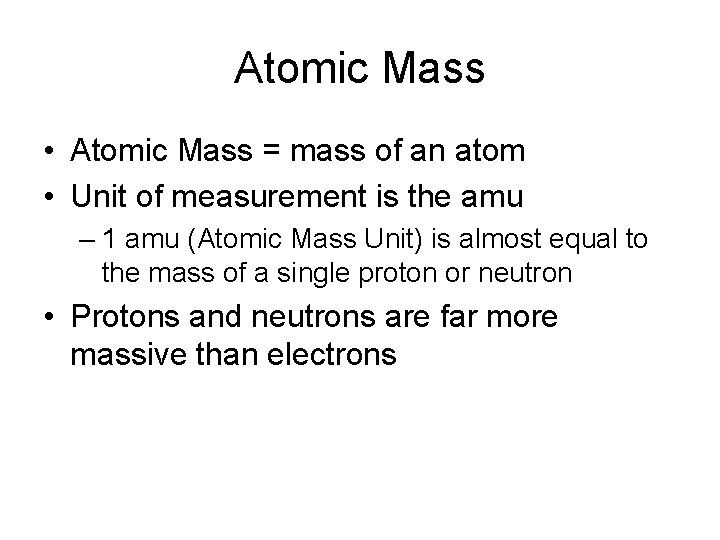
Atomic Mass • Atomic Mass = mass of an atom • Unit of measurement is the amu – 1 amu (Atomic Mass Unit) is almost equal to the mass of a single proton or neutron • Protons and neutrons are far more massive than electrons

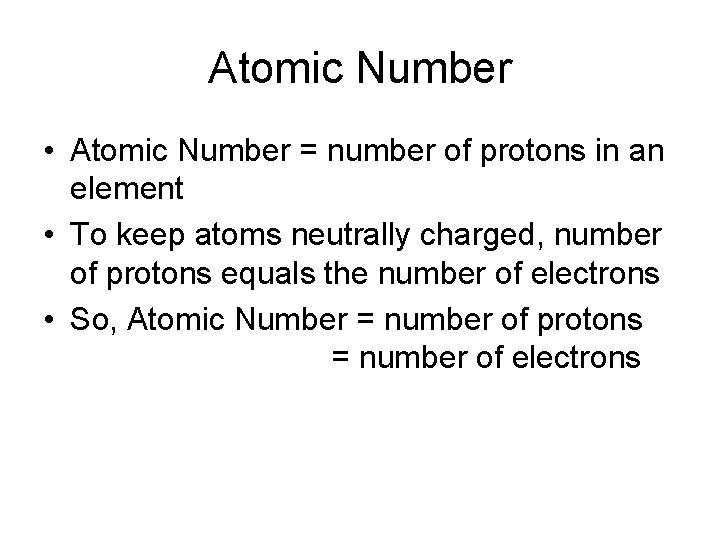
Atomic Number • Atomic Number = number of protons in an element • To keep atoms neutrally charged, number of protons equals the number of electrons • So, Atomic Number = number of protons = number of electrons

Mass Number • The Mass Number can be used to find the number of neutrons in an element. – Mass Number = sum of protons and neutrons – Neutrons = Mass Number – Atomic Number

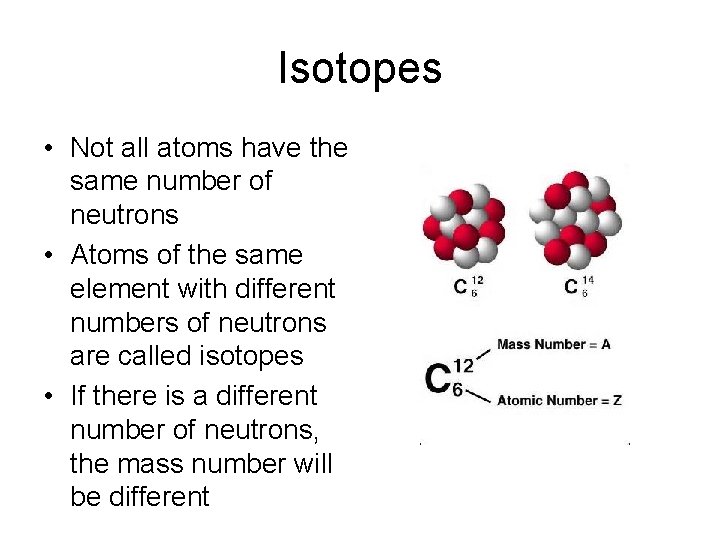
Isotopes • Not all atoms have the same number of neutrons • Atoms of the same element with different numbers of neutrons are called isotopes • If there is a different number of neutrons, the mass number will be different

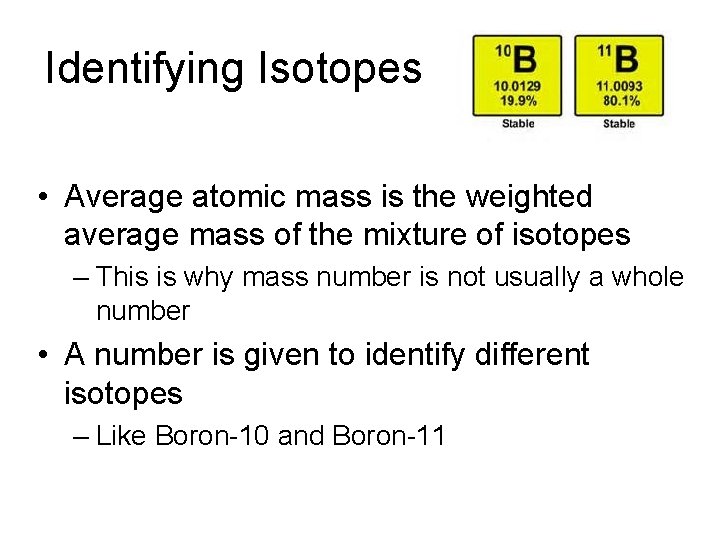
Identifying Isotopes • Average atomic mass is the weighted average mass of the mixture of isotopes – This is why mass number is not usually a whole number • A number is given to identify different isotopes – Like Boron-10 and Boron-11

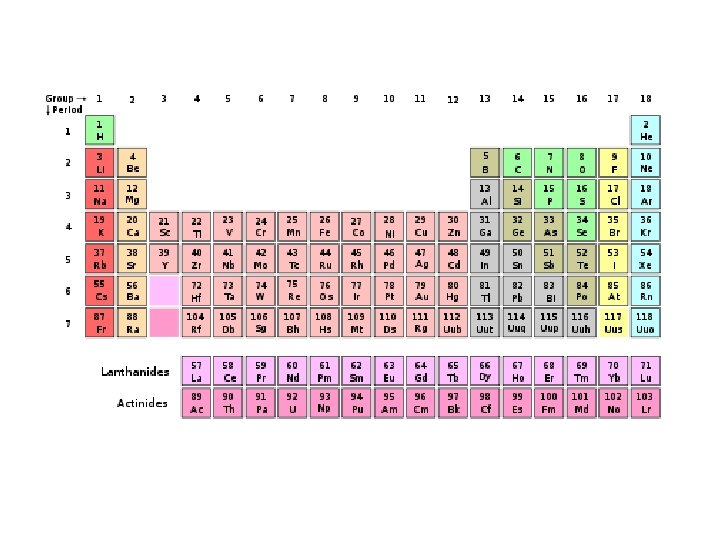
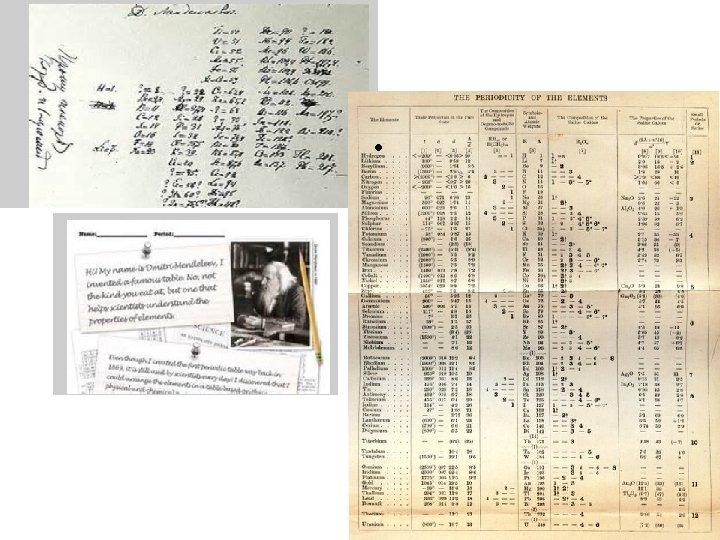
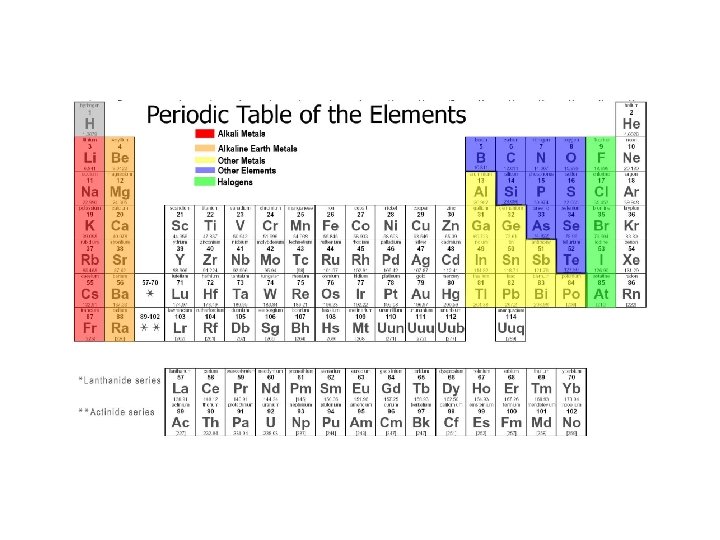
Periodic Table of Elements • Organized chart of the elements • Elements are in order by atomic number • Also arranged by differences in physical and chemical properties • Groups or Families in vertical columns have elements with similar properties. • Periods are in horizontal rows.

Dmitri Mendeleev • Father of the periodic table noticed trends in elements that were periodic, or repeated. • Arranged the first periodic table by atomic mass in 1869. • Left blanks for missing elements AND predicted their properties.


Henry Mosely • Mosely made some changes to the periodic table • He arranged all atoms by increasing atomic numbers instead of atomic masses


Electron Cloud Structure • Electrons within the electron cloud have different amounts of energy – The closer an electron is to the nucleus, the less energy it has. • Elements in a group have same number of electrons in the outer valence energy level – This determines properties of the elements

Electron Dot Diagram • The outer shell is important in determining chemical properties of elements. • Electron dot structures use dots around the symbols of the elements to represent the electrons in the outer shell. • Electron dot structures can also be used to show elements bond.

Valence Electrons • Valence electrons are the electrons in the highest occupied energy level of the atom. Usually they are in the outer shell. • Valence electrons are the only electrons generally involved in bond formation.

Groups and Periods • Each element in a Group or Family column has the same number of valence electrons in their outer energy level. • Generally, each Period row represents an energy level where electrons may be found

Energy Levels 1 – 7 (p 592) • Atoms want to be stable by filling their electron energy levels. • The 1 st energy level nearest the nucleus may contain a maximum of 2 electrons. • The 2 nd and 3 rd energy levels may contain a maximum of 8 electrons. • Beyond that, energy levels may contain 18 or even 32 electrons.

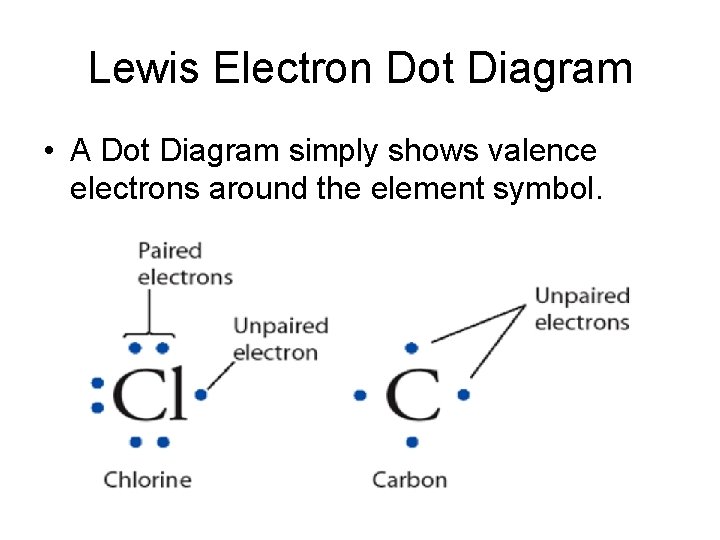
Lewis Electron Dot Diagram • A Dot Diagram simply shows valence electrons around the element symbol.

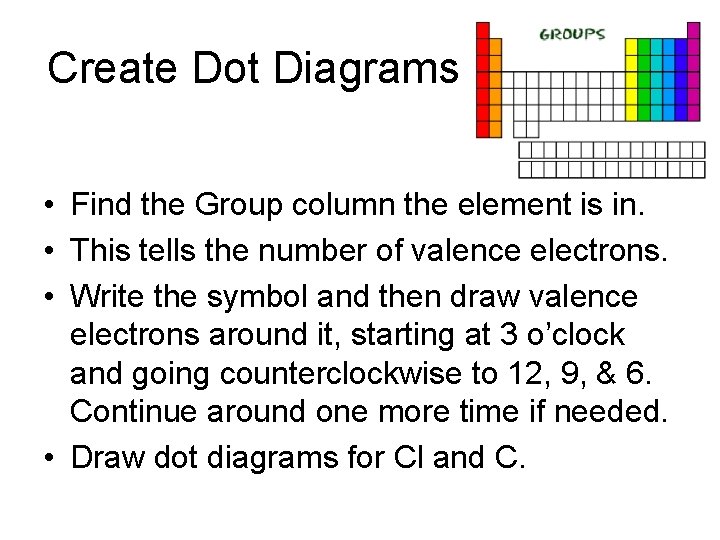
Create Dot Diagrams • Find the Group column the element is in. • This tells the number of valence electrons. • Write the symbol and then draw valence electrons around it, starting at 3 o’clock and going counterclockwise to 12, 9, & 6. Continue around one more time if needed. • Draw dot diagrams for Cl and C.

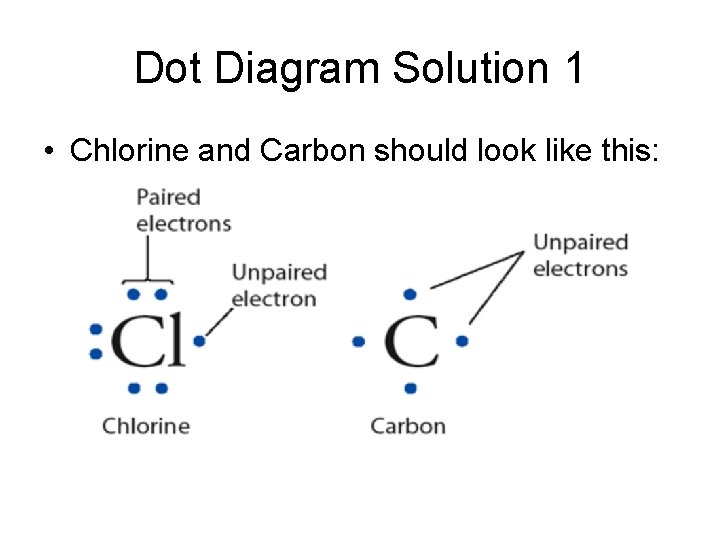
Dot Diagram Solution 1 • Chlorine and Carbon should look like this:

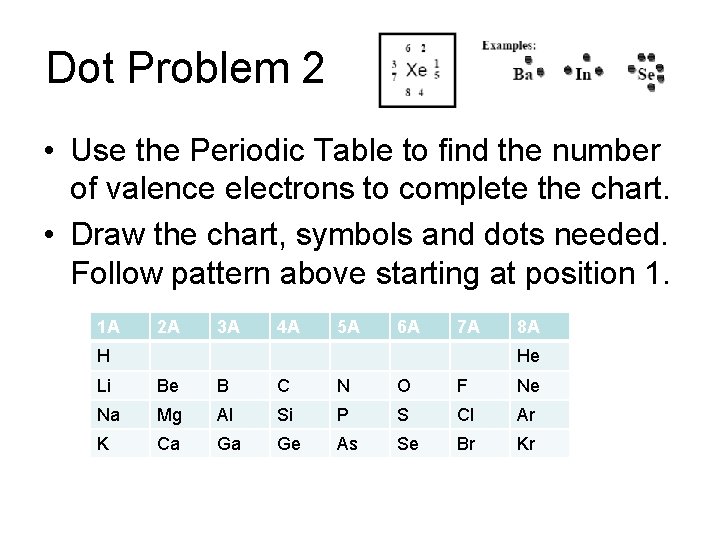
Dot Problem 2 • Use the Periodic Table to find the number of valence electrons to complete the chart. • Draw the chart, symbols and dots needed. Follow pattern above starting at position 1. 1 A 2 A 3 A 4 A 5 A 6 A 7 A H 8 A He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Ga Ge As Se Br Kr

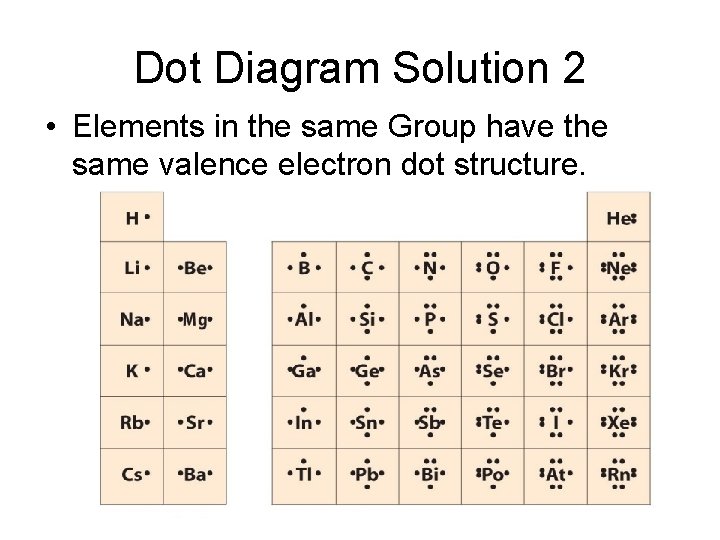
Dot Diagram Solution 2 • Elements in the same Group have the same valence electron dot structure.

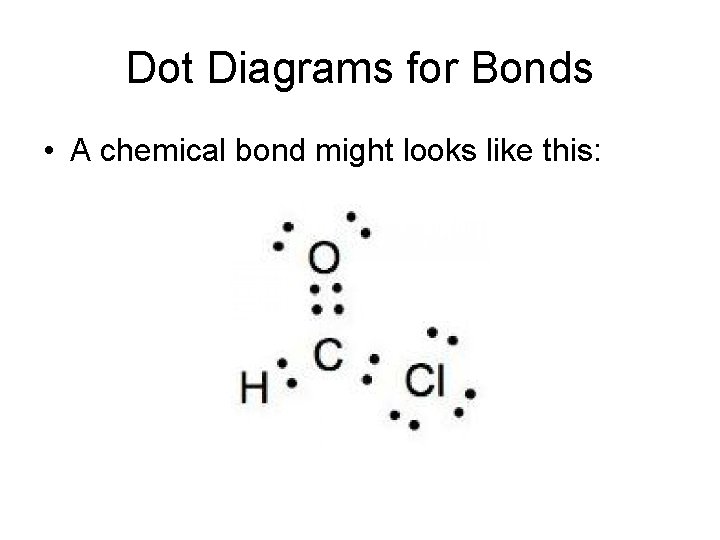
Dot Diagrams for Bonds • A chemical bond might looks like this:

Definitions • Alloy: solid solution (mixture) of metals • Aluminum: most abundant metal in Earth’s crust • Conductive: able to transfer materials like heat (thermal) and electricity easily • Ductile: able to be stretched (drawn) into wire • Ion: charged atom from electrons gained or lost • Luster: shiny and able to reflect light • Malleable: able to be shaped by hammer • Oxidation: rust caused by reacting with oxygen • Oxygen: most abundant element in Earth’s crust

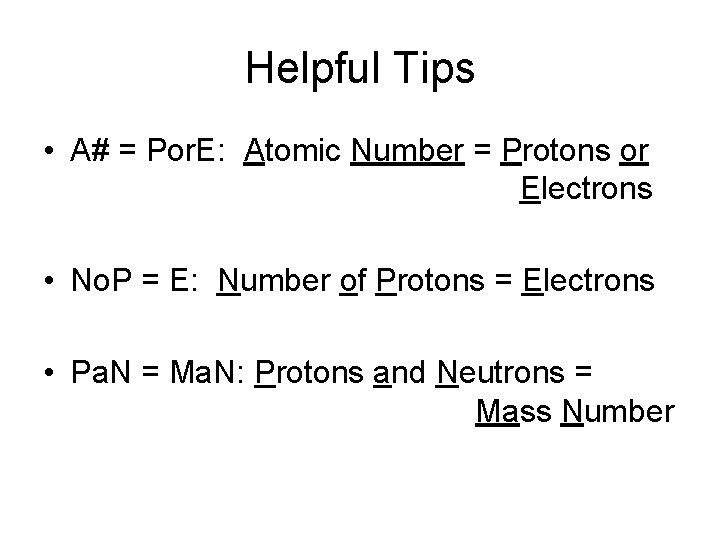
Helpful Tips • A# = Por. E: Atomic Number = Protons or Electrons • No. P = E: Number of Protons = Electrons • Pa. N = Ma. N: Protons and Neutrons = Mass Number

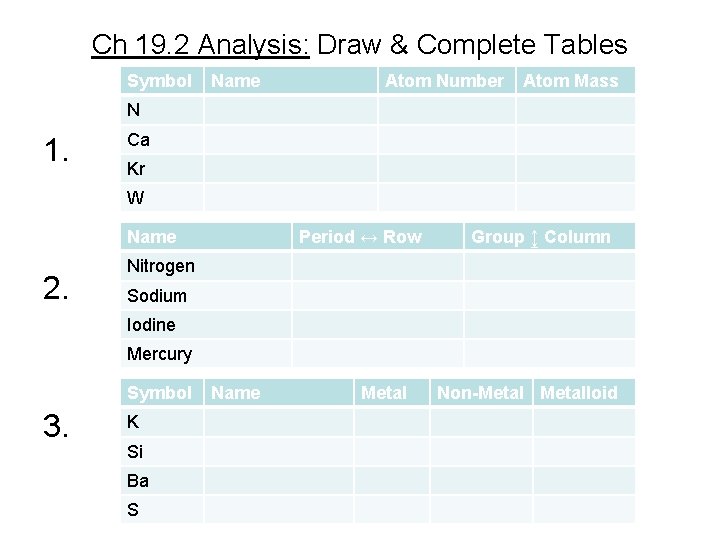
Ch 19. 2 Analysis: Make Tables 1. Use Periodic Table to find the name, atomic number, and atomic mass of the following elements: N, Ca, Kr, and W. 2. List the period and group where these elements are found: nitrogen, sodium, iodine, and mercury. 3. Give the name & classify each as metal, nonmetal, or a metalloid: K, Si, Ba, and S. See tables on next slide.

Ch 19. 2 Analysis: Draw & Complete Tables Symbol Name Atom Number Atom Mass N 1. Ca Kr W Name 2. Period ↔ Row Group ↨ Column Nitrogen Sodium Iodine Mercury Symbol 3. K Si Ba S Name Metal Non-Metalloid