Chapter 19 Second Law of Thermodynamics Time direction






- Slides: 30

Chapter 19 Second Law of Thermodynamics

Time direction • Irreversible processes – processes that cannot be reversed by means of small changes in their environment

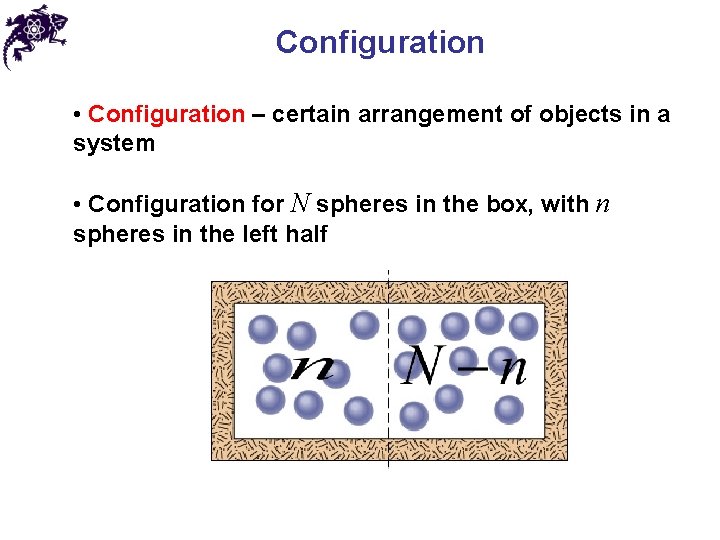
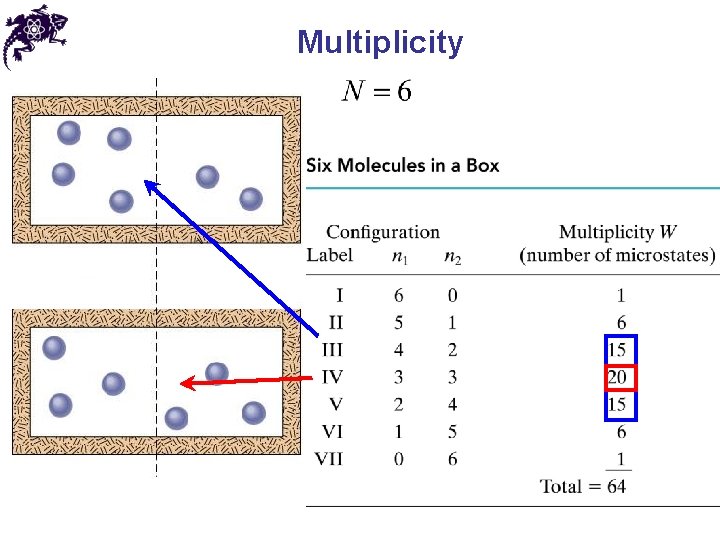
Configuration • Configuration – certain arrangement of objects in a system • Configuration for N spheres in the box, with n spheres in the left half

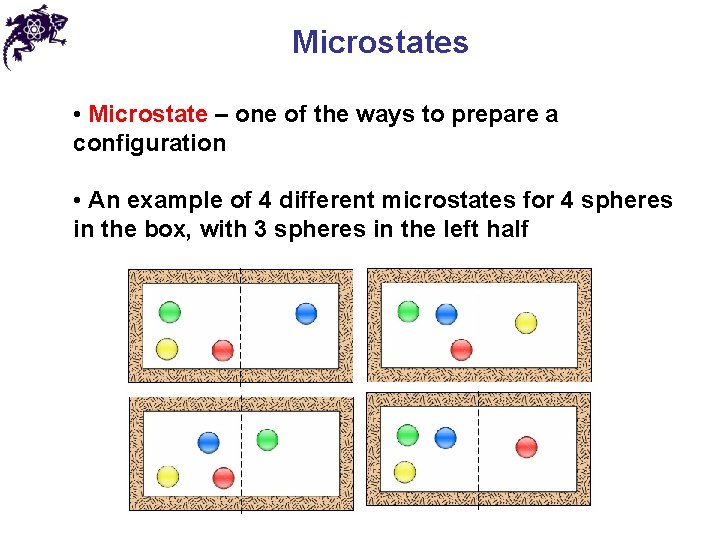
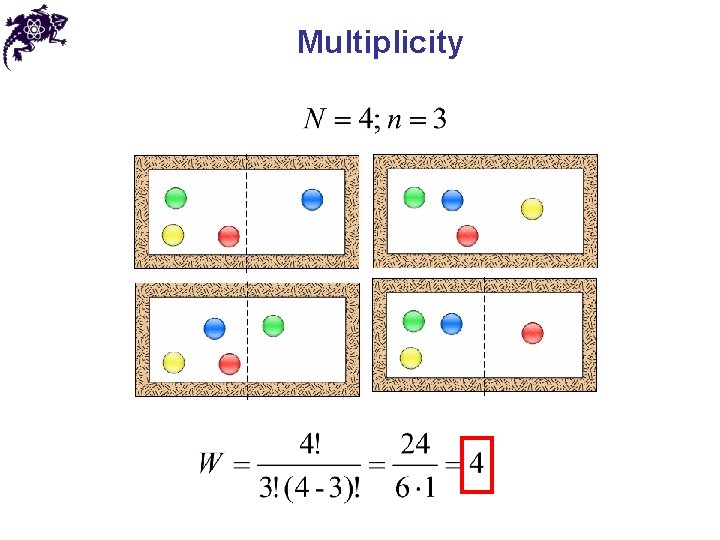
Microstates • Microstate – one of the ways to prepare a configuration • An example of 4 different microstates for 4 spheres in the box, with 3 spheres in the left half

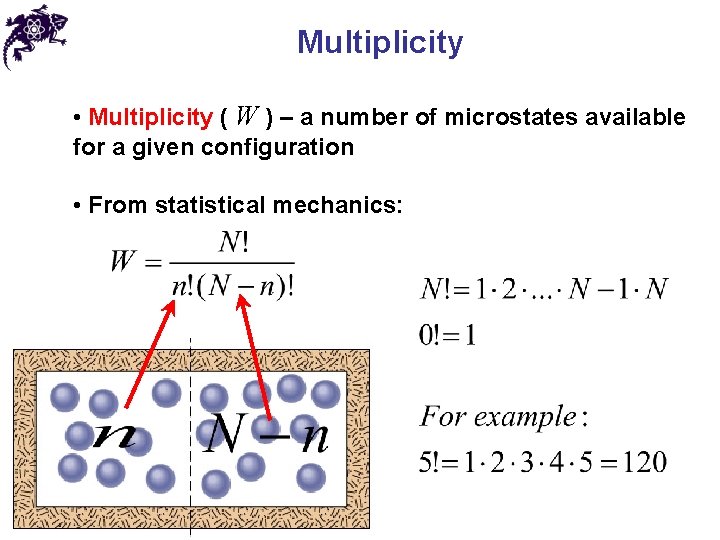
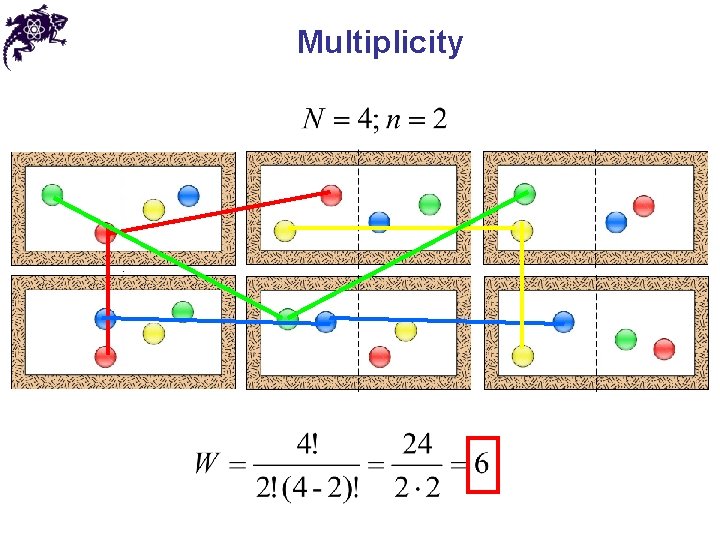
Multiplicity • Multiplicity ( W ) – a number of microstates available for a given configuration • From statistical mechanics:

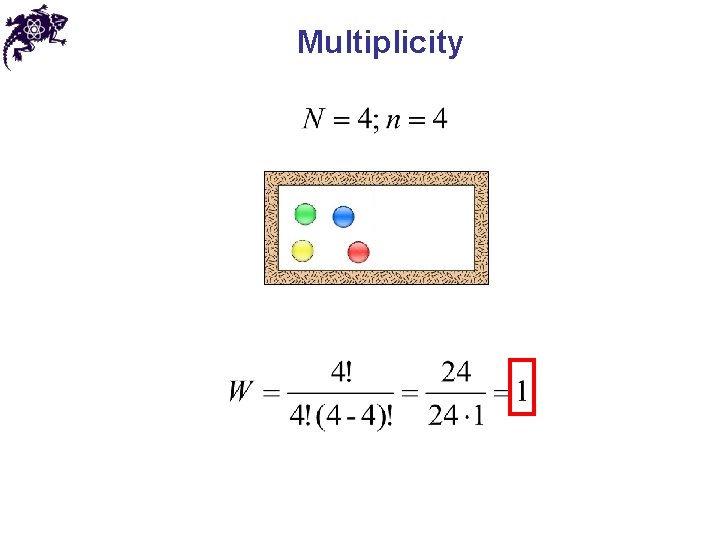
Multiplicity

Multiplicity

Multiplicity

Multiplicity

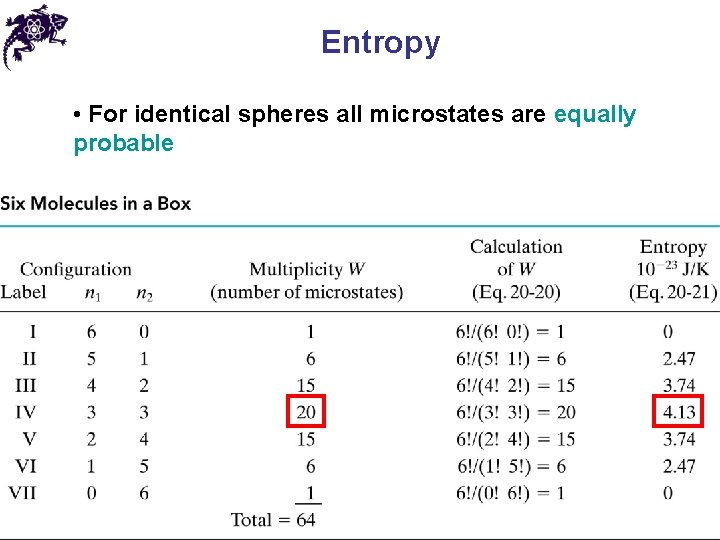
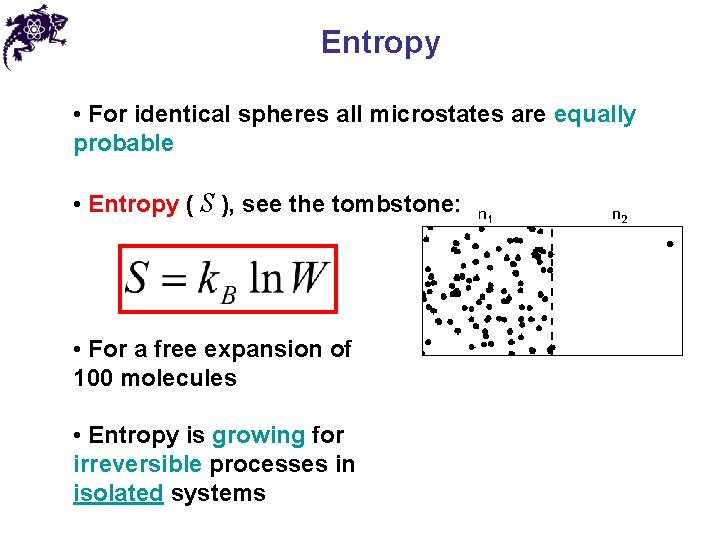
Entropy • For identical spheres all microstates are equally probable • Entropy ( S ), see the tombstone:

Entropy • For identical spheres all microstates are equally probable • Entropy ( S ), see the tombstone: • For a free expansion of 100 molecules • Entropy is growing for irreversible processes in isolated systems

Entropy • Entropy, loosely defined, is a measure of disorder in the system • Entropy is related to another fundamental concept – information. Alternative definition of irreversible processes – processes involving erasure of information • Entropy cannot noticeably decrease in isolated systems • Entropy has a tendency to increase in open systems

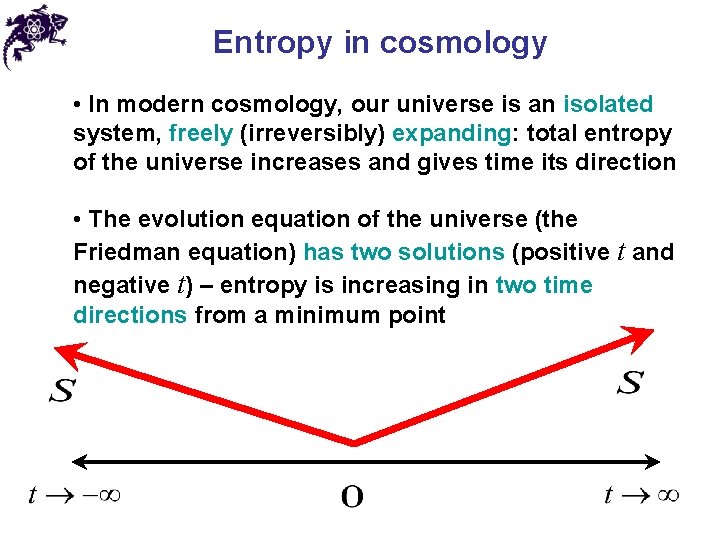
Entropy in cosmology • In modern cosmology, our universe is an isolated system, freely (irreversibly) expanding: total entropy of the universe increases and gives time its direction • The evolution equation of the universe (the Friedman equation) has two solutions (positive t and negative t) – entropy is increasing in two time directions from a minimum point

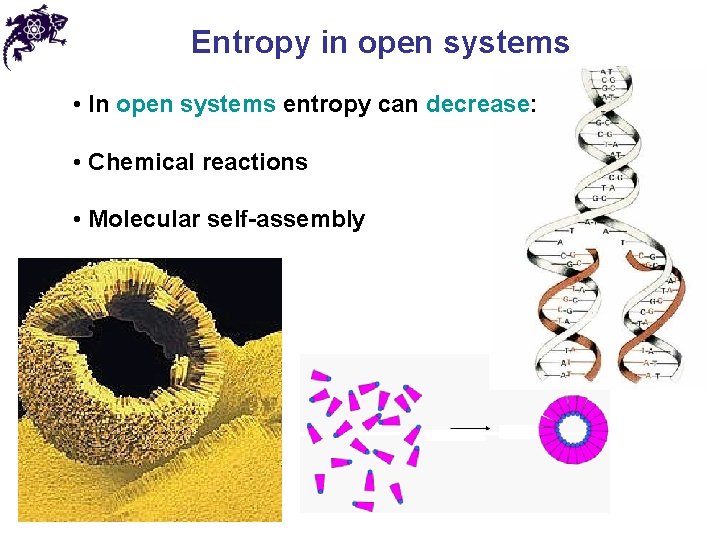
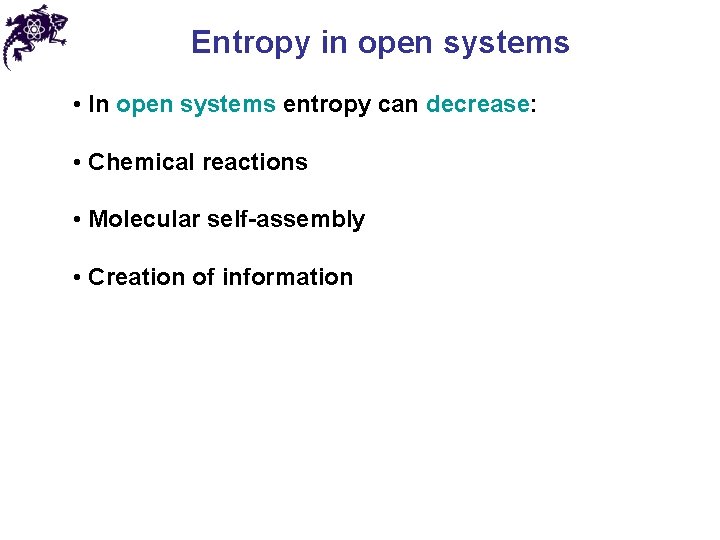
Entropy in open systems • In open systems entropy can decrease: • Chemical reactions

Entropy in open systems • In open systems entropy can decrease: • Chemical reactions • Molecular self-assembly

Entropy in open systems • In open systems entropy can decrease: • Chemical reactions • Molecular self-assembly • Creation of information

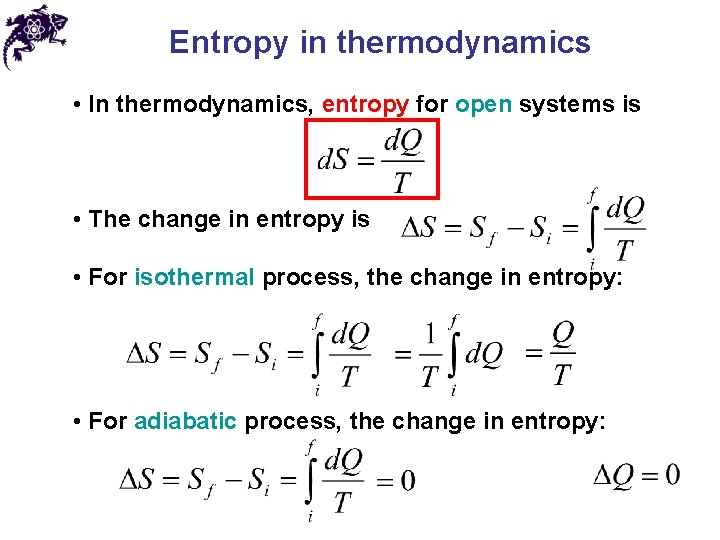
Entropy in thermodynamics • In thermodynamics, entropy for open systems is • The change in entropy is • For isothermal process, the change in entropy: • For adiabatic process, the change in entropy:

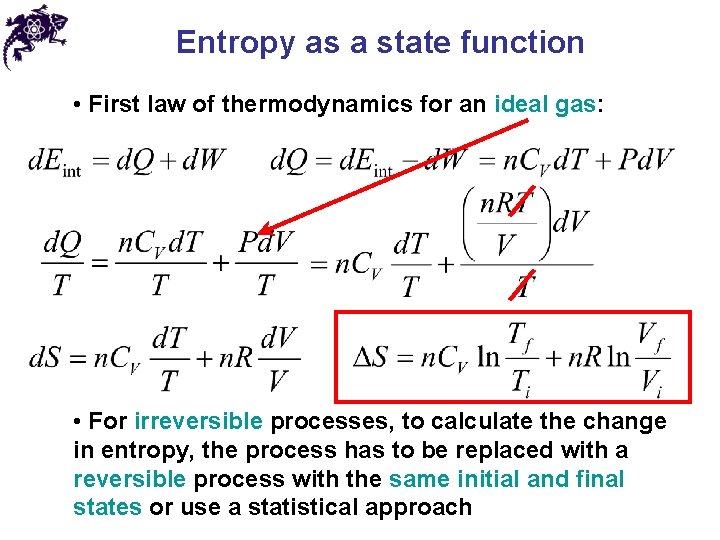
Entropy as a state function • First law of thermodynamics for an ideal gas: • For irreversible processes, to calculate the change in entropy, the process has to be replaced with a reversible process with the same initial and final states or use a statistical approach

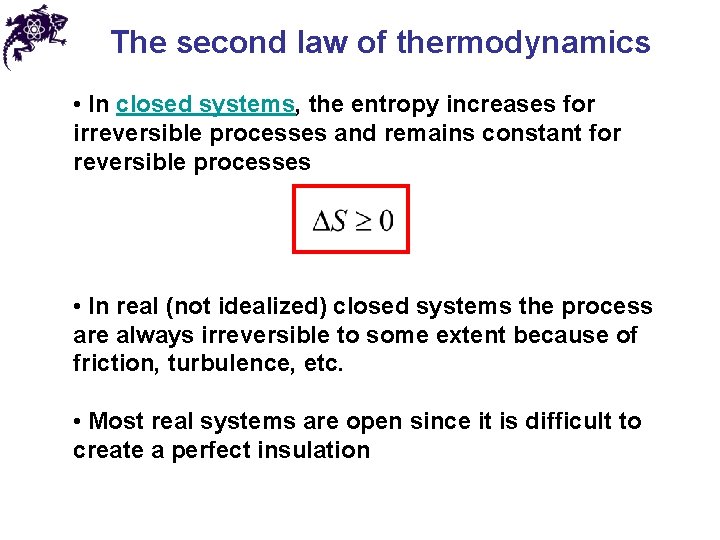
The second law of thermodynamics • In closed systems, the entropy increases for irreversible processes and remains constant for reversible processes • In real (not idealized) closed systems the process are always irreversible to some extent because of friction, turbulence, etc. • Most real systems are open since it is difficult to create a perfect insulation

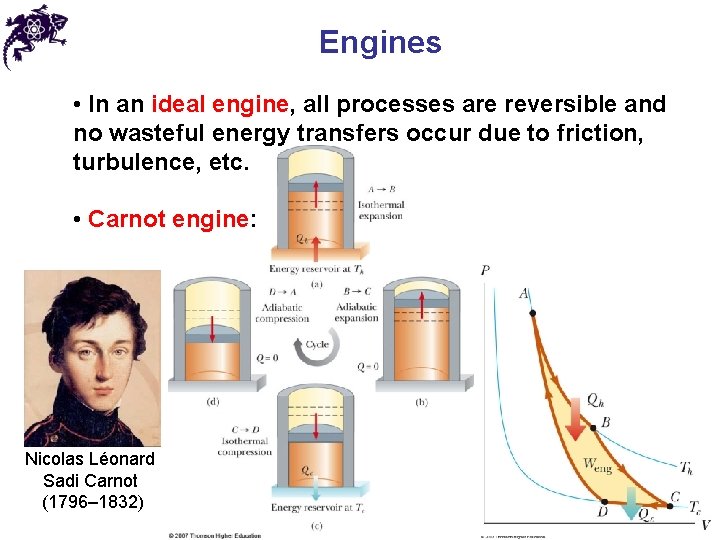
Engines • In an ideal engine, all processes are reversible and no wasteful energy transfers occur due to friction, turbulence, etc. • Carnot engine: Nicolas Léonard Sadi Carnot (1796– 1832)

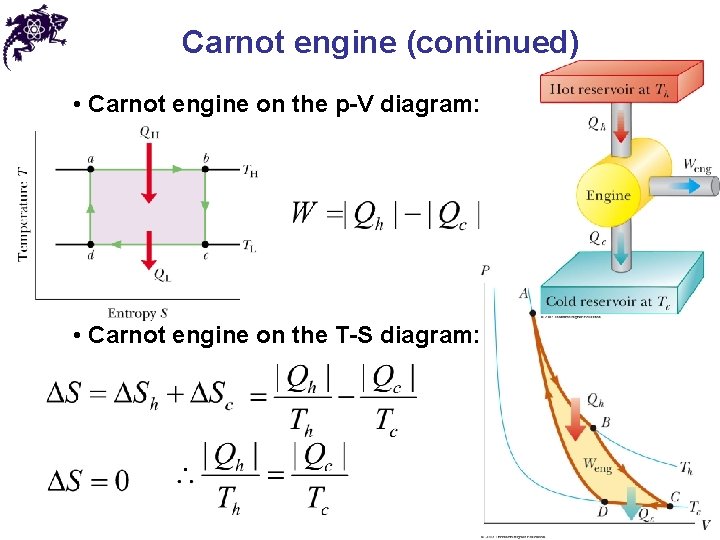
Carnot engine (continued) • Carnot engine on the p-V diagram: • Carnot engine on the T-S diagram:

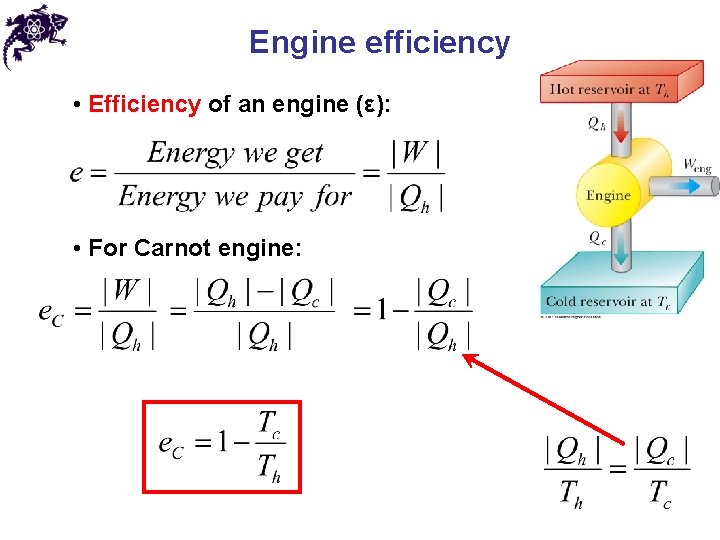
Engine efficiency • Efficiency of an engine (ε): • For Carnot engine:

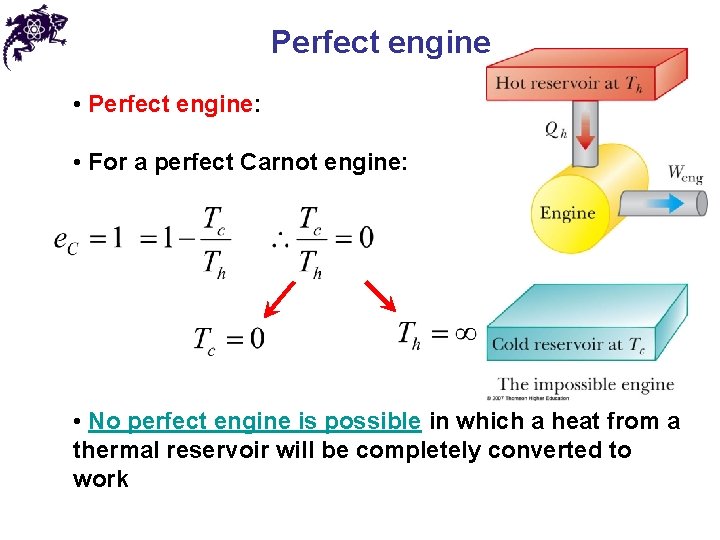
Perfect engine • Perfect engine: • For a perfect Carnot engine: • No perfect engine is possible in which a heat from a thermal reservoir will be completely converted to work

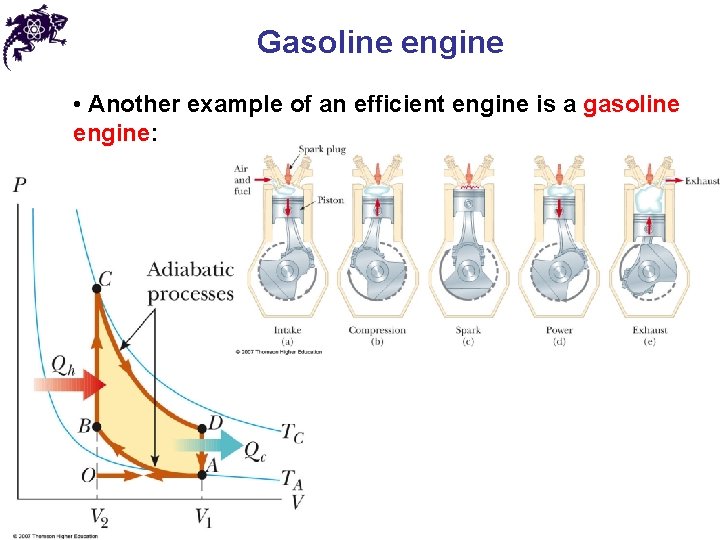
Gasoline engine • Another example of an efficient engine is a gasoline engine:

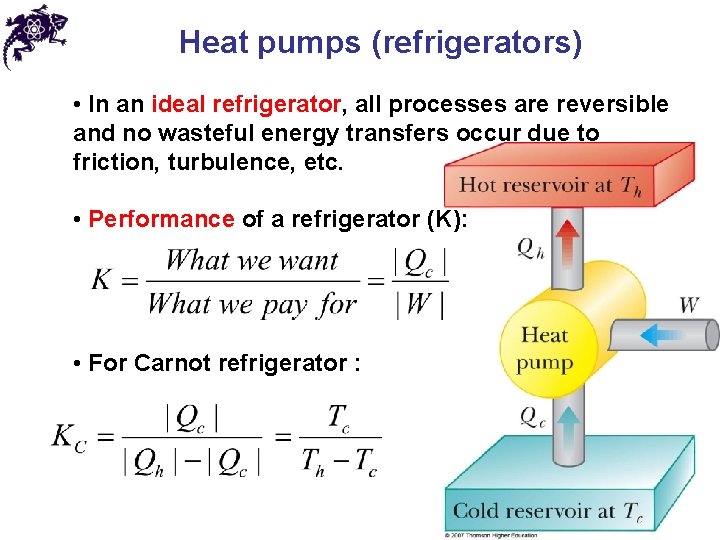
Heat pumps (refrigerators) • In an ideal refrigerator, all processes are reversible and no wasteful energy transfers occur due to friction, turbulence, etc. • Performance of a refrigerator (K): • For Carnot refrigerator :

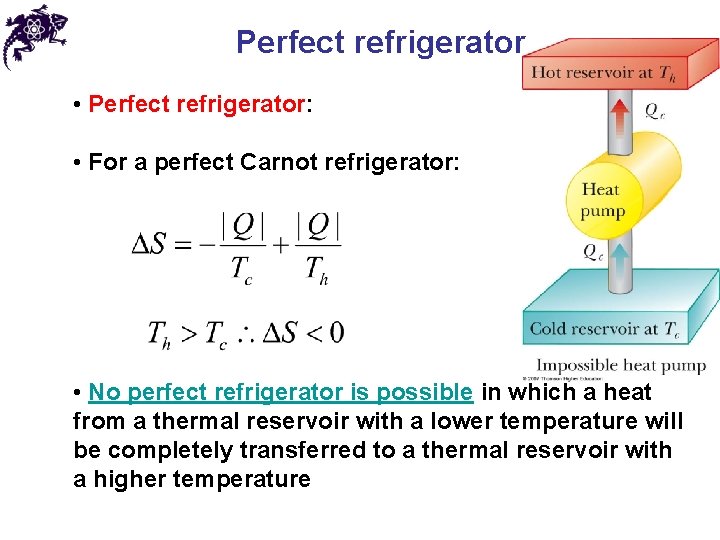
Perfect refrigerator • Perfect refrigerator: • For a perfect Carnot refrigerator: • No perfect refrigerator is possible in which a heat from a thermal reservoir with a lower temperature will be completely transferred to a thermal reservoir with a higher temperature

Questions?

Answers to the even-numbered problems Chapter 19 Problem 14 99. 95%

Answers to the even-numbered problems Chapter 19 Problem 22 280 J/K

Answers to the even-numbered problems Chapter 19 Problem 28 2. 3