CHAPTER 19 NOTES Part III p H and
![CHAPTER 19 NOTES: Part III: p. H and [H+] CHAPTER 19 NOTES: Part III: p. H and [H+]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-1.jpg)


![Kw = dissociation constant Kw =[H+]x[OH-] Kw =(1. 0 x 10 -7 M)x(1. 0 Kw = dissociation constant Kw =[H+]x[OH-] Kw =(1. 0 x 10 -7 M)x(1. 0](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-5.jpg)
![ACIDS, BASES and CONCENTRATION Even if a solution is acidic or basic, [H+][OH-] = ACIDS, BASES and CONCENTRATION Even if a solution is acidic or basic, [H+][OH-] =](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-6.jpg)
![[H+] and [OH-] Concentration Which solution is neutral? Which solution is acidic? Which solution [H+] and [OH-] Concentration Which solution is neutral? Which solution is acidic? Which solution](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-7.jpg)
![THE p. OH SCALE The p. OH scale measures the hydroxide ion concentration. Kw=[H+][OH-] THE p. OH SCALE The p. OH scale measures the hydroxide ion concentration. Kw=[H+][OH-]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-11.jpg)
![p. H/p. OH SQUARE [H+] = 10 -p. H [OH-] = 10 -p. OH p. H/p. OH SQUARE [H+] = 10 -p. H [OH-] = 10 -p. OH](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-12.jpg)
![PRACTICE PROBLEM #1 What is the p. H of a solution where [H+] = PRACTICE PROBLEM #1 What is the p. H of a solution where [H+] =](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-14.jpg)


![PRACTICE PROBLEM #4 What is the p. OH of a solution with a [H+] PRACTICE PROBLEM #4 What is the p. OH of a solution with a [H+]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-17.jpg)
![PRACTICE PROBLEM #5 Find [OH-], p. H and p. OH of a solution where PRACTICE PROBLEM #5 Find [OH-], p. H and p. OH of a solution where](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-18.jpg)
- Slides: 18
![CHAPTER 19 NOTES Part III p H and H CHAPTER 19 NOTES: Part III: p. H and [H+]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-1.jpg)
CHAPTER 19 NOTES: Part III: p. H and [H+]

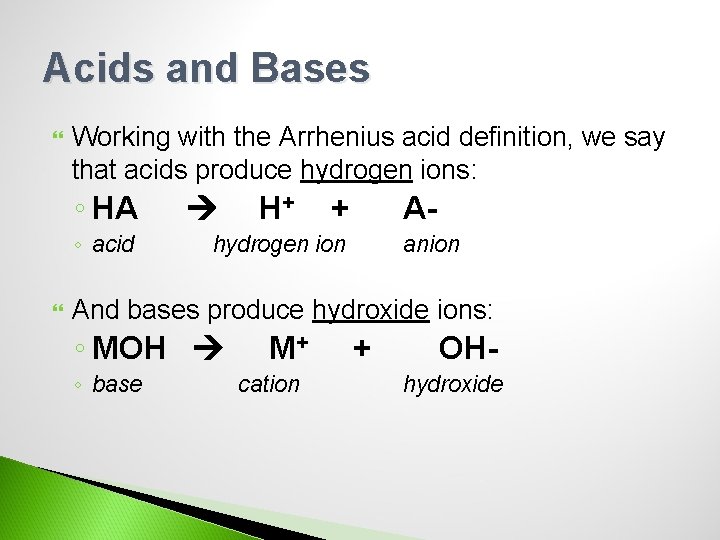
Acids and Bases Working with the Arrhenius acid definition, we say that acids produce hydrogen ions: ◦ HA ◦ acid H+ + A- hydrogen ion anion And bases produce hydroxide ions: ◦ MOH ◦ base M+ cation + OHhydroxide

Water, being made up of both H+ and OHcan dissociate giving us the formula: ◦ H 2 O H+ + OH- But it only does this once out of every 500, 000 molecules!

H 2 O We can measure how many H+ ions and OH- ions there are in regular water. ◦ [H+]=1 x 10 -7 M and [OH-]=1 x 10 -7 M From this information, we can get a dissociation constant for water.
![Kw dissociation constant Kw HxOH Kw 1 0 x 10 7 Mx1 0 Kw = dissociation constant Kw =[H+]x[OH-] Kw =(1. 0 x 10 -7 M)x(1. 0](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-5.jpg)
Kw = dissociation constant Kw =[H+]x[OH-] Kw =(1. 0 x 10 -7 M)x(1. 0 x 10 -7 M) Kw = 1. 0 x 10 -14
![ACIDS BASES and CONCENTRATION Even if a solution is acidic or basic HOH ACIDS, BASES and CONCENTRATION Even if a solution is acidic or basic, [H+][OH-] =](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-6.jpg)
ACIDS, BASES and CONCENTRATION Even if a solution is acidic or basic, [H+][OH-] = 1 x 10 -14 Like a see-saw, raising or lowering the amount of [H+] will change the amount of [OH-] in solution
![H and OH Concentration Which solution is neutral Which solution is acidic Which solution [H+] and [OH-] Concentration Which solution is neutral? Which solution is acidic? Which solution](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-7.jpg)
[H+] and [OH-] Concentration Which solution is neutral? Which solution is acidic? Which solution is basic?

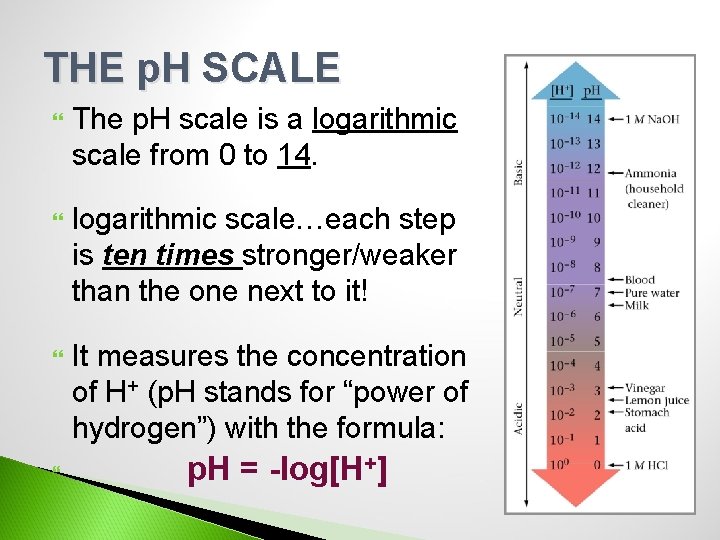
THE p. H SCALE The p. H scale is a logarithmic scale from 0 to 14. logarithmic scale…each step is ten times stronger/weaker than the one next to it! It measures the concentration of H+ (p. H stands for “power of hydrogen”) with the formula: p. H = -log[H+]

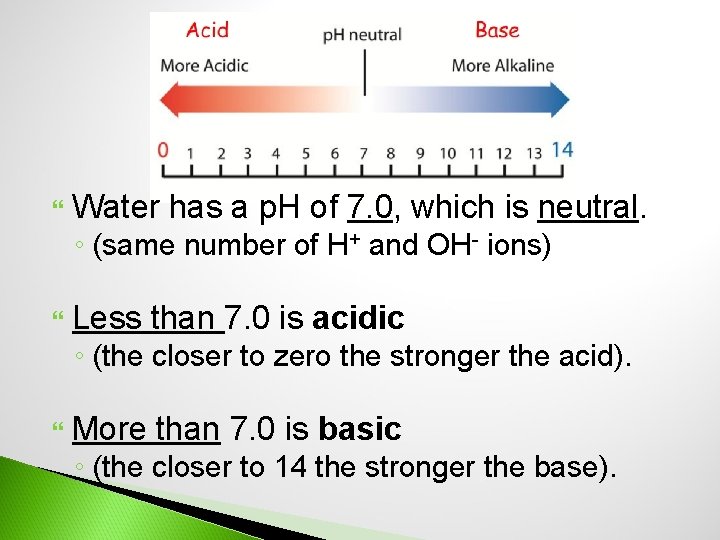
Water has a p. H of 7. 0, which is neutral. ◦ (same number of H+ and OH- ions) Less than 7. 0 is acidic ◦ (the closer to zero the stronger the acid). More than 7. 0 is basic ◦ (the closer to 14 the stronger the base).

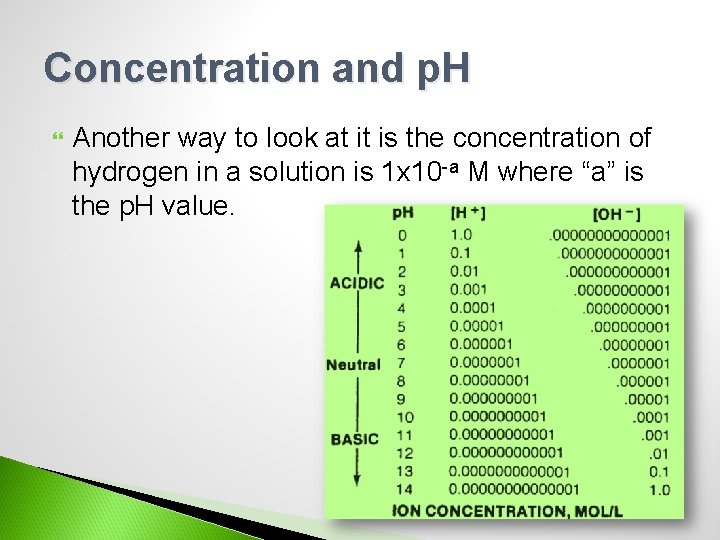
Concentration and p. H Another way to look at it is the concentration of hydrogen in a solution is 1 x 10 -a M where “a” is the p. H value.
![THE p OH SCALE The p OH scale measures the hydroxide ion concentration KwHOH THE p. OH SCALE The p. OH scale measures the hydroxide ion concentration. Kw=[H+][OH-]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-11.jpg)
THE p. OH SCALE The p. OH scale measures the hydroxide ion concentration. Kw=[H+][OH-] = 1 x 10 -14 14 = p. H + p. OH Therefore, you can solve for p. H, p. OH and [OH-] given just [H+].
![p Hp OH SQUARE H 10 p H OH 10 p OH p. H/p. OH SQUARE [H+] = 10 -p. H [OH-] = 10 -p. OH](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-12.jpg)
p. H/p. OH SQUARE [H+] = 10 -p. H [OH-] = 10 -p. OH p. H= -log [H] p. OH= -log[OH-]

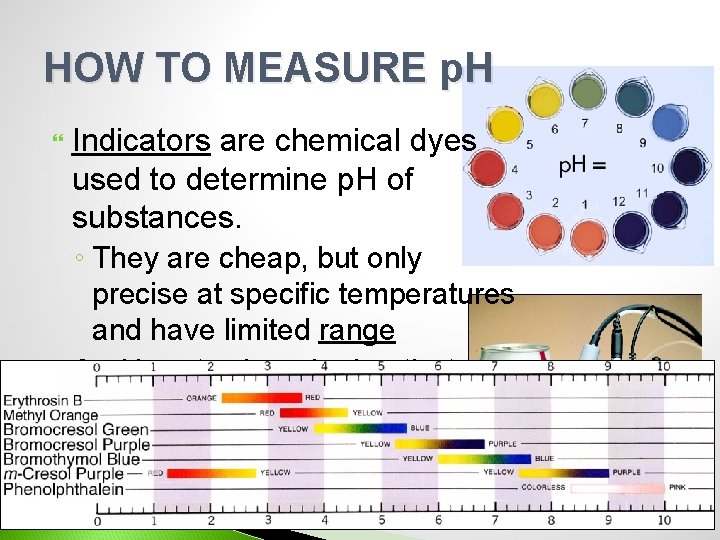
HOW TO MEASURE p. H Indicators are chemical dyes used to determine p. H of substances. ◦ They are cheap, but only precise at specific temperatures and have limited range A p. H meter is a device that uses two electrodes to measure p. H.
![PRACTICE PROBLEM 1 What is the p H of a solution where H PRACTICE PROBLEM #1 What is the p. H of a solution where [H+] =](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-14.jpg)
PRACTICE PROBLEM #1 What is the p. H of a solution where [H+] = 1. 0 x 10 -3 M?

PRACTICE PROBLEM #2 What is the hydrogen ion concentration of a solution with a p. H of 9. 6?

PRACTICE PROBLEM #3 What is the p. OH of a solution with a p. H of 6. 2?
![PRACTICE PROBLEM 4 What is the p OH of a solution with a H PRACTICE PROBLEM #4 What is the p. OH of a solution with a [H+]](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-17.jpg)
PRACTICE PROBLEM #4 What is the p. OH of a solution with a [H+] = 2. 9 x 10 -11 M?
![PRACTICE PROBLEM 5 Find OH p H and p OH of a solution where PRACTICE PROBLEM #5 Find [OH-], p. H and p. OH of a solution where](https://slidetodoc.com/presentation_image_h2/6385b185e590eb8e8a1d9899b44fbe2d/image-18.jpg)
PRACTICE PROBLEM #5 Find [OH-], p. H and p. OH of a solution where [H+]=7. 2 x 10 -2 M.