Chapter 19 Eukaryotic Genomes Organization Regulation Evolution Chapter
- Slides: 27

Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution

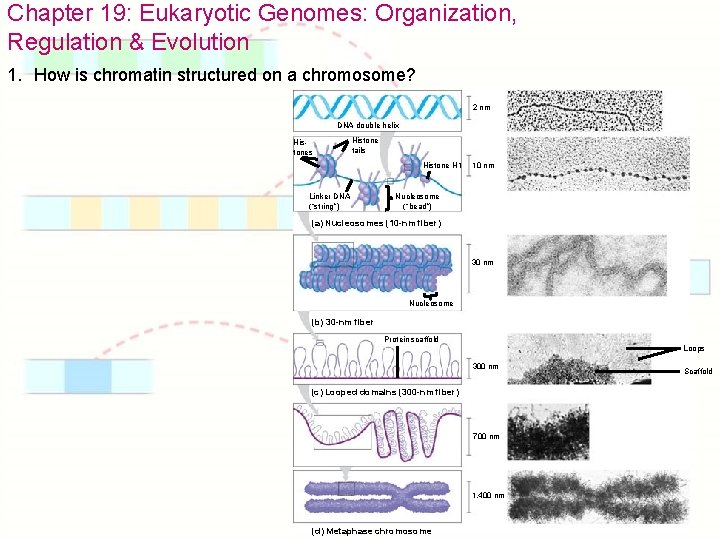
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. How is chromatin structured on a chromosome? 2 nm DNA double helix Histones Histone tails Histone H 1 Linker DNA (“string”) 10 nm Nucleosome (“bead”) (a) Nucleosomes (10 -nm fiber) 30 nm Nucleosome (b) 30 -nm fiber Protein scaffold Loops 300 nm (c) Looped domains (300 -nm fiber) 700 nm 1, 400 nm (d) Metaphase chromosome Scaffold

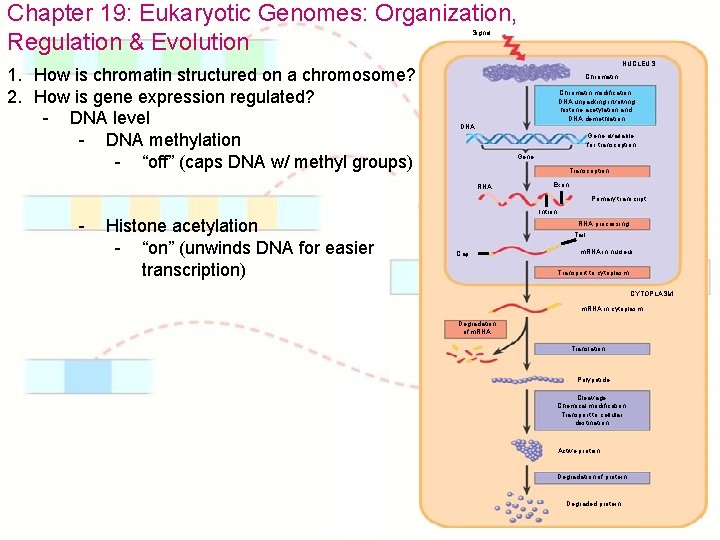
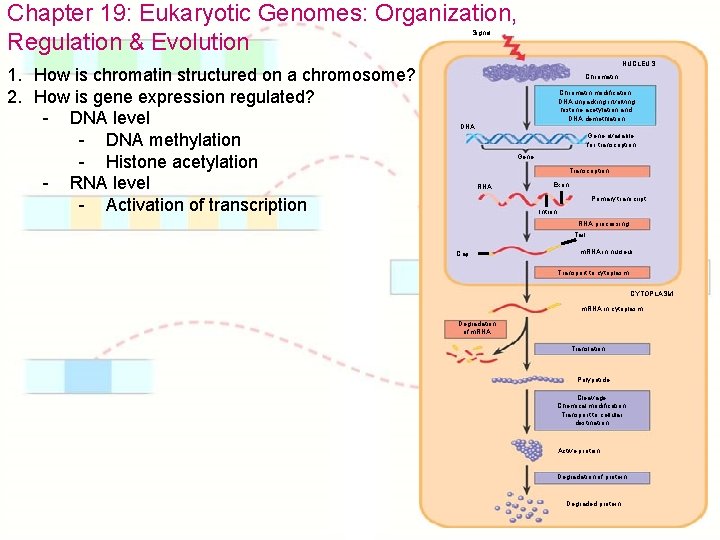
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution Signal 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? - DNA level - DNA methylation - “off” (caps DNA w/ methyl groups) NUCLEUS Chromatin modification: DNA unpacking involving histone acetylation and DNA demethlation DNA Gene available for transcription Gene Transcription RNA Exon Primary transcript Intron - Histone acetylation - “on” (unwinds DNA for easier transcription) RNA processing Tail Cap m. RNA in nucleus Transport to cytoplasm CYTOPLASM m. RNA in cytoplasm Degradation of m. RNA Translation Polypetide Cleavage Chemical modification Transport to cellular destination Active protein Degradation of protein Degraded protein

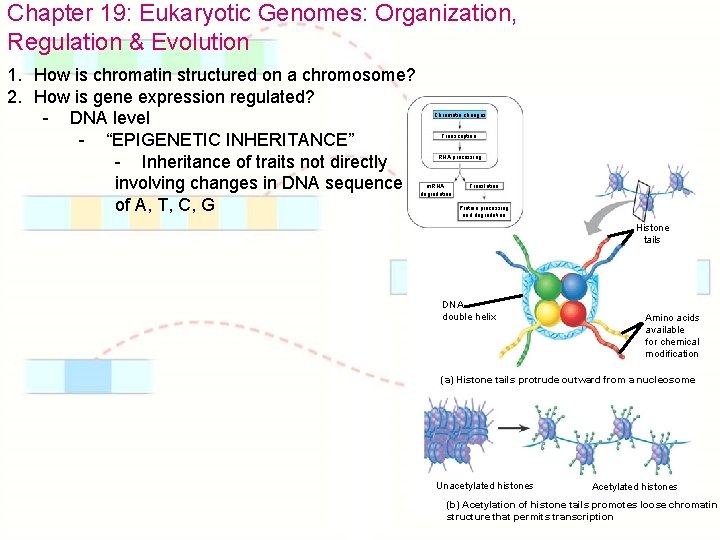
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? - DNA level - “EPIGENETIC INHERITANCE” - Inheritance of traits not directly involving changes in DNA sequence of A, T, C, G Chromatin changes Transcription RNA processing m. RNA degradation Translation Protein processing and degradation Histone tails DNA double helix Amino acids available for chemical modification (a) Histone tails protrude outward from a nucleosome Unacetylated histones Acetylated histones (b) Acetylation of histone tails promotes loose chromatin structure that permits transcription

Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution Signal 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? - DNA level - DNA methylation - Histone acetylation - RNA level - Activation of transcription NUCLEUS Chromatin modification: DNA unpacking involving histone acetylation and DNA demethlation DNA Gene available for transcription Gene Transcription RNA Exon Primary transcript Intron RNA processing Tail Cap m. RNA in nucleus Transport to cytoplasm CYTOPLASM m. RNA in cytoplasm Degradation of m. RNA Translation Polypetide Cleavage Chemical modification Transport to cellular destination Active protein Degradation of protein Degraded protein

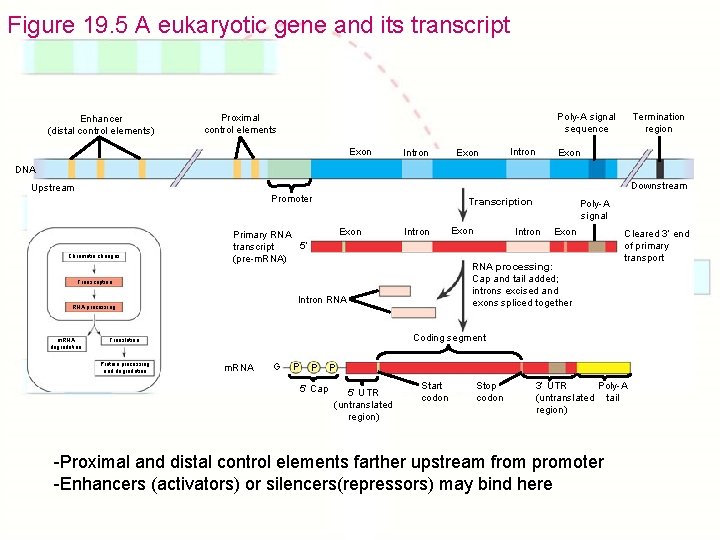
Figure 19. 5 A eukaryotic gene and its transcript Enhancer (distal control elements) Poly-A signal sequence Proximal control elements Exon Intron Termination region Exon DNA Downstream Upstream Promoter Chromatin changes Transcription Exon Primary RNA 5 transcript (pre-m. RNA) Intron m. RNA degradation Intron RNA Exon Cleared 3 end of primary transport Coding segment Translation Protein processing and degradation Intron RNA processing: Cap and tail added; introns excised and exons spliced together Transcription RNA processing Exon Poly-A signal m. RNA G P P 5 Cap P 5 UTR (untranslated region) Start codon Stop codon Poly-A 3 UTR (untranslated tail region) -Proximal and distal control elements farther upstream from promoter -Enhancers (activators) or silencers(repressors) may bind here

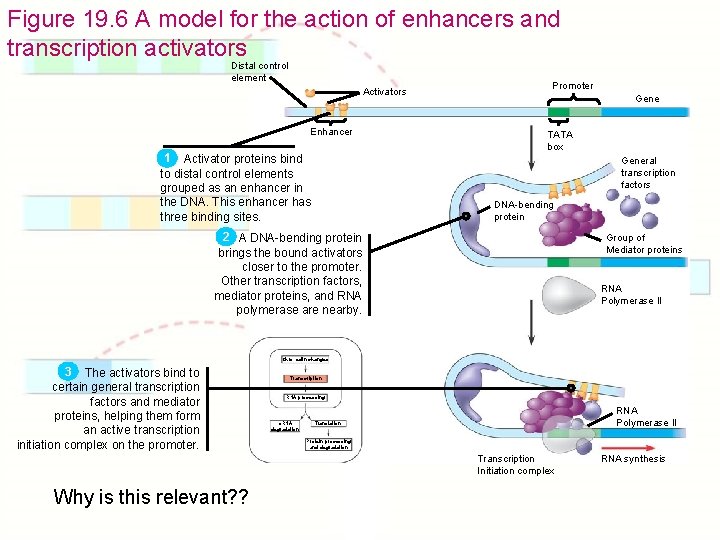
Figure 19. 6 A model for the action of enhancers and transcription activators Distal control element Activators Enhancer 1 Activator proteins bind to distal control elements grouped as an enhancer in the DNA. This enhancer has three binding sites. Promoter Gene TATA box General transcription factors DNA-bending protein 2 A DNA-bending protein brings the bound activators closer to the promoter. Other transcription factors, mediator proteins, and RNA polymerase are nearby. Group of Mediator proteins RNA Polymerase II Chromatin changes 3 The activators bind to certain general transcription factors and mediator proteins, helping them form an active transcription initiation complex on the promoter. Transcription RNA processing m. RNA degradation RNA Polymerase II Translation Protein processing and degradation Transcription Initiation complex Why is this relevant? ? RNA synthesis

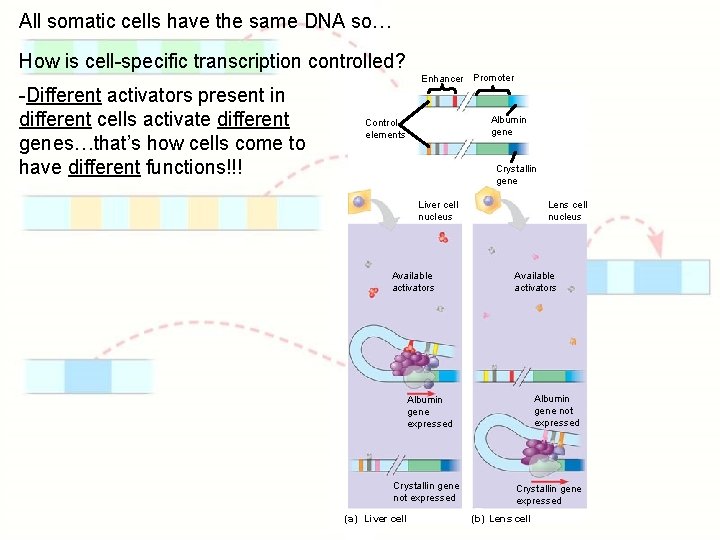
All somatic cells have the same DNA so… How is cell-specific transcription controlled? -Different activators present in different cells activate different genes…that’s how cells come to have different functions!!! Enhancer Promoter Albumin gene Control elements Crystallin gene Liver cell nucleus Available activators Lens cell nucleus Available activators Albumin gene not expressed Albumin gene expressed Crystallin gene not expressed (a) Liver cell Crystallin gene expressed (b) Lens cell

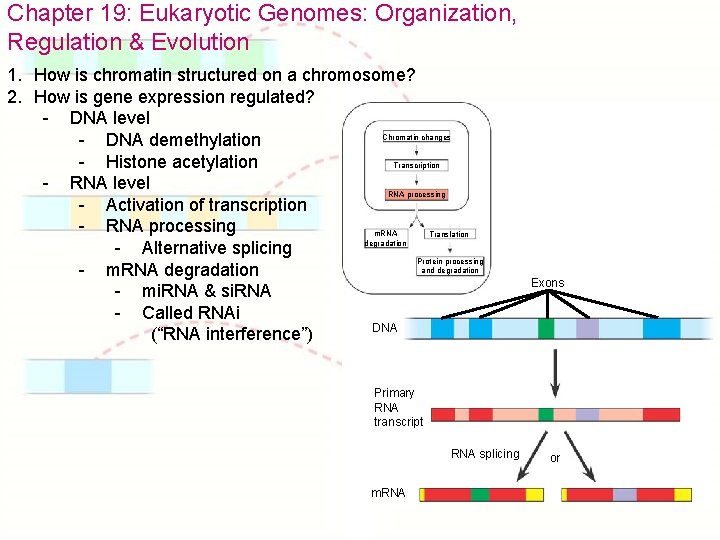
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? - DNA level Chromatin changes - DNA demethylation - Histone acetylation Transcription - RNA level RNA processing - Activation of transcription - RNA processing m. RNA Translation degradation - Alternative splicing Protein processing and degradation - m. RNA degradation - mi. RNA & si. RNA - Called RNAi DNA (“RNA interference”) Exons Primary RNA transcript RNA splicing m. RNA or

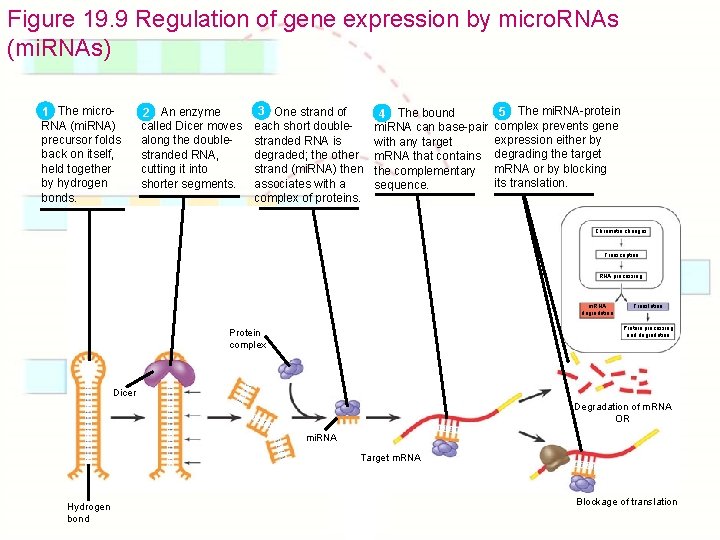
Figure 19. 9 Regulation of gene expression by micro. RNAs (mi. RNAs) The micro 1 RNA (mi. RNA) precursor folds back on itself, held together by hydrogen bonds. An enzyme 2 called Dicer moves along the doublestranded RNA, cutting it into shorter segments. 3 One strand of each short doublestranded RNA is degraded; the other strand (mi. RNA) then associates with a complex of proteins. The bound 4 mi. RNA can base-pair with any target m. RNA that contains the complementary sequence. The mi. RNA-protein 5 complex prevents gene expression either by degrading the target m. RNA or by blocking its translation. Chromatin changes Transcription RNA processing m. RNA degradation Translation Protein processing and degradation Protein complex Dicer Degradation of m. RNA OR mi. RNA Target m. RNA Hydrogen bond Blockage of translation

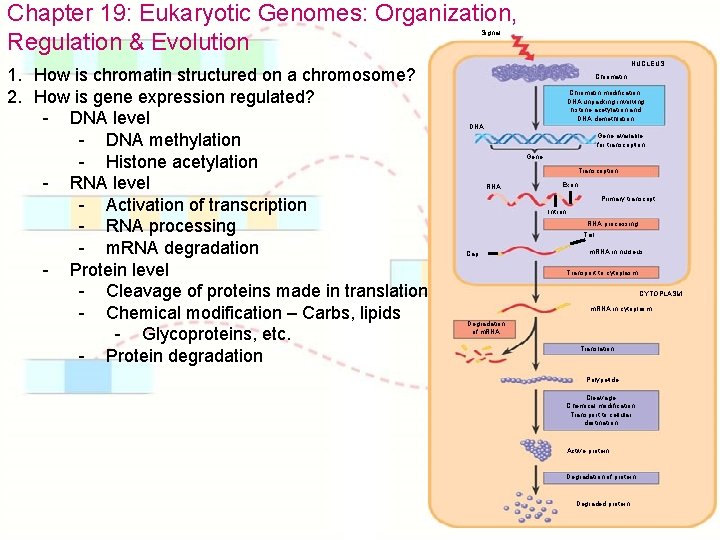
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution Signal 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? - DNA level - DNA methylation - Histone acetylation - RNA level - Activation of transcription - RNA processing - m. RNA degradation - Protein level - Cleavage of proteins made in translation - Chemical modification – Carbs, lipids - Glycoproteins, etc. - Protein degradation NUCLEUS Chromatin modification: DNA unpacking involving histone acetylation and DNA demethlation DNA Gene available for transcription Gene Transcription RNA Exon Primary transcript Intron RNA processing Tail Cap m. RNA in nucleus Transport to cytoplasm CYTOPLASM m. RNA in cytoplasm Degradation of m. RNA Translation Polypetide Cleavage Chemical modification Transport to cellular destination Active protein Degradation of protein Degraded protein

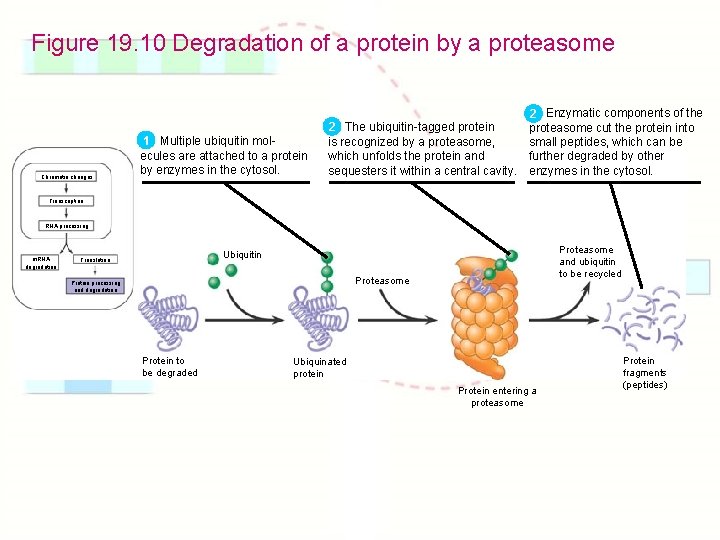
Figure 19. 10 Degradation of a protein by a proteasome Chromatin changes 1 Multiple ubiquitin molecules are attached to a protein by enzymes in the cytosol. 2 The ubiquitin-tagged protein is recognized by a proteasome, which unfolds the protein and sequesters it within a central cavity. Enzymatic components of the 2 proteasome cut the protein into small peptides, which can be further degraded by other enzymes in the cytosol. Transcription RNA processing m. RNA degradation Proteasome and ubiquitin to be recycled Ubiquitin Translation Proteasome Protein processing and degradation Protein to be degraded Ubiquinated protein Protein entering a proteasome Protein fragments (peptides)

Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? 3. How can proto-oncogenes become oncogenes? Proto-oncogene DNA Translocation or transposition: gene moved to new locus, under new controls Gene amplification: multiple copies of the gene New promoter Normal growth-stimulating protein in excess Point mutation within a control element Point mutation within the gene Oncogene Normal growth-stimulating protein in excess Hyperactive or degradationresistant protein

Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer?

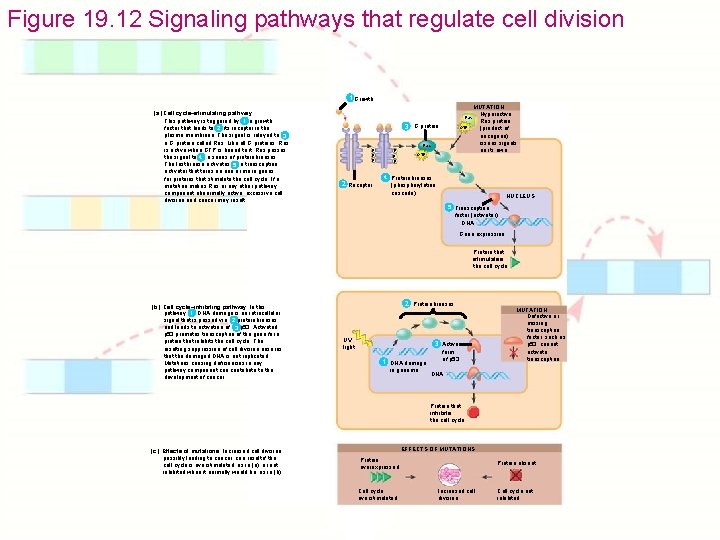
Figure 19. 12 Signaling pathways that regulate cell division 1 Growth factor (a) Cell cycle–stimulating pathway. This pathway is triggered by a growth 1 2 factor that binds to its receptor in the plasma membrane. The signal is relayed to 3 a G protein called Ras. Like all G proteins, Ras is active when GTP is bound to it. Ras passes the signal to a series of protein kinases. 4 The last kinase activates a transcription 5 activator that turns on one or more genes for proteins that stimulate the cell cycle. If a mutation makes Ras or any other pathway component abnormally active, excessive cell division and cancer may result. Ras 3 G protein p p p 2 Receptor GTP Ras p p p GTP MUTATION Hyperactive Ras protein (product of oncogene) issues signals on its own 4 Protein kinases (phosphorylation cascade) NUCLEUS 5 Transcription factor (activator) DNA Gene expression Protein that stimulates the cell cycle (b) Cell cycle–inhibiting pathway. In this pathway, DNA damage is an intracellular 1 signal that is passed via protein kinases 2 and leads to activation of p 53. Activated 3 p 53 promotes transcription of the gene for a protein that inhibits the cell cycle. The resulting suppression of cell division ensures that the damaged DNA is not replicated. Mutations causing deficiencies in any pathway component can contribute to the development of cancer. 2 Protein kinases UV light 3 Active 1 DNA damage in genome form of p 53 MUTATION Defective or missing transcription factor, such as p 53, cannot activate transcription DNA Protein that inhibits the cell cycle (c) Effects of mutations. Increased cell division, possibly leading to cancer, can result if the cell cycle is overstimulated, as in (a), or not inhibited when it normally would be, as in (b). EFFECTS OF MUTATIONS Protein overexpressed Cell cycle overstimulated Protein absent Increased cell division Cell cycle not inhibited

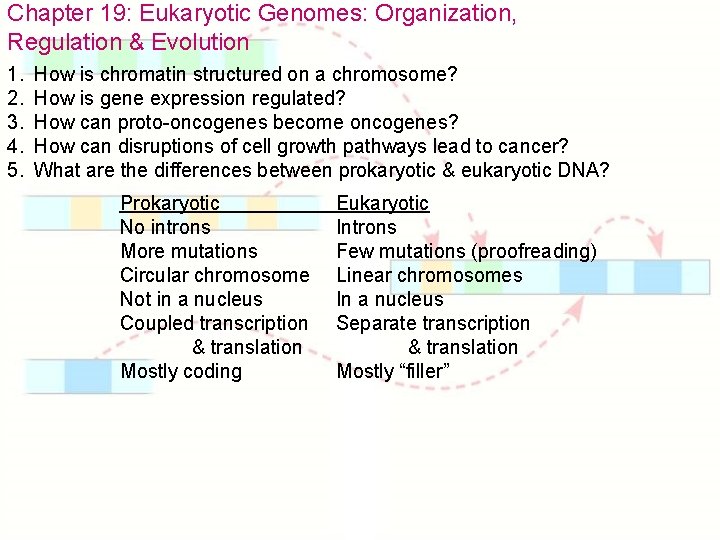
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. 5. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer? What are the differences between prokaryotic & eukaryotic DNA? Prokaryotic No introns More mutations Circular chromosome Not in a nucleus Coupled transcription & translation Mostly coding Eukaryotic Introns Few mutations (proofreading) Linear chromosomes In a nucleus Separate transcription & translation Mostly “filler”

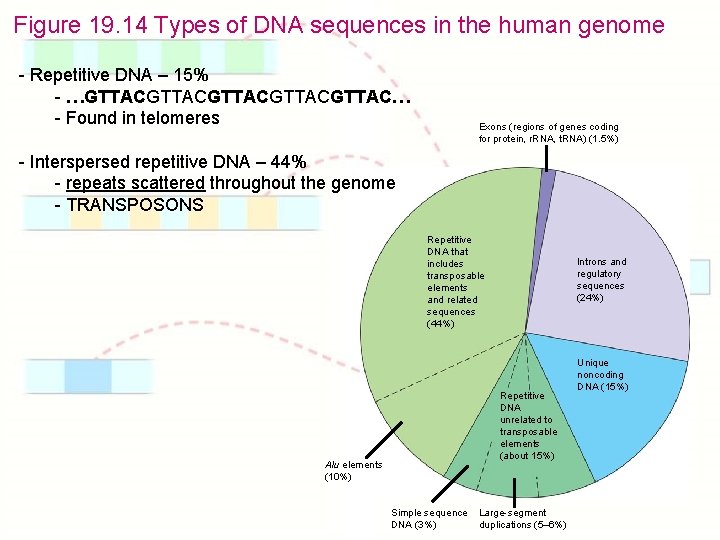
Figure 19. 14 Types of DNA sequences in the human genome - Repetitive DNA – 15% - …GTTACGTTACGTTAC… - Found in telomeres Exons (regions of genes coding for protein, r. RNA, t. RNA) (1. 5%) - Interspersed repetitive DNA – 44% - repeats scattered throughout the genome - TRANSPOSONS Repetitive DNA that includes transposable elements and related sequences (44%) Introns and regulatory sequences (24%) Repetitive DNA unrelated to transposable elements (about 15%) Alu elements (10%) Simple sequence DNA (3%) Large-segment duplications (5– 6%) Unique noncoding DNA (15%)

Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. 5. 6. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer? What are the differences between prokaryotic & eukaryotic DNA? How do eukaryotic transposable elements become repetitive?

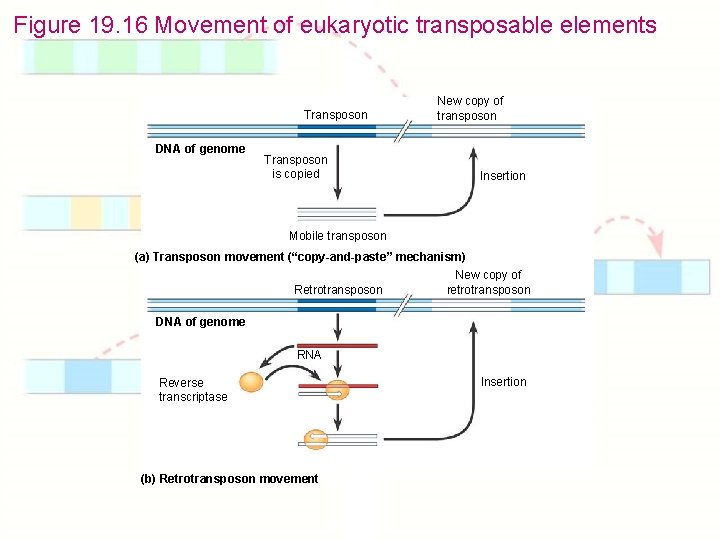
Figure 19. 16 Movement of eukaryotic transposable elements Transposon DNA of genome Transposon is copied New copy of transposon Insertion Mobile transposon (a) Transposon movement (“copy-and-paste” mechanism) New copy of Retrotransposon retrotransposon DNA of genome RNA Reverse transcriptase (b) Retrotransposon movement Insertion

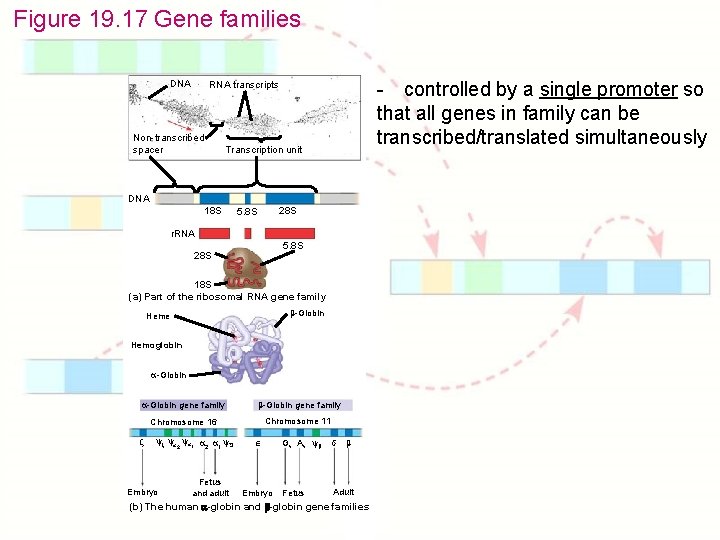
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. 5. 6. 7. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer? What are the differences between prokaryotic & eukaryotic DNA? How do eukaryotic transposable elements become repetitive? What are gene families? - Collection of identical or very similar genes that are clustered or dispersed throughout the genome

Figure 19. 17 Gene families DNA - controlled by a single promoter so that all genes in family can be transcribed/translated simultaneously RNA transcripts Non-transcribed spacer Transcription unit DNA 18 S 28 S 5. 8 S r. RNA 5. 8 S 28 S 18 S (a) Part of the ribosomal RNA gene family -Globin Heme Hemoglobin -Globin gene family Chromosome 16 Chromosome 11 2 1 2 1 Embryo Fetus and adult Embryo G A Fetus Adult (b) The human -globin and -globin gene families

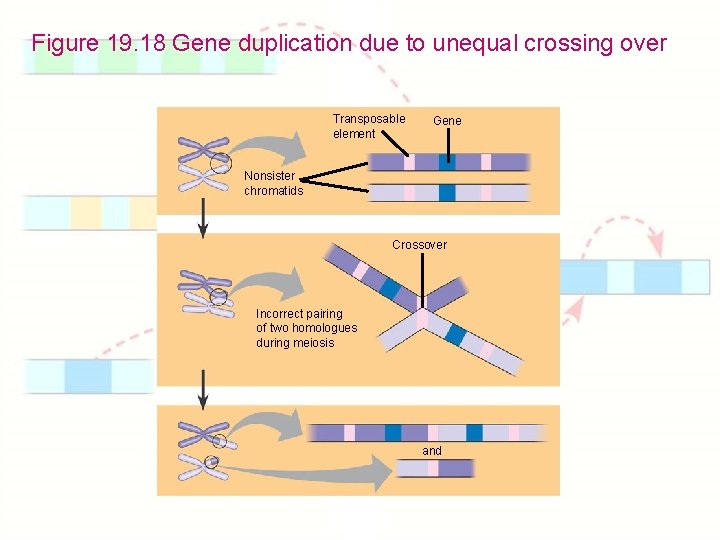
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. 5. 6. 7. 8. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer? What are the differences between prokaryotic & eukaryotic DNA? How do eukaryotic transposable elements become repetitive? What are gene families? How do genes get duplicated or deleted?

Figure 19. 18 Gene duplication due to unequal crossing over Transposable element Gene Nonsister chromatids Crossover Incorrect pairing of two homologues during meiosis and

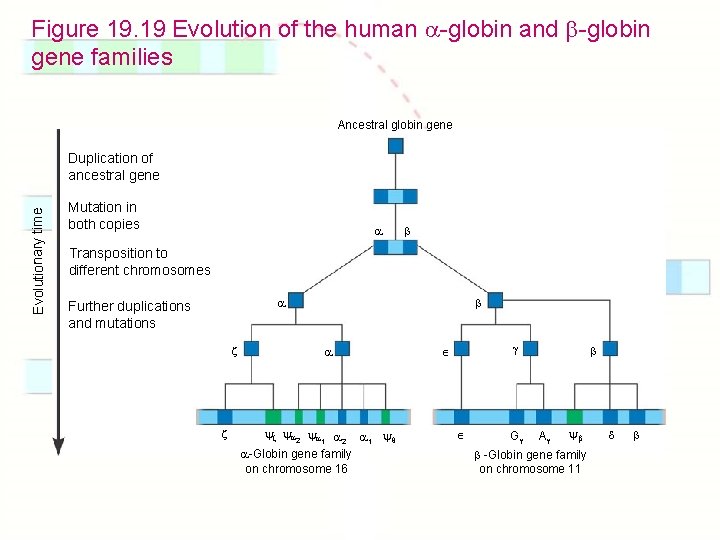
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. 2. 3. 4. 5. 6. 7. 8. 9. How is chromatin structured on a chromosome? How is gene expression regulated? How can proto-oncogenes become oncogenes? How can disruptions of cell growth pathways lead to cancer? What are the differences between prokaryotic & eukaryotic DNA? How do eukaryotic transposable elements become repetitive? What are gene families? How do genes get duplicated or deleted? How did the globin gene family evolve?

Figure 19. 19 Evolution of the human -globin and -globin gene families Ancestral globin gene Evolutionary time Duplication of ancestral gene Mutation in both copies Transposition to different chromosomes Further duplications and mutations 2 1 2 1 -Globin gene family on chromosome 16 G A -Globin gene family on chromosome 11

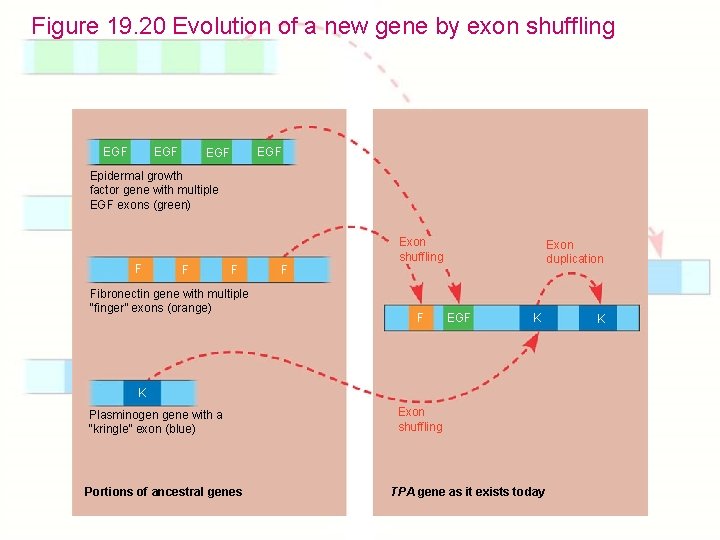
Chapter 19: Eukaryotic Genomes: Organization, Regulation & Evolution 1. How is chromatin structured on a chromosome? 2. How is gene expression regulated? 3. How can proto-oncogenes become oncogenes? 4. How can disruptions of cell growth pathways lead to cancer? 5. What are the differences between prokaryotic & eukaryotic DNA? 6. How do eukaryotic transposable elements become repetitive? 7. What are gene families? 8. How do genes get duplicated or deleted? 9. How did the globin gene family evolve? 10. How do new genes evolve? - Exon shuffling

Figure 19. 20 Evolution of a new gene by exon shuffling EGF EGF Epidermal growth factor gene with multiple EGF exons (green) F Fibronectin gene with multiple “finger” exons (orange) F Exon shuffling F Exon duplication EGF K K Plasminogen gene with a “kringle” exon (blue) Portions of ancestral genes Exon shuffling TPA gene as it exists today K