Chapter 19 Entropy and Free Energy Thermodynamics Spontaneity


- Slides: 9

Chapter 19 Entropy and Free Energy

Thermodynamics: Spontaneity, Entropy and Free energy Definitions: Spontaneous process: a process that can proceed without any outside intervention. Remember: Kinetics tells us about a rates of a reaction Entropy (S)- a measurement of the distribution of energy (“randomness”) of a system. Enthalpy (H)- the heat flow in a process occurring at constant pressure (no work performed) Free energy (G)- a thermodynamic function that relates entropy and enthalpy to spontaneity.

Law of Thermodynamics: • 1 st Law: Energy in conserved (neither created nor destroyed in any process) • 2 nd Law: the total entropy of the universe increases in any spontaneous process. • 3 rd Law: the entropy of a pure crystalline substance at absolute zero is zero (S =0)

Understanding Entropy • Is a state function so that ΔS = Sfinal- Sinitial • For an isothermal process: ΔS = qrev/T – Affect of entropy decreases with increasing temp. – Units to describe entropy = J/K qrev. - a pathway in which heat is added/removed in extremely small increments so that surrounding is not altered. • ΔS total = ΔS system + ΔS surrounding Reversible process: ΔSuniv. = 0 Irreversible process ΔSuniv. >0

Factors that increase in Entropy: • Phase change which increased randomness Solid < liquid < Gas • • Increase in temperature for any substance. Increase in the number of moles of a gas. Increase in volume of a gas. Formation of a solution from a liquid or solid.

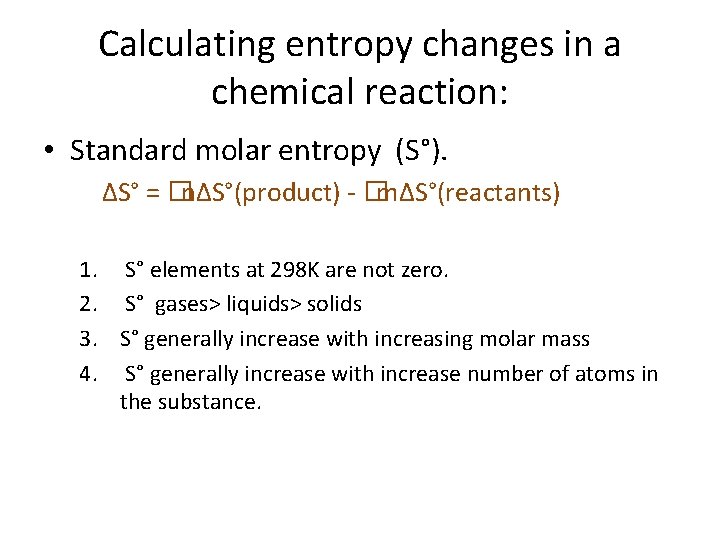
Calculating entropy changes in a chemical reaction: • Standard molar entropy (S°). ΔS° = �nΔS°(product) - �mΔS°(reactants) 1. S° elements at 298 K are not zero. 2. S° gases> liquids> solids 3. S° generally increase with increasing molar mass 4. S° generally increase with increase number of atoms in the substance.

Gibb’s Free Energy: • Describes the “free energy” a system possesses to a drive a spontaneous reaction and takes into account both enthalpy (H) and entropy (S) ΔG = Δ H –T Δ S ΔG < 0 spontaneous ΔG > 0 non spontaneous ΔG = 0 equilibrium

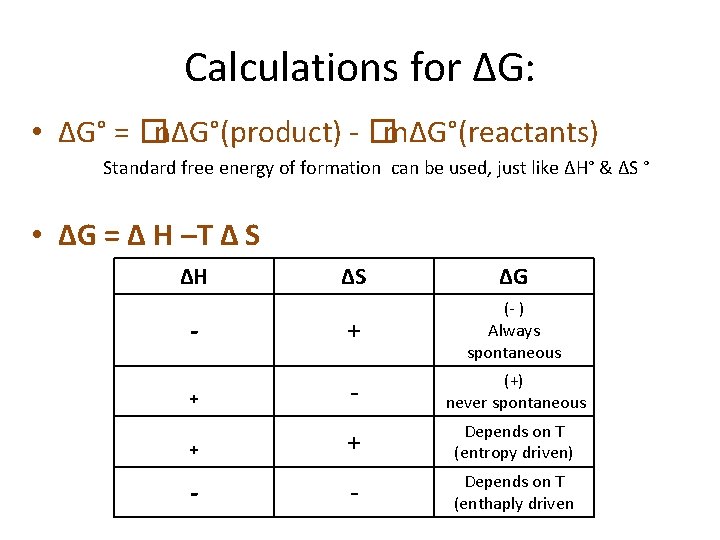
Calculations for ΔG: • ΔG° = �nΔG°(product) - �mΔG°(reactants) Standard free energy of formation can be used, just like ΔH° & ΔS ° • ΔG = Δ H –T Δ S ΔH ΔS ΔG + (- ) Always spontaneous + - (+) never spontaneous + + Depends on T (entropy driven) - Depends on T (enthaply driven - -

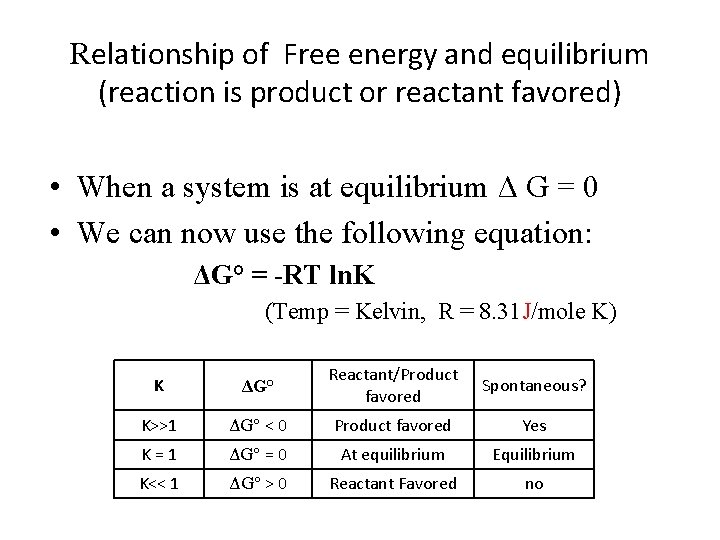
Relationship of Free energy and equilibrium (reaction is product or reactant favored) • When a system is at equilibrium Δ G = 0 • We can now use the following equation: ΔG° = -RT ln. K (Temp = Kelvin, R = 8. 31 J/mole K) K ΔG° Reactant/Product favored Spontaneous? K>>1 ΔG° < 0 Product favored Yes K=1 ΔG° = 0 At equilibrium Equilibrium K<< 1 ΔG° > 0 Reactant Favored no