Chapter 19 Elements and their Properties Periodic Table

Chapter 19 Elements and their Properties
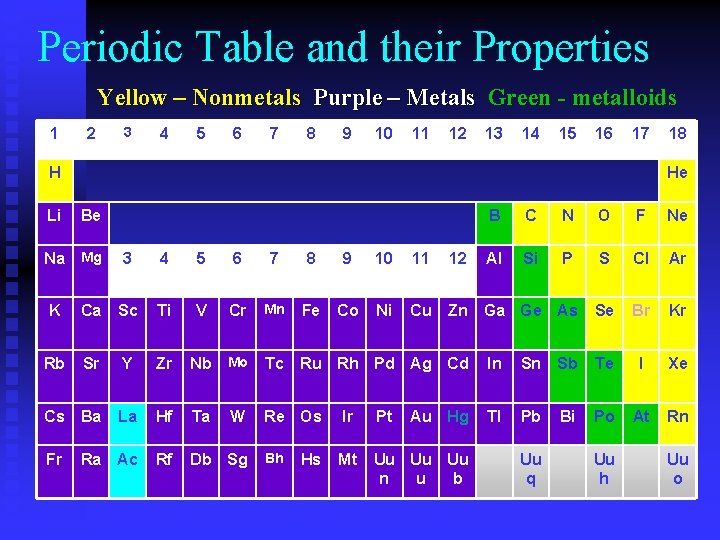
Periodic Table and their Properties Yellow – Nonmetals Purple – Metals Green - metalloids 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H 18 He Li Be Na Mg 3 4 5 6 7 8 9 10 11 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd Cs Ba La Hf Ta Re Os Ir Pt Fr Ra Ac Rf Db Sg Bh Mt Uu Uu Uu n u b W Hs B C N O F Ne 12 Al Si P S Cl Ar Zn Ga Ge As Se Br Kr In Sn Sb Te I Xe Tl Pb Bi Po At Rn Au Hg Uu q Uu h Uu o

Properties of Metals • Conduct heat and electricity • Luster - shiny • Malleable – thin sheets • Ductile - wires

Bonds formed by Metals • Ionic bonds – metal combines with a nonmetal • Metallic bonding – next slide…
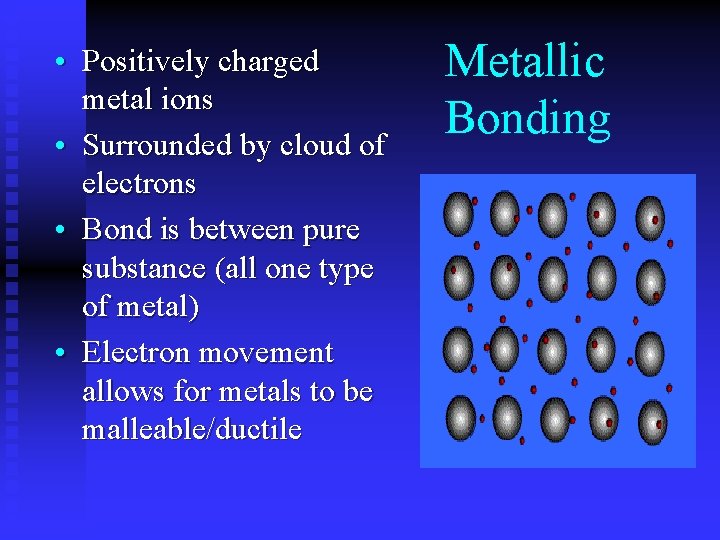
• Positively charged metal ions • Surrounded by cloud of electrons • Bond is between pure substance (all one type of metal) • Electron movement allows for metals to be malleable/ductile Metallic Bonding
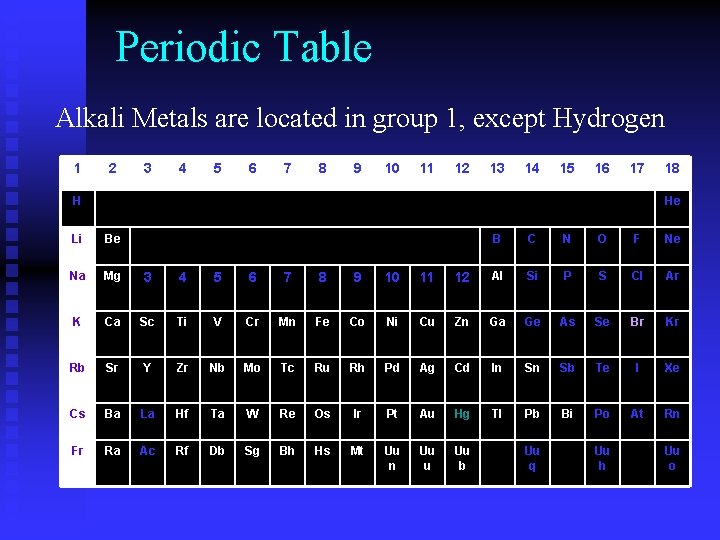
Periodic Table Alkali Metals are located in group 1, except Hydrogen 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H 18 He Li Be B C N O F Ne Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Uu n Uu u Uu b Uu q Uu h Uu o

Alkali Metals • Soft, silvery white • Most reactive of all metals • Highly reactive w/ oxygen & water • Never found uncombined in nature – 1 valence electron • Sodium is the most abundant

Lithium Sodium Rubidium Colors of flame given off by Alkali Metals Cesium Potassium

Uses of Alkali Metals • Table Salt (Na. Cl) Lite Salt : (KCl) • Baking Soda (Na. HCO 3) • Fertilizers ( K 2 O) • Matches (KCl. O 3) • Make glass stronger (K) • Fireworks (Rb {purple})
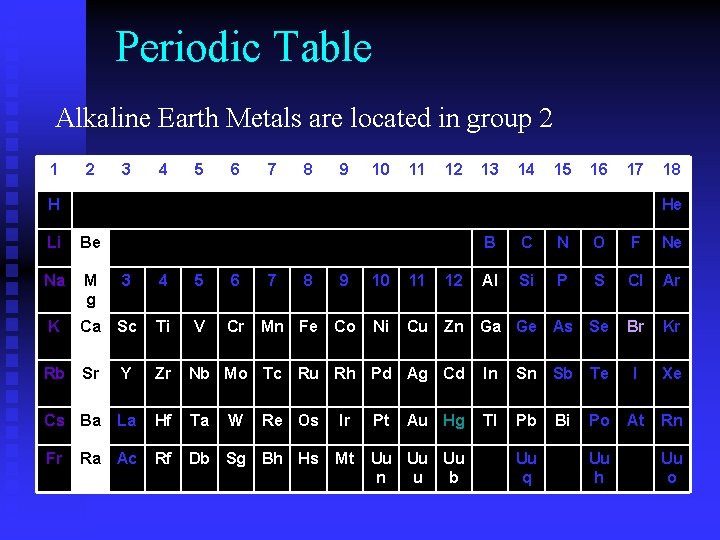
Periodic Table Alkaline Earth Metals are located in group 2 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H He Li Be Na M g 3 4 5 6 K Ca Sc Ti V Cr Mn Fe Rb Sr Y Zr Nb Mo Tc Cs Ba La Hf Ta Re Os Ir Pt Ra Ac Rf Db Sg Bh Hs Mt Uu Uu Uu n u b Fr 18 W 7 8 12 B C N O F Ne Al Si P S Cl Ar Se Br Kr 9 10 11 Co Ni Cu Zn Ga Ge As Ru Rh Pd Ag Cd Au Hg In Sn Sb Te I Xe Tl Pb Po At Rn Uu q Bi Uu h Uu o

Alkaline Earth Metals • Never found uncombined in nature • 2 nd most reactive group of metals • React w/Halogens to form salts • 2 valence electrons

Uses of Alkaline Earth Metals • Fireworks (Mg{silver}, Ba {green}, Sr{red}) • Ba – sparkplugs, paint (white) • Mg – makes aluminum cans stronger, engine components • Ca – strong bones/teeth, chalk, cement mortar
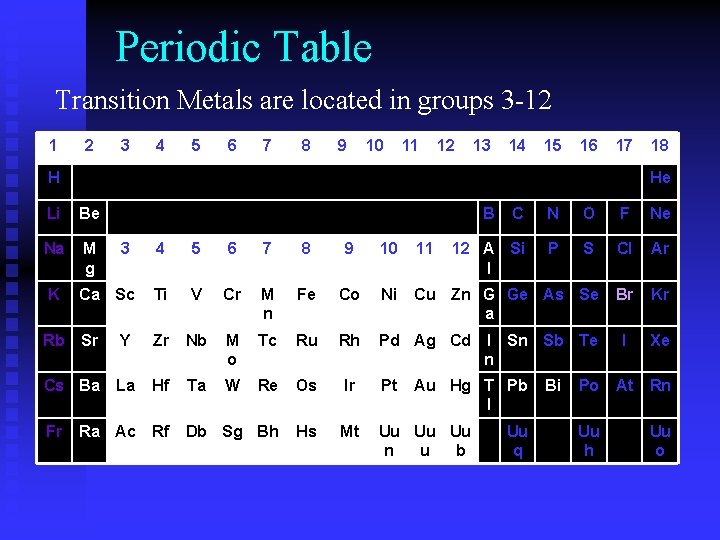
Periodic Table Transition Metals are located in groups 3 -12 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H 18 He Li Be Na M g K B N O F Ne 12 A Si l P 4 5 6 7 8 9 10 11 S Cl Ar Ca Sc Ti V Cr M n Fe Co Ni Cu Zn G Ge As Se a Br Kr Sr Y Zr Nb M o Tc Ru Rh Pd Ag Cd I Sn Sb n Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Po At Rn Ra Ac Rf Db Sg Bh Hs Mt Uu Uu Uu n u b Rb Fr 3 C Au Hg T Pb l Uu q Bi Uu h Uu o

Transition Elements (3 -12) • Typically form colored compounds • Good conductors • High Luster • Iron Triad (Fe, Co, Ni) • Coinage Metals (Cu, Ag, Au) • Group 12 Metals (Zn, Hg, Cd)

Iron Triad: Fe IRON • Fe – cheapest, most abundant, useful of all metals - steel • Iron is vital to human and plant life. Ex: hemoglobin/blood • Core of earth is 90% iron

Red appearance of rocks is due to iron

Iron Triad: Co COBALT • Co – important metal in magnetic applications • Cobalt salts (combined with halogens) used as blue pigment in porcelain, glass, pottery and enamels (paint).

Cobalt Glass

Iron Triad: Ni NICKEL • Ni – used to make stainless steel and coins (a nickle is 25% nickel) • Gives glass a green color • Found in baked beans (navy bean) • Nickel is also used for rocket engines

Coinage Metals COPPER • Cu – reddish gold metal • Used extensively in wiring and motors • Used as alloy to make coins, all coins now contain some copper. • Brass and bronze are Cu alloys

Coinage Metals SILVER • Silver – symbol comes from Latin word “argentum” • Sterling Silver (alloy) 92. 5% Ag • Used in mirrors – best reflector of light; Photography (Ag. NO 3) – to develop film and electrical contacts, batteries, jewelry

Coinage Metals GOLD • Gold – Au, symbol comes from Latin word “Aurum” • Non reactive • Used in electronics, jewelry • Most malleable and ductile metal

Group 12 Metals • Zinc – used in brass, making pennies (since 1982), battery containers, Zinc Oxide is used in paint (white pigment) and to protect from sunburn , deodorant (Zn. O 2). • Lack of zinc in diet can cause hair loss

Group 12 Metals • Cadmium – used in coating metals to prevent rust/corrosion • Used in rechargeable batteries • Cadmium compounds are used as blue and green phosphors in TV’s

Group 12 Metals • Mercury – Only metal that is a liquid at room temperature • Symbol is Hg from Latin word “hydragyrum” • Used in thermometers, switches and batteries

Inner Transition Metals • Also called “Rare Earth Elements” • Consists of two groups: • Lanthanides • Actinides
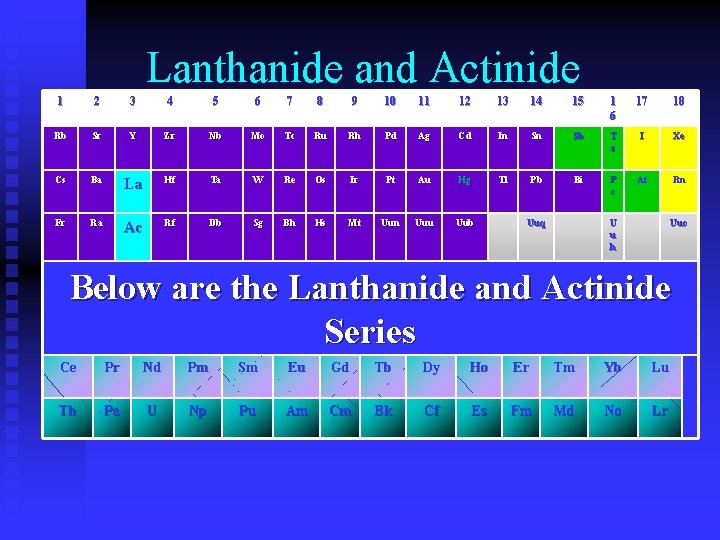
Lanthanide and Actinide 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 1 6 17 18 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi P o At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq U u h Uuo Below are the Lanthanide and Actinide Series Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

Lanthanides • Europium – used in fluorescent paints and glass. If you see red on your TV – it is Europium phosphor • Gadolinium – used in making CD’s and computer memory • Terbium – Green phosphors for TV, electronic circuits

Actinides • All actinides are radioactive • Thorium – used in making camera lenses • Uranium – nuclear reactors as fuel and in nuclear weapons
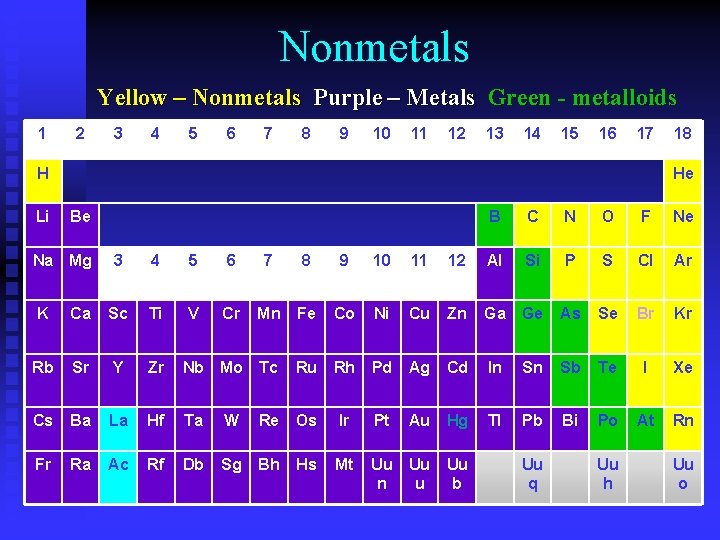
Nonmetals Yellow – Nonmetals Purple – Metals Green - metalloids 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H Li 18 He Be Na Mg B C N O F Ne 3 4 5 6 7 8 9 10 11 12 Al Si P S Cl Ar V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr K Ca Sc Ti Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Uu n Uu u Uu b Uu q Uu h Uu o

Properties of Nonmetals • Usually gas or brittle solid at room temperature • Poor conductors • Dull • Just the opposite of metals

Bonds formed by Nonmetals • Ionic bonds – formed when a metal combines with a nonmetal • Covalent bonds – formed when two nonmetals combine

Hydrogen • Most common in universe • Diatomic molecule in nature • H 2 • Highly reactive • Found mostly as water

Halogens – Group 17 • Forms a salt when it combines with a metal • Uses: • Chlorine – disinfectant • Bromine – dyes in cosmetics • Iodine – hormone regulation

Sublimation – Part 1 • When a solid changes directly into a gas w/o becoming a liquid • Iodine • Frozen CO 2 (Dry Ice) • Solid Air Fresheners • Mothballs

Sublimation – Part 2 • It also describes the reverse process – solid from gas w/o becoming a liquid • Frost • Snowflakes

Noble Gases – Group 18 • Only group that is both neutral and stable. • Helium is used in blimps, balloons • Neon, argon, krypton used in making “Neon” lights

3 Ways Neon Signs get Color • Inert gas. We use 2 inert gasses, neon and argon/mercury. Neon gives off a reddishorange color, while argon/mercury is a light blue.

3 Ways Neon Signs get Color • Fluorescent powders. Many neon tubes are coated on the inside with fluorescent powders that filter out different colors from the light spectrum. In combination with the different gasses, an even greater number of colors are achieved. For instance, a green tube, filled with argon/mercury (blue gas) will light up green. Fill the same tube with neon (red gas) and it lights up orange. Likewise a blue tube, filled with blue gas will light up blue, and the same blue tube filled with red gas will light up pink.

3 Ways Neon Signs get Color • Finally, the use of colored "classic" glass is an old world method which achieves the most vivid colors (reddest reds, deepest blues, etc. ) but they are not as bright as other neon. They are also more costly, so are not used as widely for signs as they once were. However, for neon artwork, they are unparalleled in beauty.
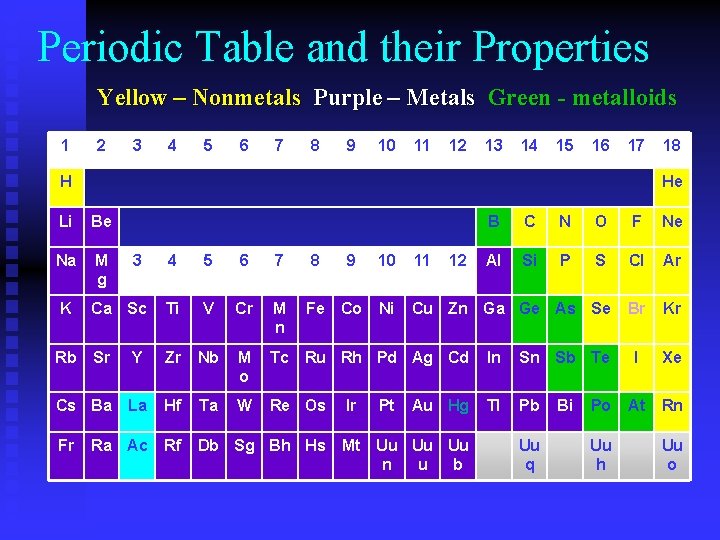
Periodic Table and their Properties Yellow – Nonmetals Purple – Metals Green - metalloids 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 H He Li Be Na M g K 8 9 N O F Ne Al Si P 6 7 Ca Sc Ti V Cr M n Sr Y Zr Nb M o Tc Ru Rh Pd Ag Cd In Sn Sb Cs Ba La Hf Ta W Re Os Tl Pb Ra Ac Rf Db Sg Bh Hs Fe Co Ir 10 11 S Cl Ar Ni Cu Zn Ga Ge As Se Br Kr Te I Xe Po At Rn Pt 12 C 5 Fr 3 B 4 Rb 18 Au Hg Mt Uu Uu Uu n u b Uu q Bi Uu h Uu o
- Slides: 41