Chapter 19 Electrochemistry Voltaic Cells Generate Electricity which












- Slides: 52

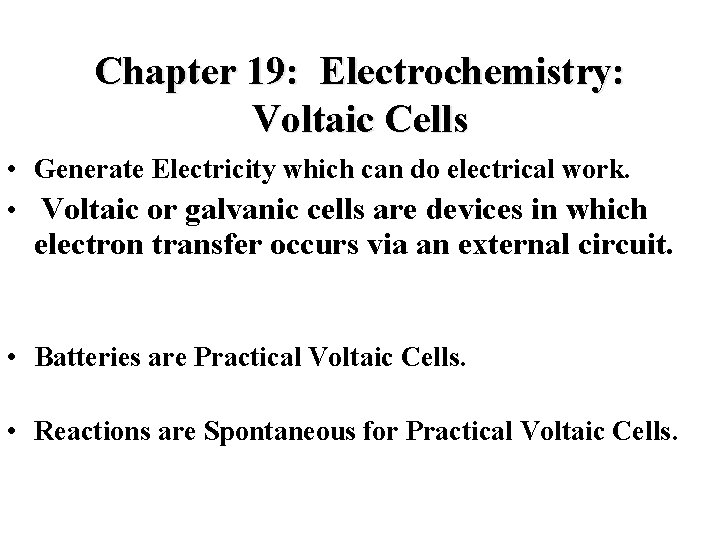
Chapter 19: Electrochemistry: Voltaic Cells • Generate Electricity which can do electrical work. • Voltaic or galvanic cells are devices in which electron transfer occurs via an external circuit. • Batteries are Practical Voltaic Cells. • Reactions are Spontaneous for Practical Voltaic Cells.

Voltaic Cell/Battery • Batteries are Practical Voltaic Cells. • Reactions are Spontaneous for Practical Voltaic Cells. – Commonly Refer to Half Reactions. • Oxidation Half-Reaction • Reduction Half-Reaction

Chapter 21: Voltaic Cells • Voltaic cells have: – Anode – Cathode – Salt bridge (used to complete the electrical circuit) – Electrolyte – Switch – Voltmeter

Voltaic Cells

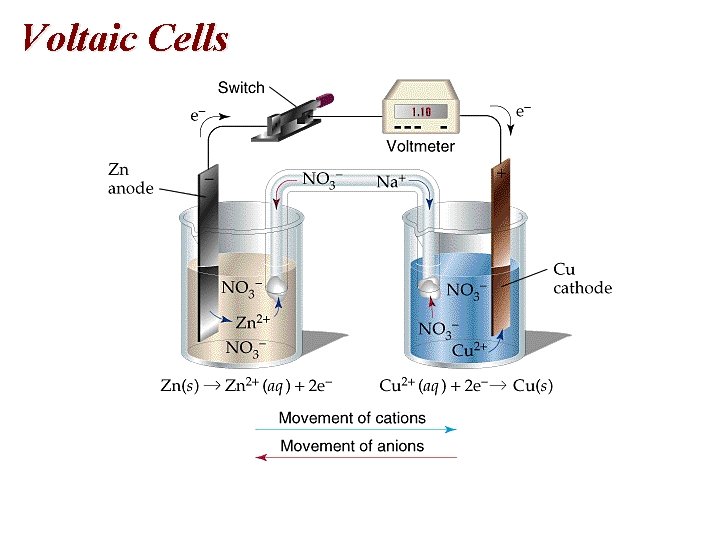
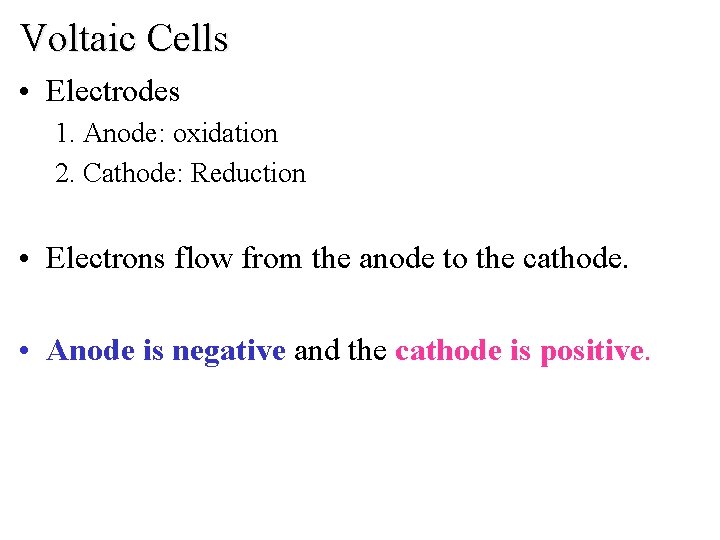
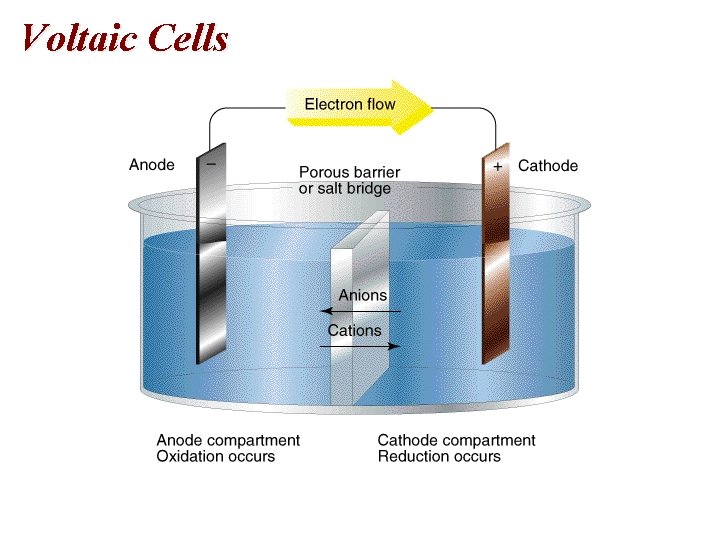
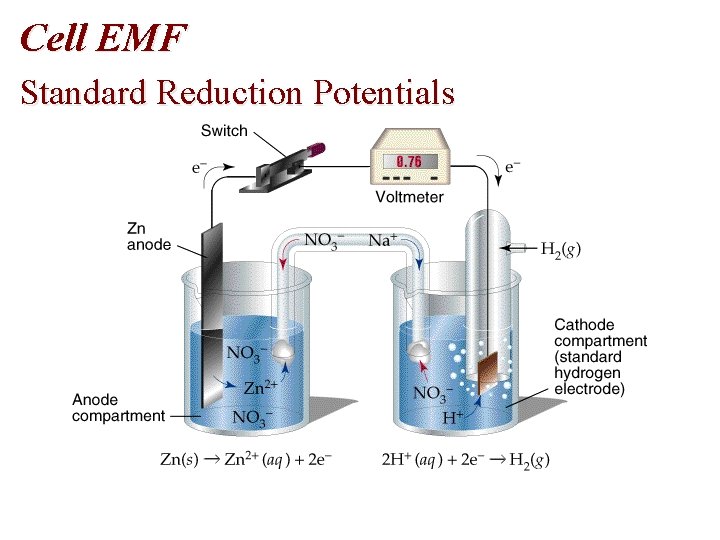
Voltaic Cells • Electrodes 1. Anode: oxidation 2. Cathode: Reduction • Electrons flow from the anode to the cathode. • Anode is negative and the cathode is positive.

Voltaic Cells

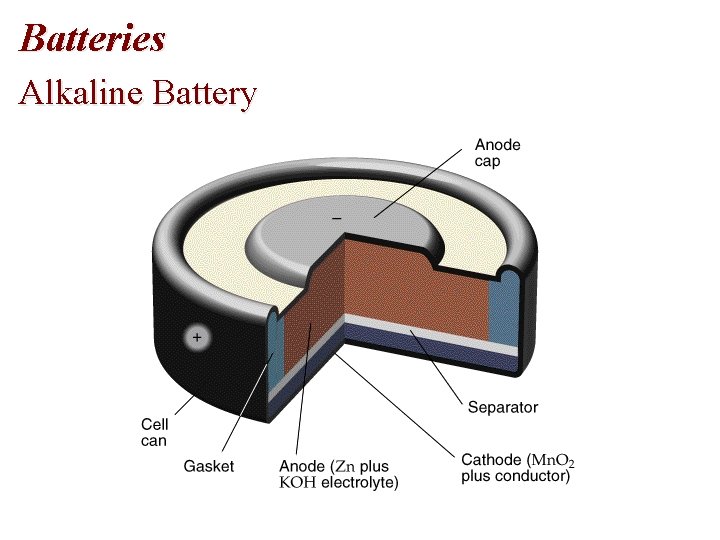
Batteries Alkaline Battery

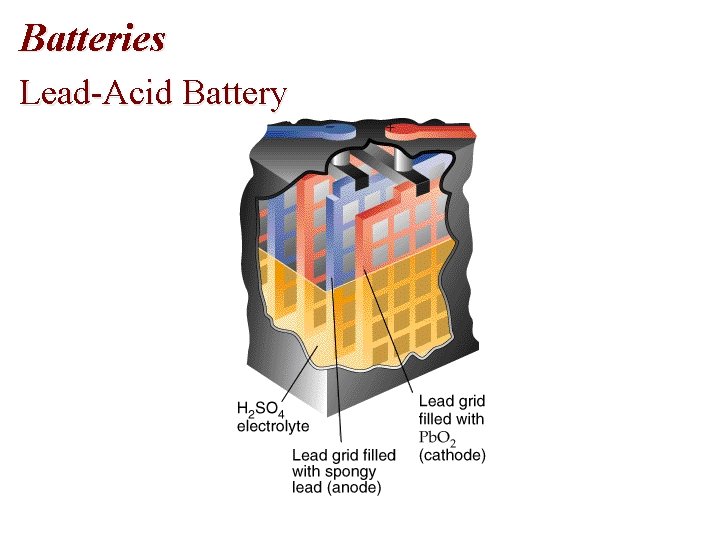
Batteries Lead-Acid Battery

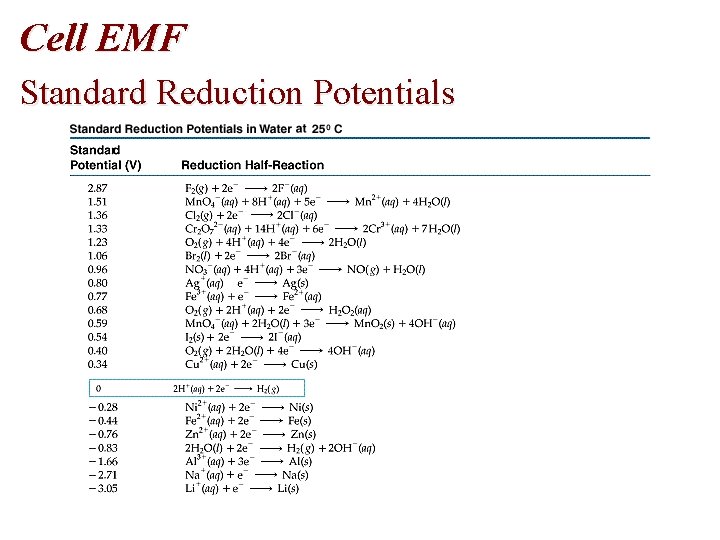
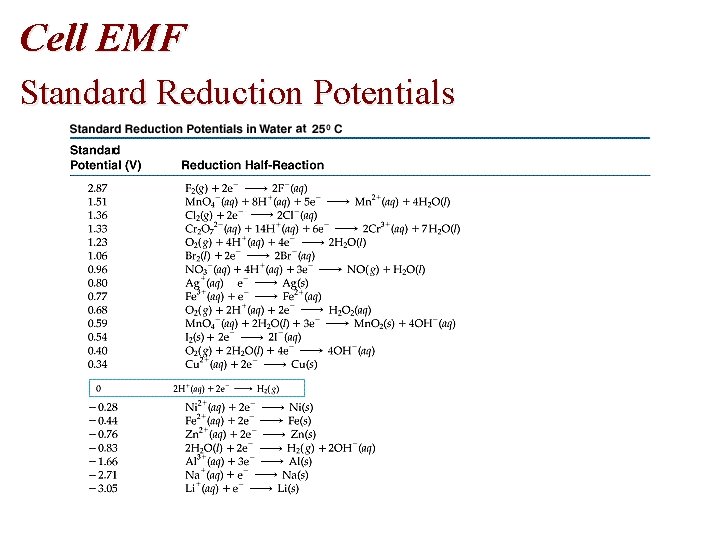
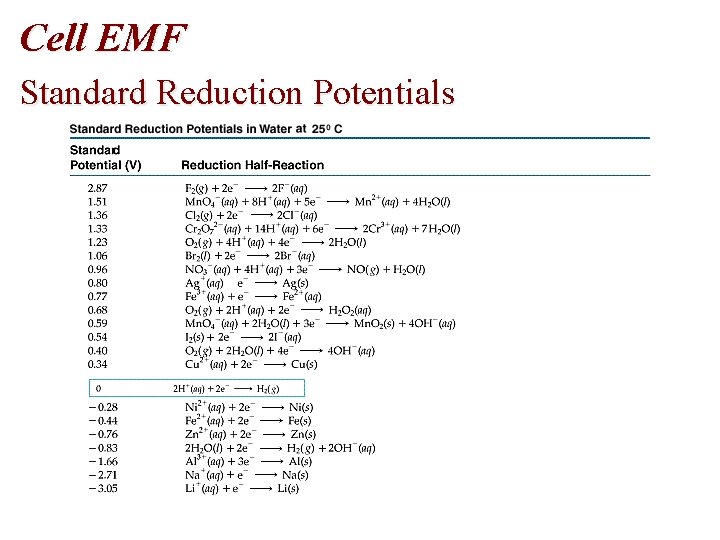
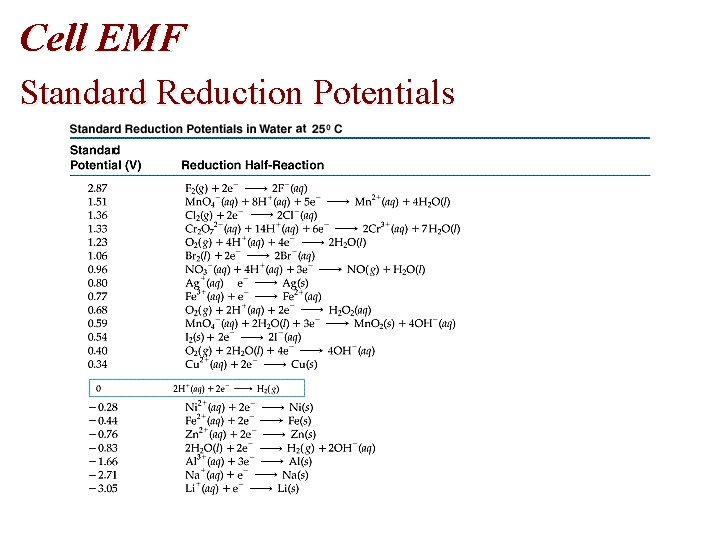
Cell EMF Standard Reduction Potentials

Cell EMF: Potential (E) • The flow of electrons from anode to cathode is spontaneous. • Electrons flow from anode to cathode because the cathode has a lower electrical potential energy than the anode. • Potential difference: difference in electrical potential. Measured in volts. • One volt is the potential difference required to impart one joule of energy to a charge of one coulomb:

Cell EMF • Electromotive force (emf) is the force required to push electrons through the external circuit. • Cell potential: Ecell is the emf of a cell. • For 1 M solutions at 25 C (standard conditions), the standard emf (standard cell potential) is called E cell. • E cell = E oxidation + E reduction

Cell EMF Standard Reduction Potentials

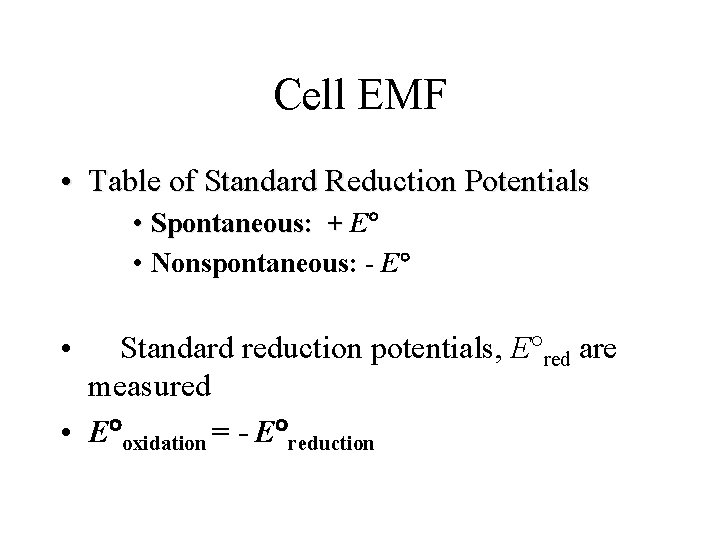
Cell EMF • Table of Standard Reduction Potentials • Spontaneous: + E • Nonspontaneous: - E • Standard reduction potentials, E red are measured • E oxidation = - E reduction

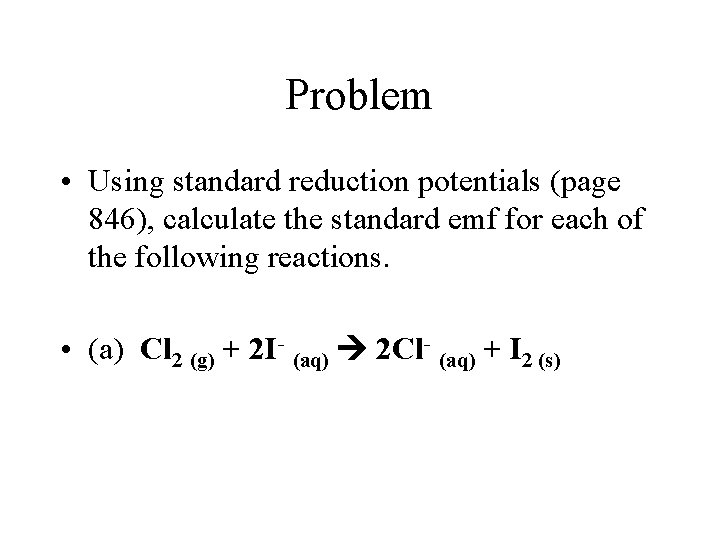
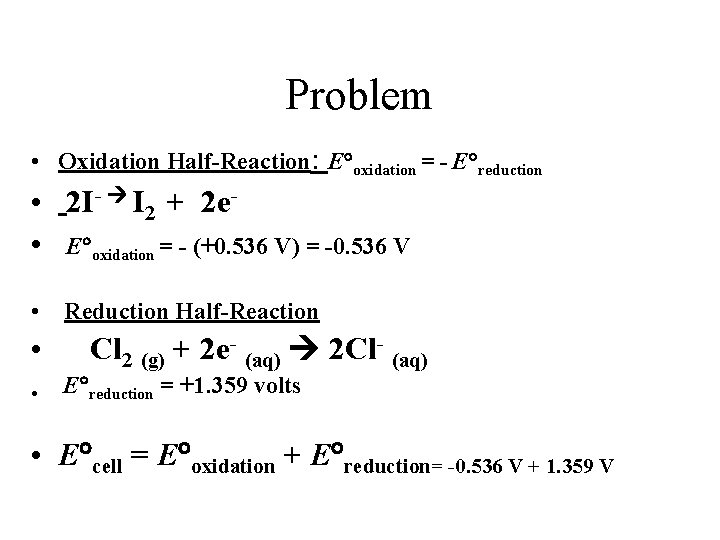
Problem • Using standard reduction potentials (page 846), calculate the standard emf for each of the following reactions. • (a) Cl 2 (g) + 2 I- (aq) 2 Cl- (aq) + I 2 (s)

Problem • Oxidation Half-Reaction: E oxidation = - E reduction • 2 I- I 2 + 2 e • E oxidation = - (+0. 536 V) = -0. 536 V • Reduction Half-Reaction • • Cl 2 (g) + 2 e- (aq) 2 Cl- (aq) E reduction = +1. 359 volts • E cell = E oxidation + E reduction= -0. 536 V + 1. 359 V

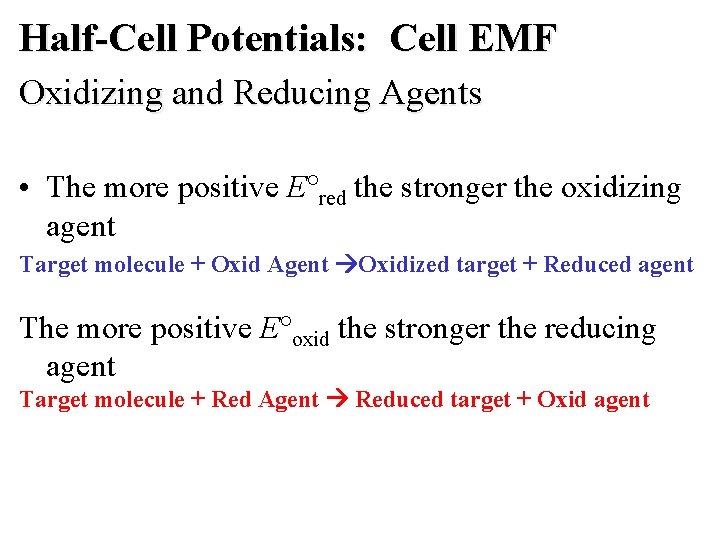
Half-Cell Potentials: Cell EMF Oxidizing and Reducing Agents • The more positive E red the stronger the oxidizing agent Target molecule + Oxid Agent Oxidized target + Reduced agent The more positive E oxid the stronger the reducing agent Target molecule + Red Agent Reduced target + Oxid agent

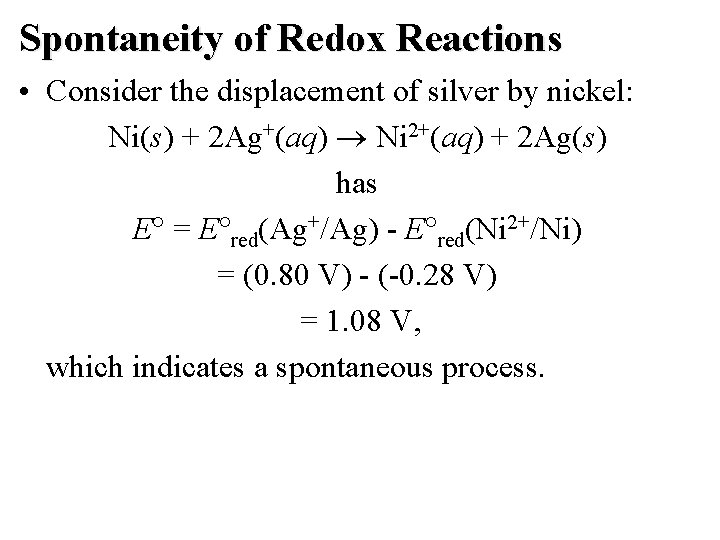
Spontaneity of Redox Reactions • Consider the displacement of silver by nickel: Ni(s) + 2 Ag+(aq) Ni 2+(aq) + 2 Ag(s) has E = E red(Ag+/Ag) - E red(Ni 2+/Ni) = (0. 80 V) - (-0. 28 V) = 1. 08 V, which indicates a spontaneous process.

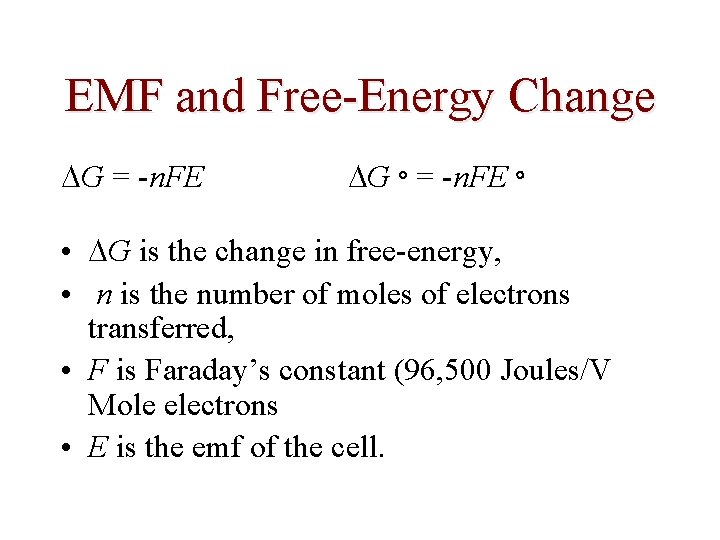
EMF and Free-Energy Change G = -n. FE G = -n. FE • G is the change in free-energy, • n is the number of moles of electrons transferred, • F is Faraday’s constant (96, 500 Joules/V Mole electrons • E is the emf of the cell.

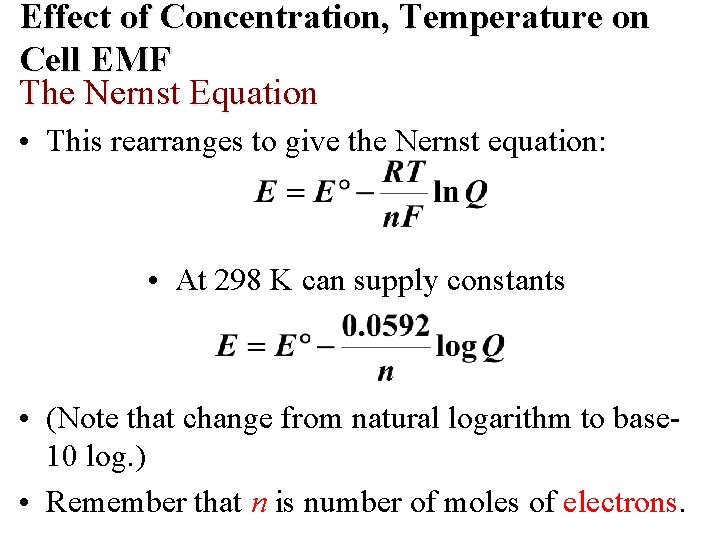
Effect of Concentration, Temperature on Cell EMF E cell calculated under standard state conditions. The Nernst Equation • The Nernst equation relates emf to concentrations and temperatures which are not standard state.

Effect of Concentration, Temperature on Cell EMF The Nernst Equation • This rearranges to give the Nernst equation: • At 298 K can supply constants • (Note that change from natural logarithm to base 10 log. ) • Remember that n is number of moles of electrons.

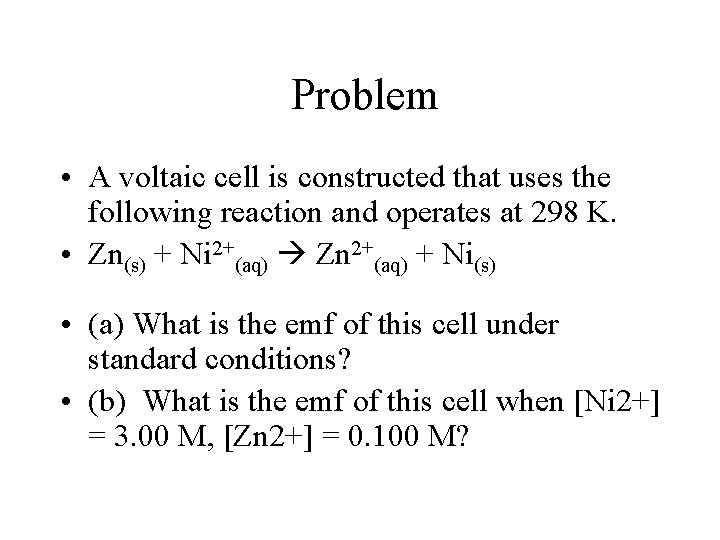
Problem • A voltaic cell is constructed that uses the following reaction and operates at 298 K. • Zn(s) + Ni 2+(aq) Zn 2+(aq) + Ni(s) • (a) What is the emf of this cell under standard conditions? • (b) What is the emf of this cell when [Ni 2+] = 3. 00 M, [Zn 2+] = 0. 100 M?

Chapter 19: Voltaic Cells • Voltaic cells have: – Anode – Cathode – Salt bridge (used to complete the electrical circuit) – Electrolyte – Switch – Voltmeter – HALF REACTIONS ARE USUALLY SPONTANEOUS.

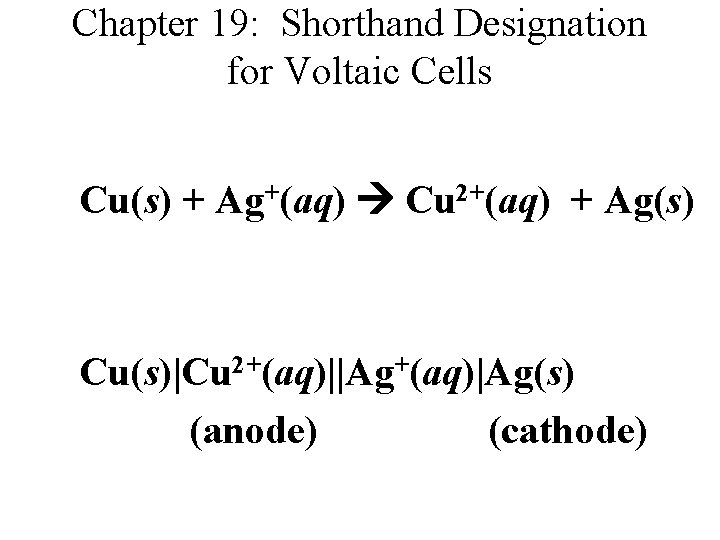
Chapter 19: Shorthand Designation for Voltaic Cells Cu(s) + Ag+(aq) Cu 2+(aq) + Ag(s) Cu(s)|Cu 2+(aq)||Ag+(aq)|Ag(s) (anode) (cathode)

Problem 19. 30 -A: Page 875 • Calculate E , E, and G for the following. Mg (s) + Sn 2+ (aq) Mg 2+ (aq) +Sn (s) [Mg 2+] = 0. 045 M, [Sn 2+] = 0. 035 M

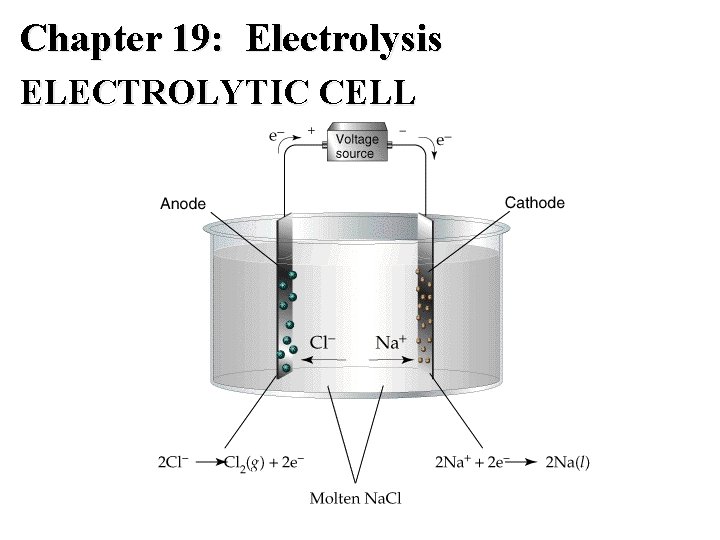
Chapter 19: Electrolysis • Electrolytic cells have: – Anode – Cathode – Electrolyte – Source of DC Voltage – Voltmeter – REDOX REACTIONS NOT NECESSARILY SPONTANEOUS.

Chapter 19: Electrolysis ELECTROLYTIC CELL

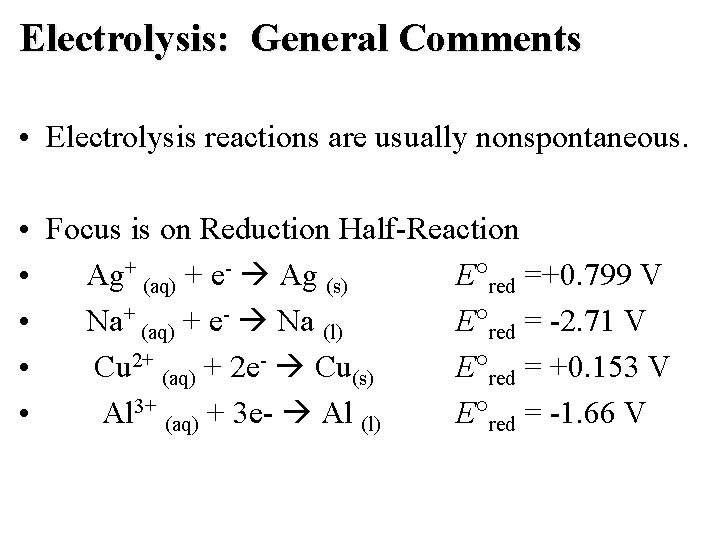
Electrolysis: General Comments • Electrolysis reactions are usually nonspontaneous. • Focus is on Reduction Half-Reaction • Ag+ (aq) + e- Ag (s) E red =+0. 799 V • Na+ (aq) + e- Na (l) E red = -2. 71 V • Cu 2+ (aq) + 2 e- Cu(s) E red = +0. 153 V • Al 3+ (aq) + 3 e- Al (l) E red = -1. 66 V

Chapter 19: Electrolysis • In voltaic and electrolytic cells: – reduction occurs at the cathode, and – oxidation occurs at the anode. – However, in electrolytic cells, electrons are forced to flow from the anode to cathode. – In electrolytic cells the anode is positive and the cathode is negative. – In galvanic cells the anode is negative and the cathode is positive

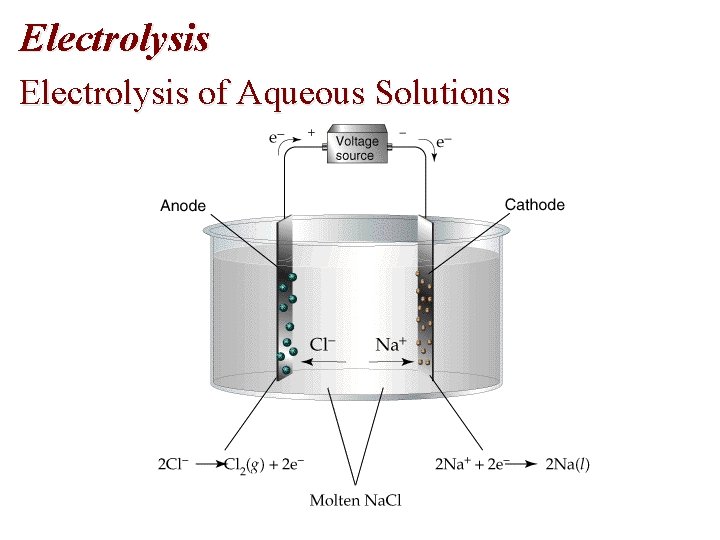
Electrolysis of Aqueous Solutions

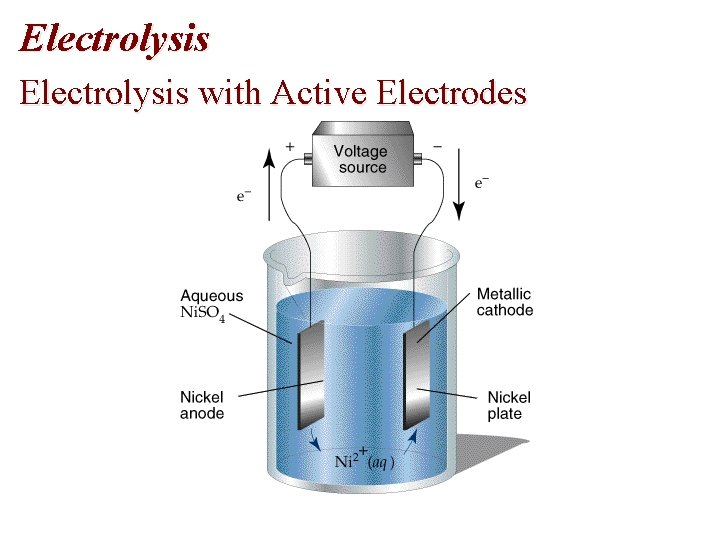
Chapter 19: Electrolysis with Active Electrodes • Active electrodes: electrodes that take part in electrolysis. • Example: electrolytic plating.

Electrolysis with Active Electrodes

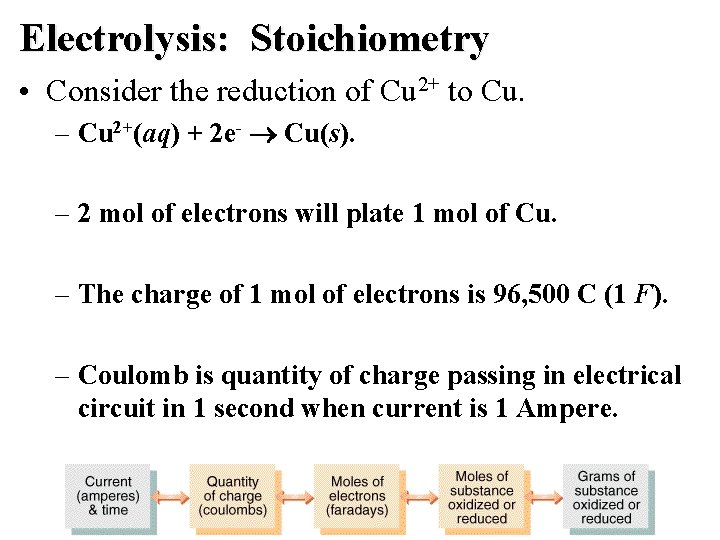
Electrolysis: Stoichiometry • Consider the reduction of Cu 2+ to Cu. – Cu 2+(aq) + 2 e- Cu(s). – 2 mol of electrons will plate 1 mol of Cu. – The charge of 1 mol of electrons is 96, 500 C (1 F). – Coulomb is quantity of charge passing in electrical circuit in 1 second when current is 1 Ampere.

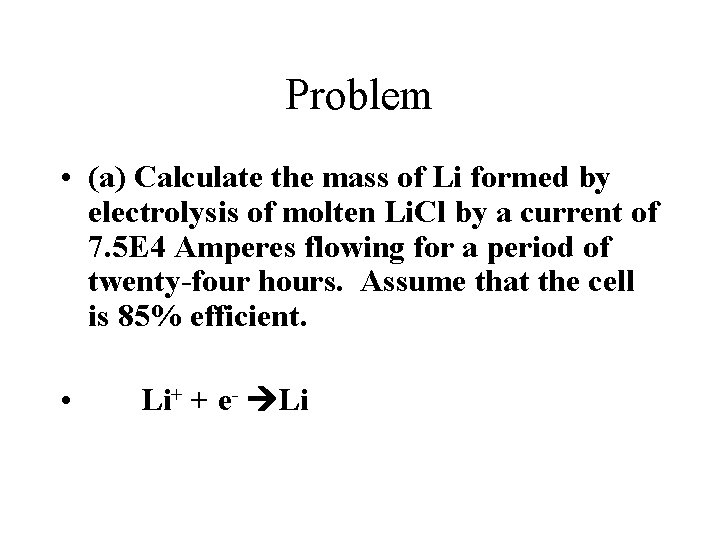
Problem • (a) Calculate the mass of Li formed by electrolysis of molten Li. Cl by a current of 7. 5 E 4 Amperes flowing for a period of twenty-four hours. Assume that the cell is 85% efficient. • Li+ + e- Li

Stoichiometry of Electrolysis –What current must be supplied to deposit 3. 00 g Au from a solution of Au. Cl 3 in 200. 0 s?

Cell EMF Standard Reduction Potentials

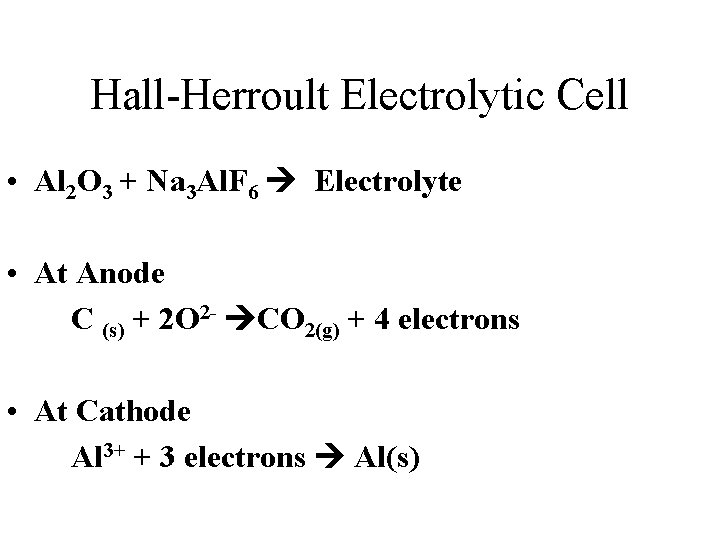
Hall-Herroult Electrolytic Cell • Al 2 O 3 + Na 3 Al. F 6 Electrolyte • At Anode C (s) + 2 O 2 - CO 2(g) + 4 electrons • At Cathode Al 3+ + 3 electrons Al(s)

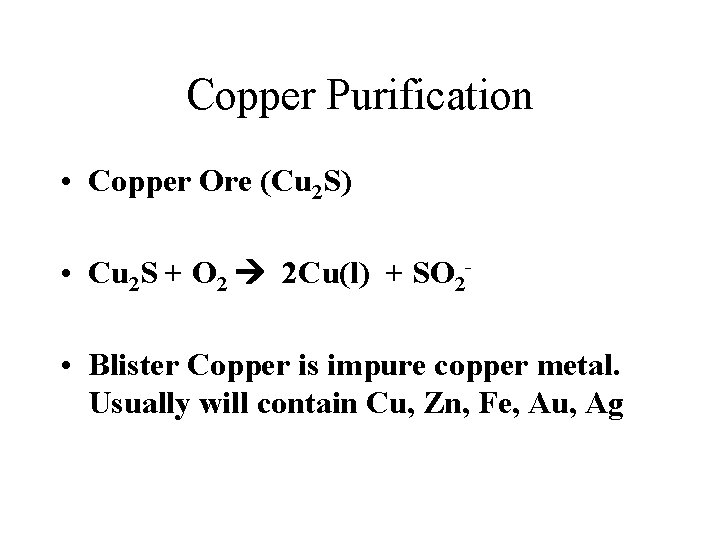
Copper Purification • Copper Ore (Cu 2 S) • Cu 2 S + O 2 2 Cu(l) + SO 2 • Blister Copper is impure copper metal. Usually will contain Cu, Zn, Fe, Au, Ag

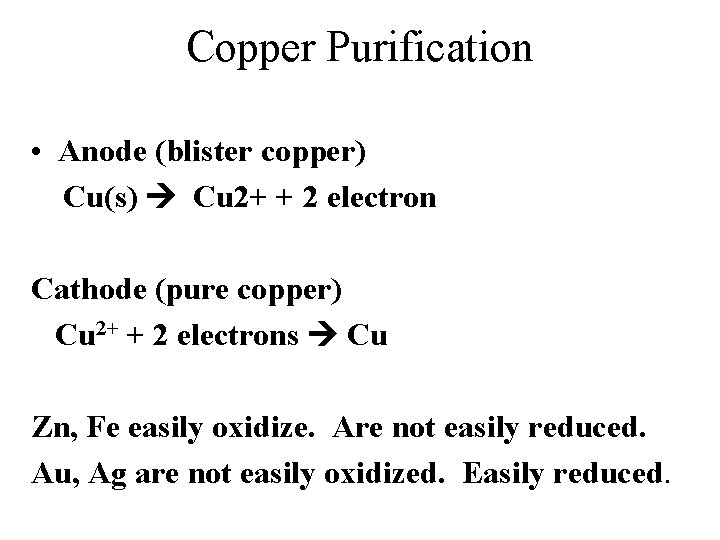
Copper Purification • Anode (blister copper) Cu(s) Cu 2+ + 2 electron Cathode (pure copper) Cu 2+ + 2 electrons Cu Zn, Fe easily oxidize. Are not easily reduced. Au, Ag are not easily oxidized. Easily reduced.

Cell EMF Standard Reduction Potentials

Problem 19. 58: Page 876 A quantity of 0. 300 grams of copper was deposited from a Cu. SO 4 solution by passing a current of 3. 00 A through the solution for 304 seconds. Calculate the value of the Faraday constant.

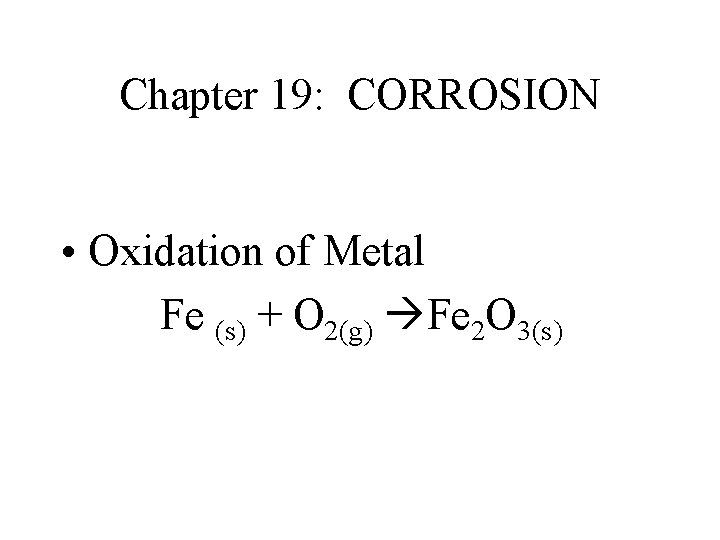
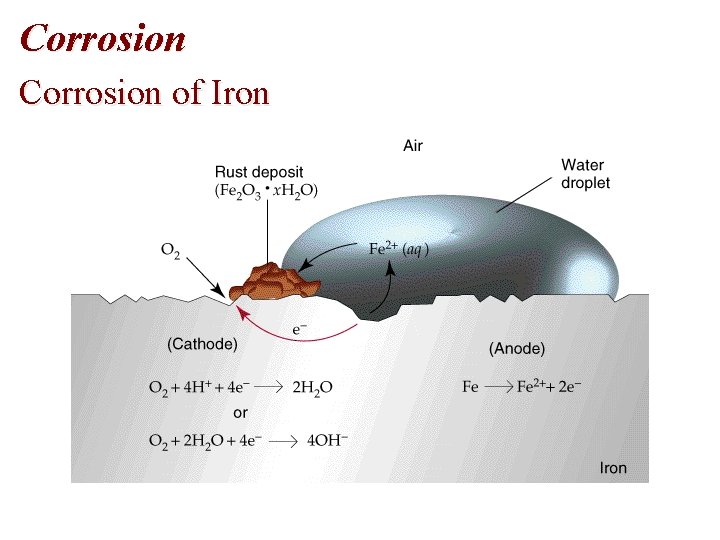
Chapter 19: CORROSION • Oxidation of Metal Fe (s) + O 2(g) Fe 2 O 3(s)

Cell EMF Standard Reduction Potentials

Fig. 19. 13 a

Fig. 19. 13 b

Fig. 19. 13 c

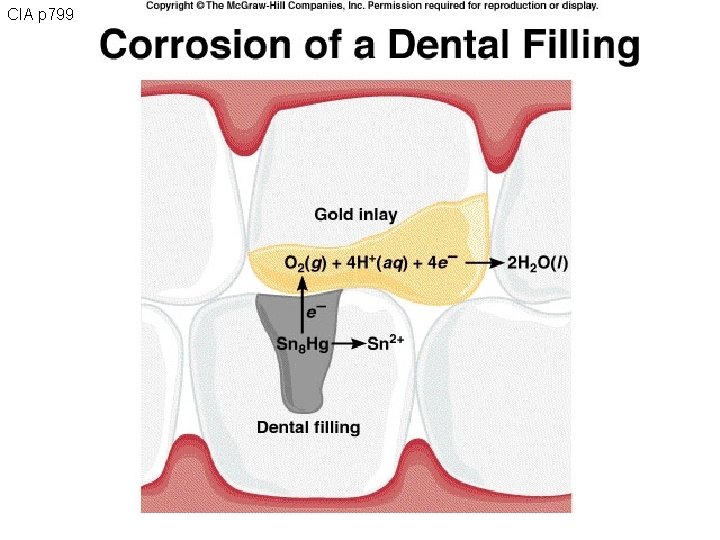
CIA p 799

Corrosion: • 1. Oxidation of Metal • Fe (s) + O 2(g) Fe 2 O 3(s) • 2. Factors which facilitate Corrosion • Aqueous Solution • Electrolyte • Acidic or Basis Solution

Corrosion of Iron

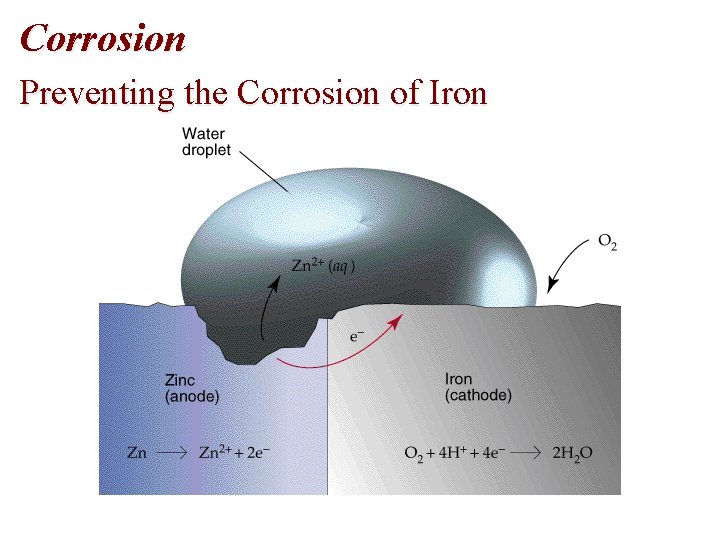
Corrosion: Preventing • Corrosion can be prevented by coating the iron with paint or another metal. • Galvanized iron is coated with a thin layer of zinc • . • Zinc protects the iron since Zn is the anode and Fe the cathode: Zn 2+(aq) +2 e- Zn(s), E red = -0. 76 V Fe 2+(aq) + 2 e- Fe(s), E red = -0. 44 V

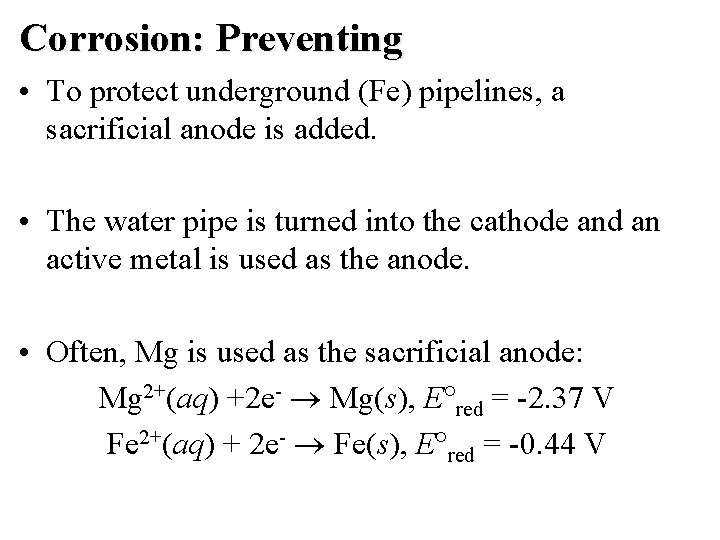
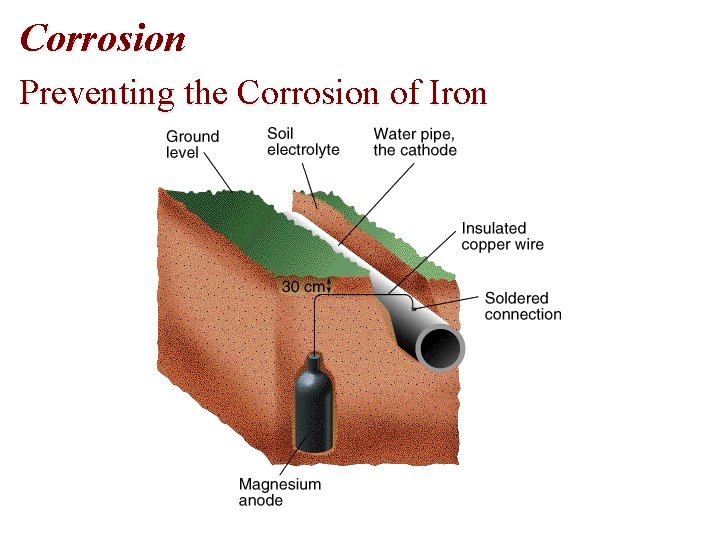
Corrosion: Preventing • To protect underground (Fe) pipelines, a sacrificial anode is added. • The water pipe is turned into the cathode and an active metal is used as the anode. • Often, Mg is used as the sacrificial anode: Mg 2+(aq) +2 e- Mg(s), E red = -2. 37 V Fe 2+(aq) + 2 e- Fe(s), E red = -0. 44 V

Corrosion Preventing the Corrosion of Iron

Corrosion Preventing the Corrosion of Iron