Chapter 19 Electrochemistry Lecture Topics 1 Balancing RedoxOxidationReduction

Chapter 19: Electrochemistry • Lecture Topics – 1. Balancing Redox/Oxidation-Reduction Reactions. – 2. Voltaic Cells – 3. Electrolysis – 4. Corrosion
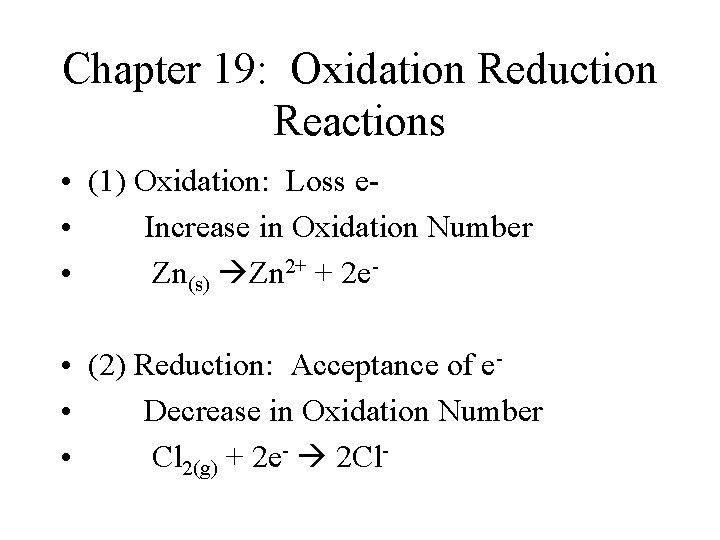
Chapter 19: Oxidation Reduction Reactions • (1) Oxidation: Loss e • Increase in Oxidation Number • Zn(s) Zn 2+ + 2 e • (2) Reduction: Acceptance of e • Decrease in Oxidation Number • Cl 2(g) + 2 e- 2 Cl-

Balancing Oxidation-Reduction Equations: Use Half-Reaction Method The half-reactions for Sn 2+(aq) + 2 Fe 3+(aq) Sn 4+(aq) + 2 Fe 3+(aq) are Sn 2+(aq) Sn 4+(aq) +2 e 2 Fe 3+(aq) + 2 e- 2 Fe 2+(aq) • Oxidation Half-Reaction: electrons are products. • Reduction Half-Reaction: electrons are reactants.

Half-Reaction Method for Balancing Oxidation-Reduction Equations 1. Separate the equation into the two half-reactions. Write down the two half reactions. 2. Balance each half reaction: a. First balance all elements other than H and O. b. Then balance O by adding water. c. Then balance H by adding H+ if have acidic solution. d. Finish by balancing charge by adding electrons.

Half-Reaction Method for Balancing Oxidation-Reduction Equations 3. Multiply each half reaction to make the number of electrons equal. 4. Add the two half-reactions and simplify. To simplify, remove components common to both reactant and product sides. 5. Check!
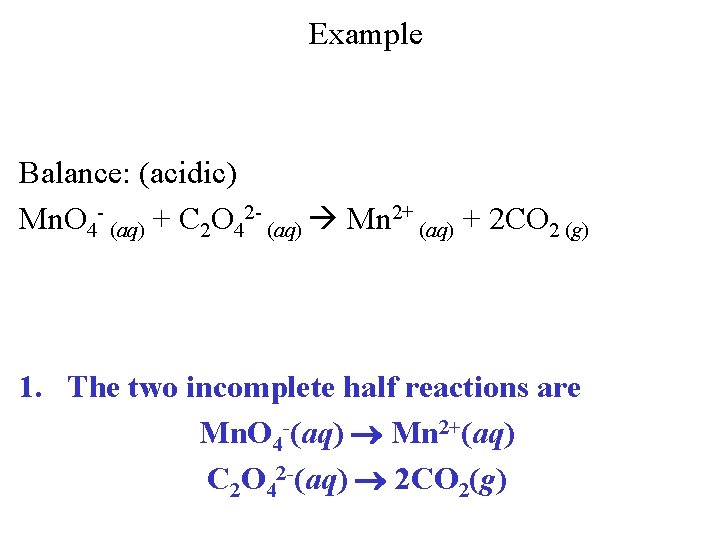
Example Balance: (acidic) Mn. O 4 - (aq) + C 2 O 42 - (aq) Mn 2+ (aq) + 2 CO 2 (g) 1. The two incomplete half reactions are Mn. O 4 -(aq) Mn 2+(aq) C 2 O 42 -(aq) 2 CO 2(g)
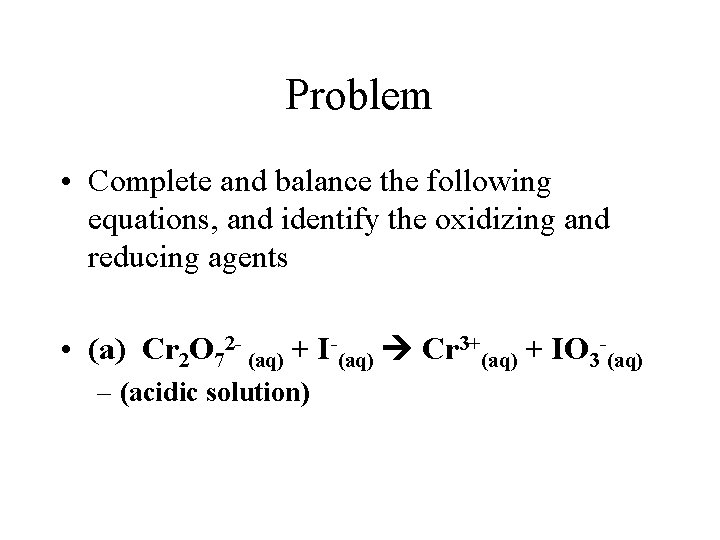
Problem • Complete and balance the following equations, and identify the oxidizing and reducing agents • (a) Cr 2 O 72 - (aq) + I-(aq) Cr 3+(aq) + IO 3 -(aq) – (acidic solution)
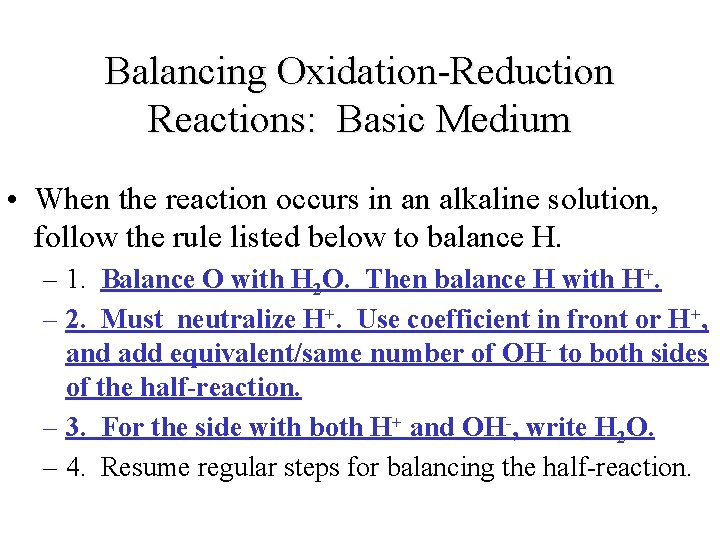
Balancing Oxidation-Reduction Reactions: Basic Medium • When the reaction occurs in an alkaline solution, follow the rule listed below to balance H. – 1. Balance O with H 2 O. Then balance H with H+. – 2. Must neutralize H+. Use coefficient in front or H+, and add equivalent/same number of OH- to both sides of the half-reaction. – 3. For the side with both H+ and OH-, write H 2 O. – 4. Resume regular steps for balancing the half-reaction.
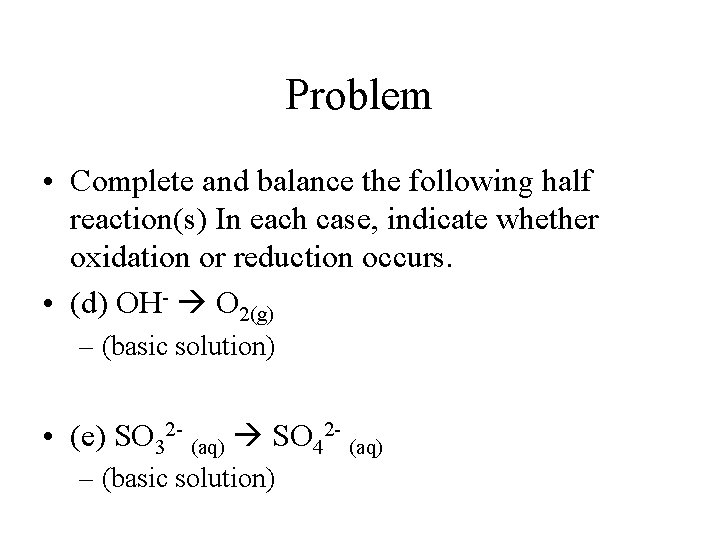
Problem • Complete and balance the following half reaction(s) In each case, indicate whether oxidation or reduction occurs. • (d) OH- O 2(g) – (basic solution) • (e) SO 32 - (aq) SO 42 - (aq) – (basic solution)
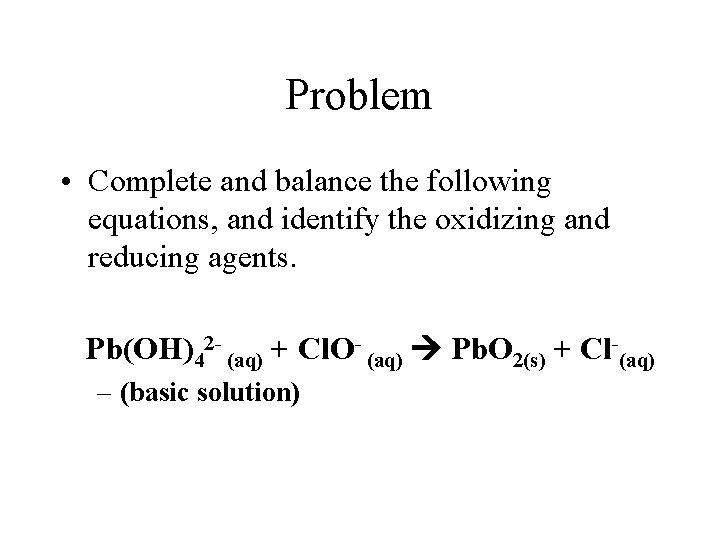
Problem • Complete and balance the following equations, and identify the oxidizing and reducing agents. Pb(OH)42 - (aq) + Cl. O- (aq) Pb. O 2(s) + Cl-(aq) – (basic solution)
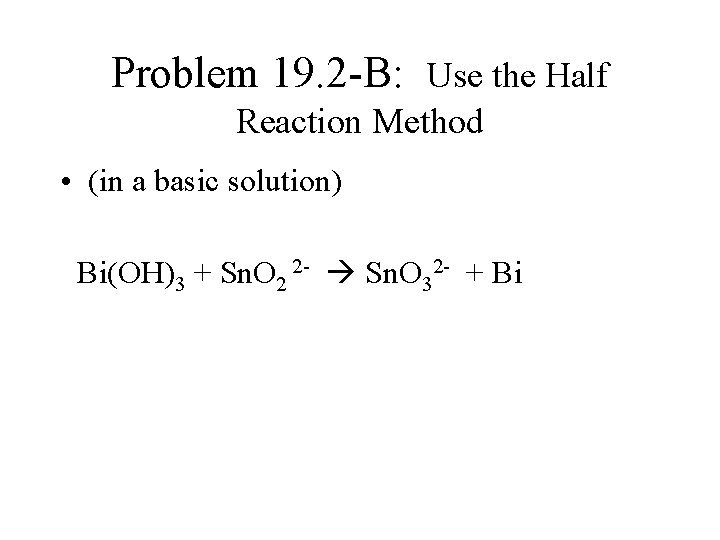
Problem 19. 2 -B: Use the Half Reaction Method • (in a basic solution) Bi(OH)3 + Sn. O 2 2 - Sn. O 32 - + Bi
- Slides: 11