Chapter 19 Chemical Bonds cartoon Section 2 Types

Chapter 19: Chemical Bonds cartoon

Section 2: Types of Bonds Gain or Loss of Electrons • ion: an atom that has gained or lost electrons to become more stable • the positive charge of the protons and the negative charge of the electrons is unbalanced • more protons than electrons = positive ion • more electrons than protons = negative ion

• How do we know how many electrons an atom will lose or gain? • The periodic table tells us! – Group 1: loses 1 electron – Group 2: loses 2 electrons – Group 13: loses 3 electrons – Group 14: shares 4 electrons – Group 15: gains 3 electrons – Group 16: gains 2 electrons – Group 17: gains 1 electron – transition elements vary

cartoon http: //www. lab-initio. com/screen_res/nz 069. jpg

• A bond forms when elements that need to lose electrons come in contact with atoms that needs to gain electrons to become stable • These are all ions: – Na + sodium ion – Cl - chlorine ion – K + potassium ion – Mg 2+ magnesium ion – Al 3+ aluminum ion – S 2 - sulfur ion • The + and – are superscripts, “written above”

Example: Potassium Iodide • How many electrons does potassium (K) gain or lose to become stable? – It loses 1 electron • How many electrons does iodine (I) gain or lose to become stable? • Potassium (K+) gives iodine (I-) its lone electron and both are stable + = Webelements. com Wikimedia. org – It gains 1 electron

• Positive ions and negative ions are always attracted to each other • As a result, they form an ionic bond: the force of attraction between opposite charges of ions • The result of the bond is a neutral compound • Occurs between a metal (+) and a nonmetal (-) • Wait: what if the charges are not the same number of electrons, like Mg 2+ and Cl - ?

Example: Magnesium Chloride • Magnesium wants to lose 2 electrons • Chlorine wants to gain only 1 electron • What happens to that extra electron from magnesium? • Another chlorine will take it! Mg. Cl 2 + = Webelements. com Wikimedia. org Mg
cartoon

Sharing Electrons • Some nonmetals are unlikely to lose or gain electrons • Group 14 elements have 4 electrons in their outer energy level: they could gain four or lose four • When it takes more energy to gain or lose electrons rather than just share them, a covalent bond is formed by sharing e • The new compound is a molecule, and is only formed between nonmetals • Common molecules: Si. O 2 H 2 O C 12 H 22 O 11

Sharing Electrons • Some elements can share more than one pair of electrons – CO 2 Two pairs shared: double bond O=C=O – N 2 Three pairs shared: triple bond N≡N • However, atoms don’t always share nicely • Some atoms “hog” the electrons to themselves, like oxygen • Perfect example: CO 2 : O=C=O: • Oxygen pulls the electrons away from carbon
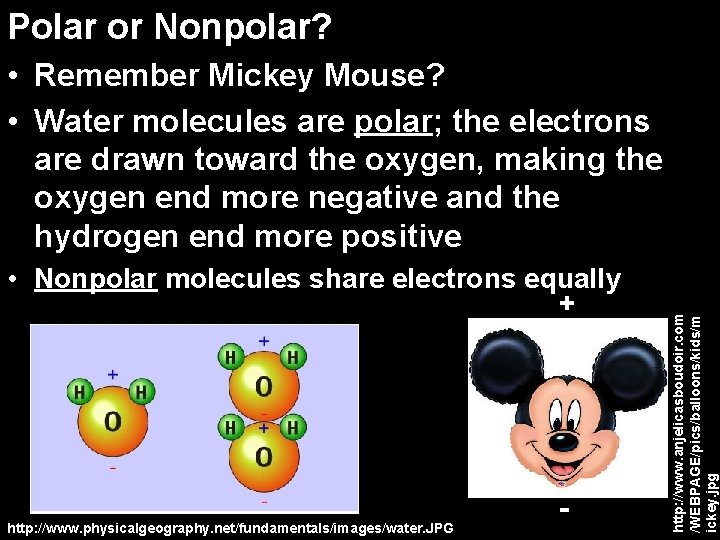
Polar or Nonpolar? • Nonpolar molecules share electrons equally + http: //www. physicalgeography. net/fundamentals/images/water. JPG - http: //www. anjelicasboudoir. com /WEBPAGE/pics/balloons/kids/m ickey. jpg • Remember Mickey Mouse? • Water molecules are polar; the electrons are drawn toward the oxygen, making the oxygen end more negative and the hydrogen end more positive

Review Questions, p. 586 Q 1 -4 Answer these questions for tomorrow: 1. Why does an atom make an ionic bond only with certain other atoms? 2. Compare the possession of electrons in ionic and covalent bonds. 3. What types of particles are formed by ionic bonds? Covalent bonds? 4. What is the difference between polar and nonpolar molecules?
- Slides: 13