Chapter 19 Avogadros Principle Avogadros Principle Avogadros Principle








- Slides: 24

Chapter 19 Avogadro’s Principle

Avogadro’s Principle • Avogadro's Principle states that equal volumes of gases at the same temperature and pressure contain an equal number of particles. • What is this really saying? – There are four related factors for gases, P, V, T and number of particles. If any three of these factors are equivalent then the fourth factor must be equivalent as well.

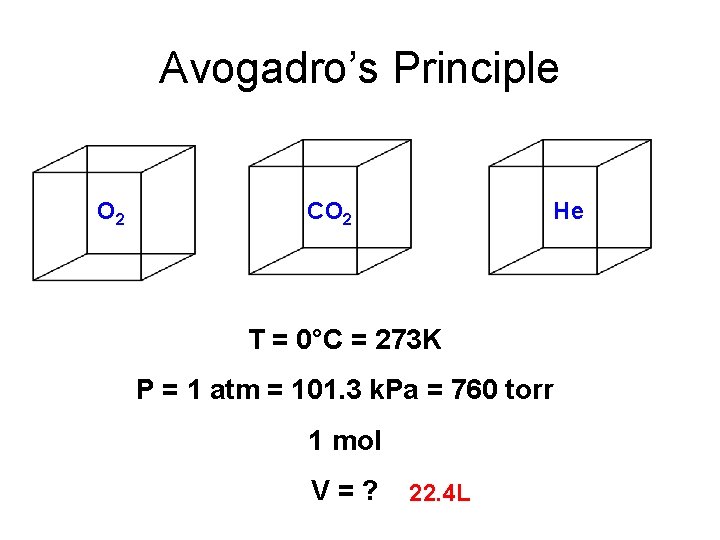
Avogadro’s Principle O 2 CO 2 He T = 0°C = 273 K P = 1 atm = 101. 3 k. Pa = 760 torr 1 mol V=? 22. 4 L

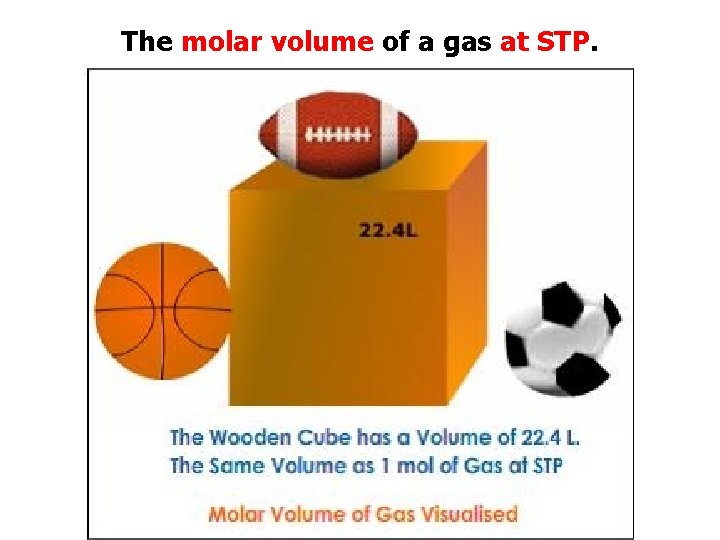
The molar volume of a gas at STP.

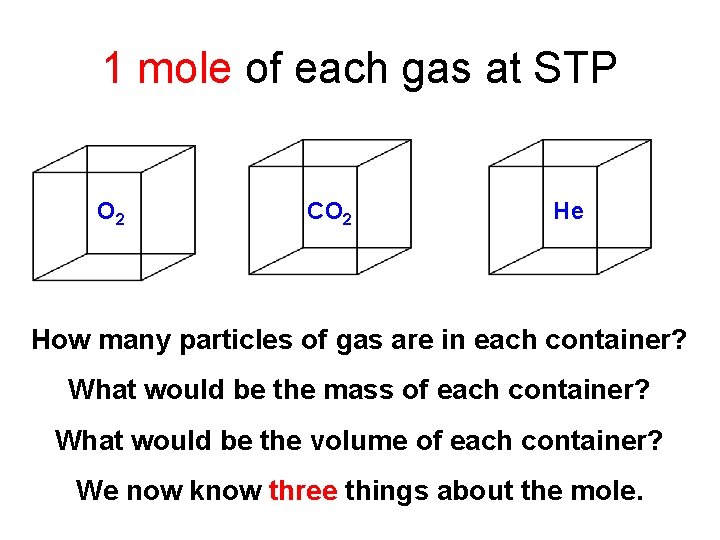
1 mole of each gas at STP O 2 CO 2 He How many particles of gas are in each container? What would be the mass of each container? What would be the volume of each container? We now know three things about the mole.

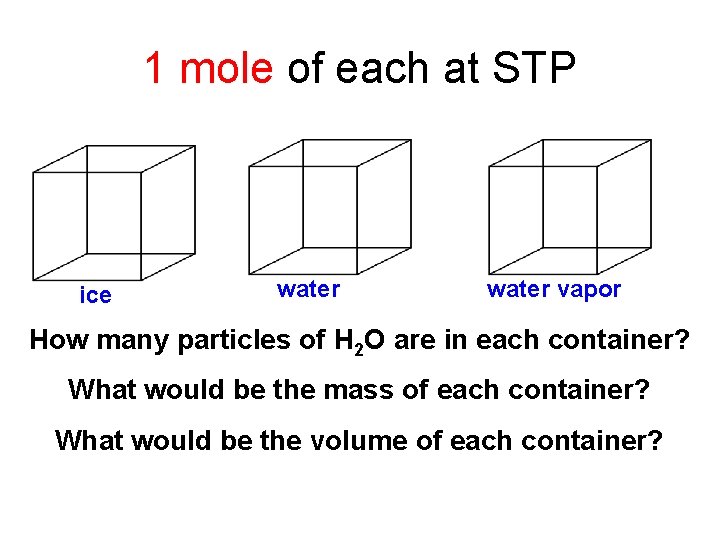
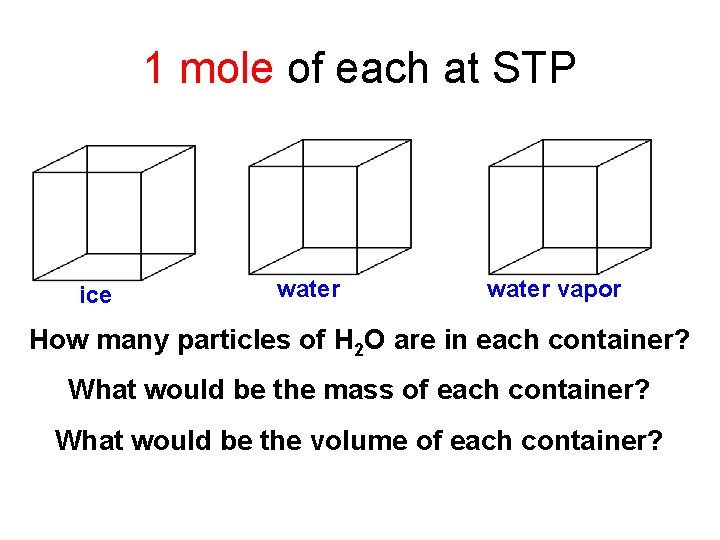
1 mole of each at STP ice water vapor How many particles of H 2 O are in each container? What would be the mass of each container? What would be the volume of each container?

The molar volume of a gas at STP. 22. 4 L

1 mole of each at STP ice water vapor How many particles of H 2 O are in each container? What would be the mass of each container? What would be the volume of each container?

1 mole of each at STP ice water vapor How many particles of H 2 O are in each container? What would be the mass of each container? What would be the volume of each container?

Mole Conversions

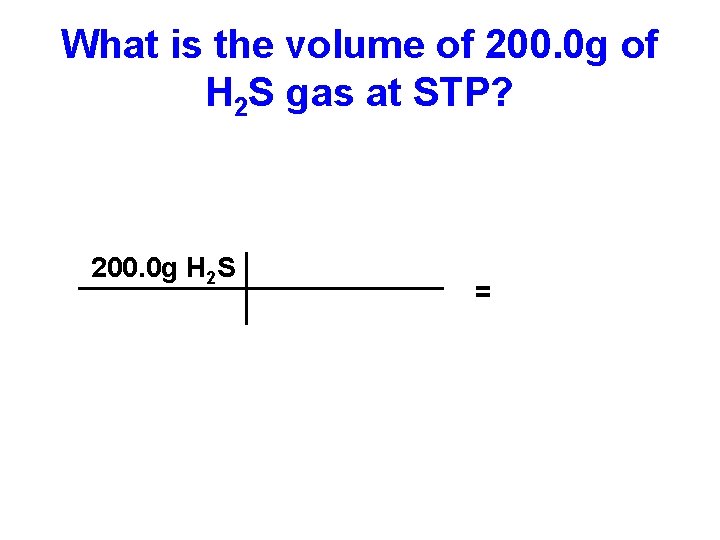
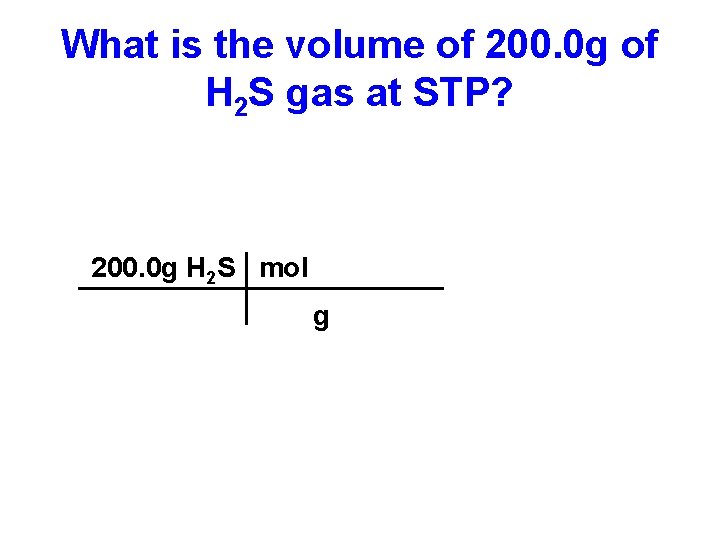
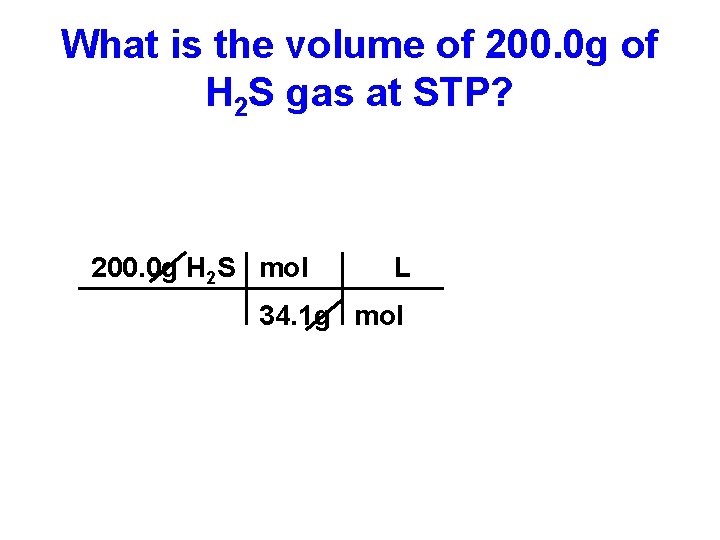
What is the volume of 200. 0 g of H 2 S gas at STP?

What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S =

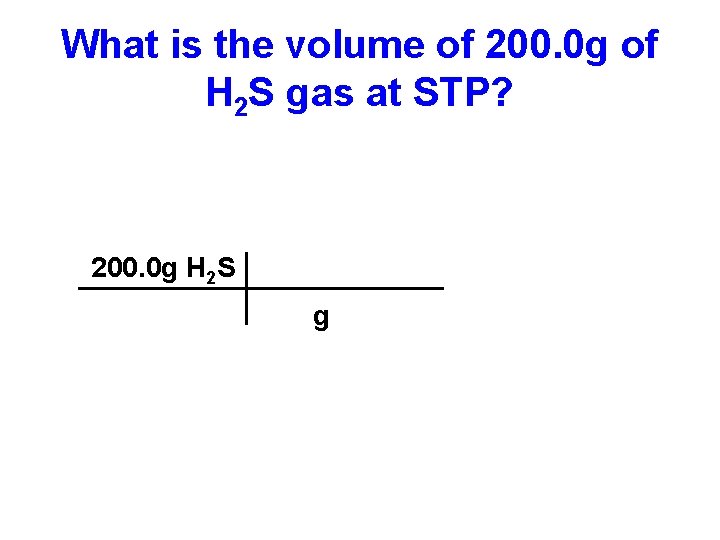
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S g

What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol g

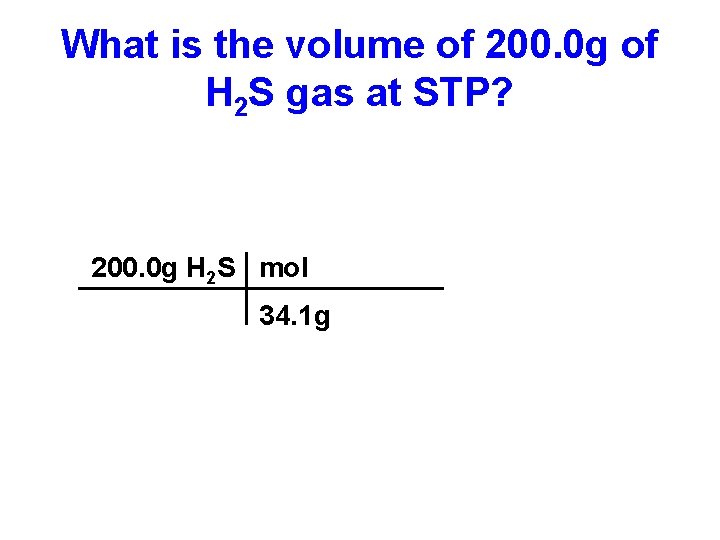
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol 34. 1 g

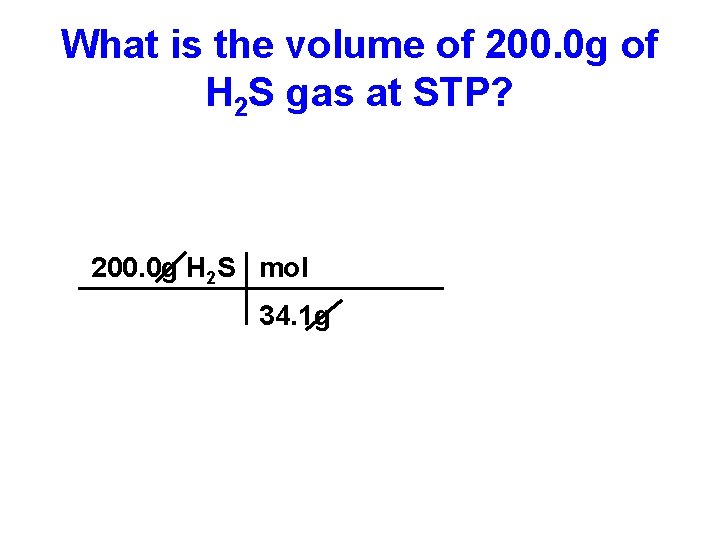
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol 34. 1 g

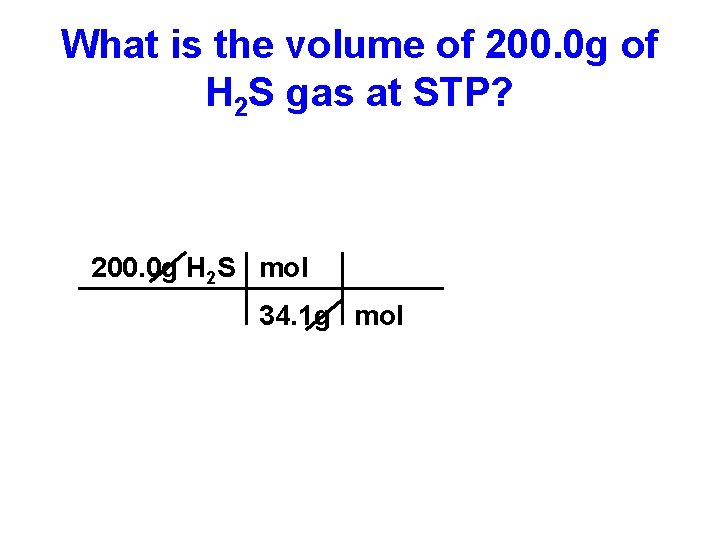
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol 34. 1 g mol

What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol L 34. 1 g mol

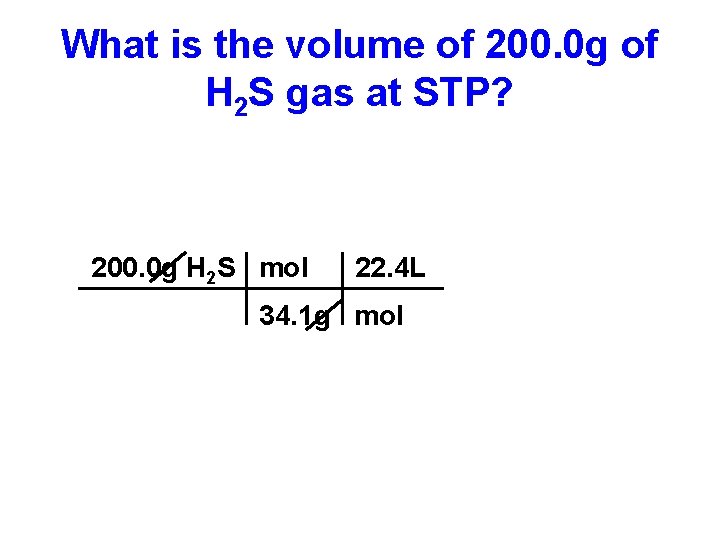
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol 22. 4 L 34. 1 g mol

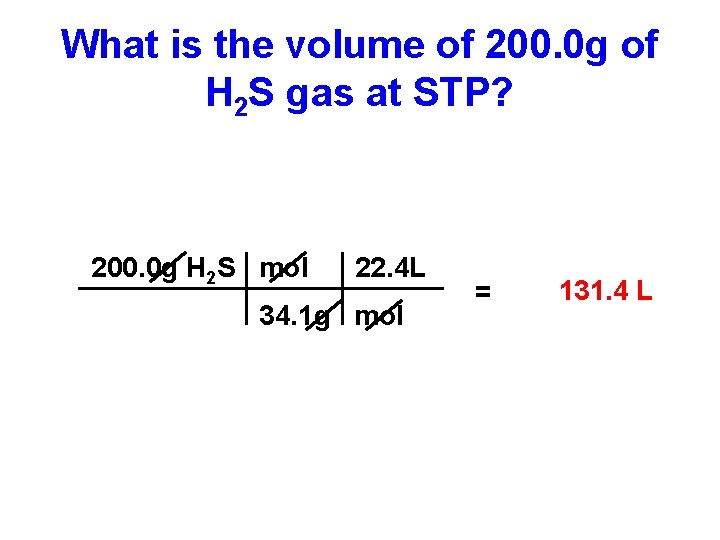
What is the volume of 200. 0 g of H 2 S gas at STP? 200. 0 g H 2 S mol 22. 4 L 34. 1 g mol = 131. 4 L

What is the mass of 14. 7 L of CO 2 at STP? 28. 9 grams

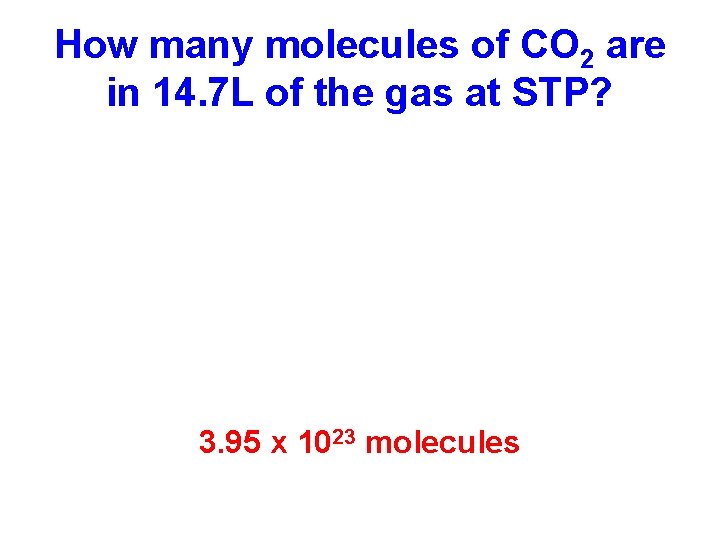
How many molecules of CO 2 are in 14. 7 L of the gas at STP? 3. 95 x 1023 molecules

How many atoms are in 14. 7 L of CO 2 gas at STP? 1. 19 x 1024 atoms

Homework • Summarize the (I) Purpose and (II) Procedure of the “Gas Laws” Lab (due tomorrow). • Study Guide Chapter 18. (due tomorrow). • Worksheet: Combined Gas Law & Graham’s Law. (due tomorrow). Mole-Volume Conversions Worksheet (due in two days).