Chapter 19 Amino Acids and Proteins 19 2
- Slides: 13

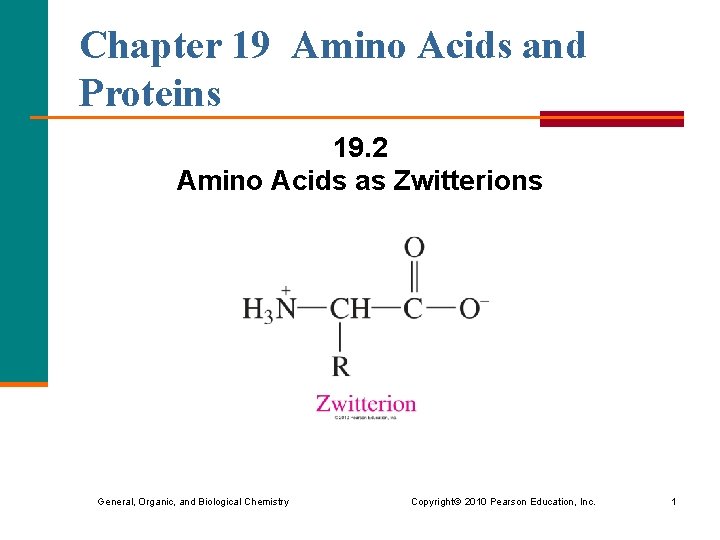
Chapter 19 Amino Acids and Proteins 19. 2 Amino Acids as Zwitterions General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

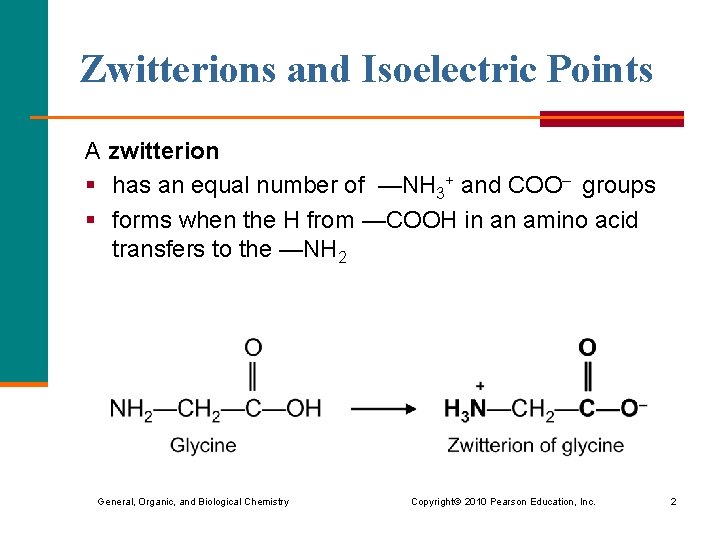
Zwitterions and Isoelectric Points A zwitterion § has an equal number of —NH 3+ and COO– groups § forms when the H from —COOH in an amino acid transfers to the —NH 2 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

Isoelectric Point (p. I) The isoelectric points (p. I) § are the p. H at which zwitterions have an overall zero charge § of nonpolar and polar (neutral) amino acids exist at p. H values from 5. 1 to 6. 3 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

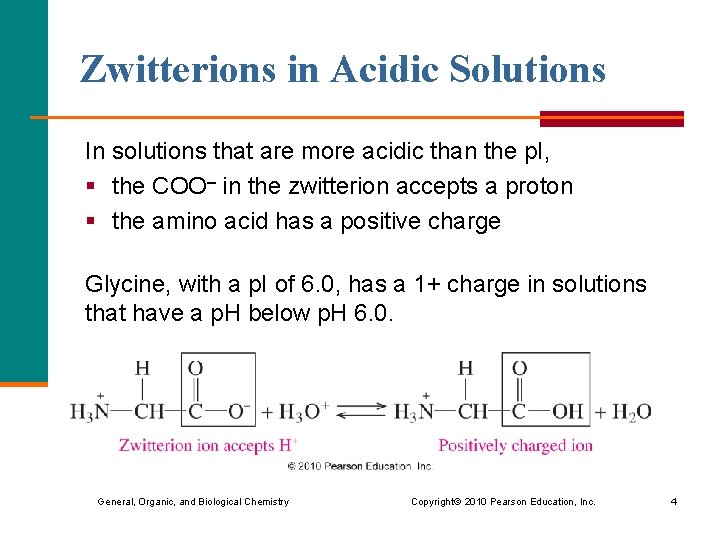
Zwitterions in Acidic Solutions In solutions that are more acidic than the p. I, § the COO– in the zwitterion accepts a proton § the amino acid has a positive charge Glycine, with a p. I of 6. 0, has a 1+ charge in solutions that have a p. H below p. H 6. 0. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

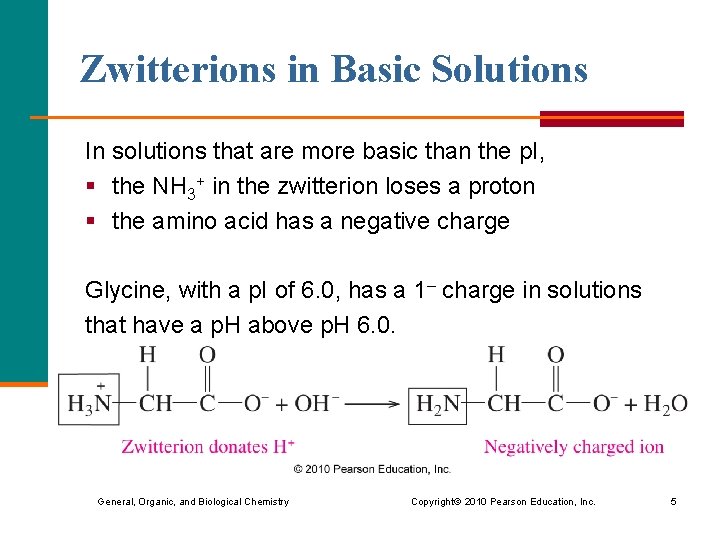
Zwitterions in Basic Solutions In solutions that are more basic than the p. I, § the NH 3+ in the zwitterion loses a proton § the amino acid has a negative charge Glycine, with a p. I of 6. 0, has a 1– charge in solutions that have a p. H above p. H 6. 0. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

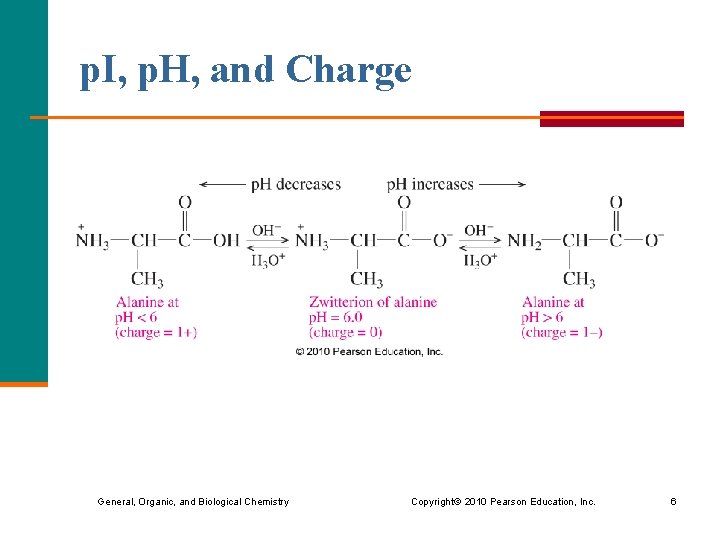
p. I, p. H, and Charge General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

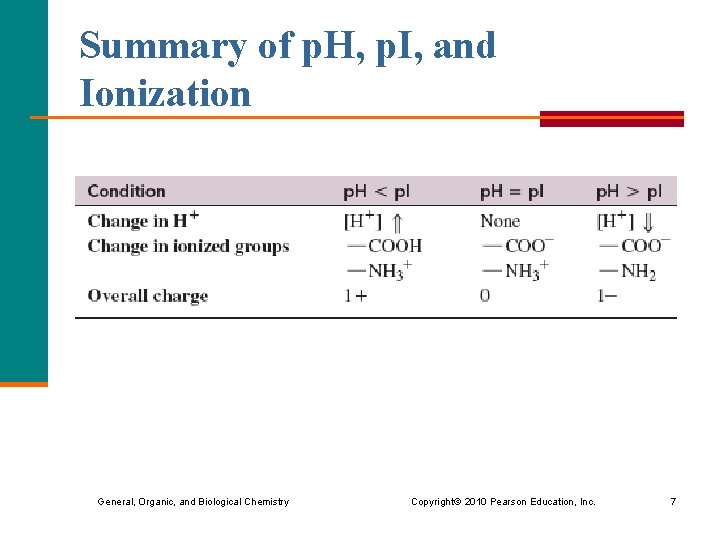
Summary of p. H, p. I, and Ionization General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

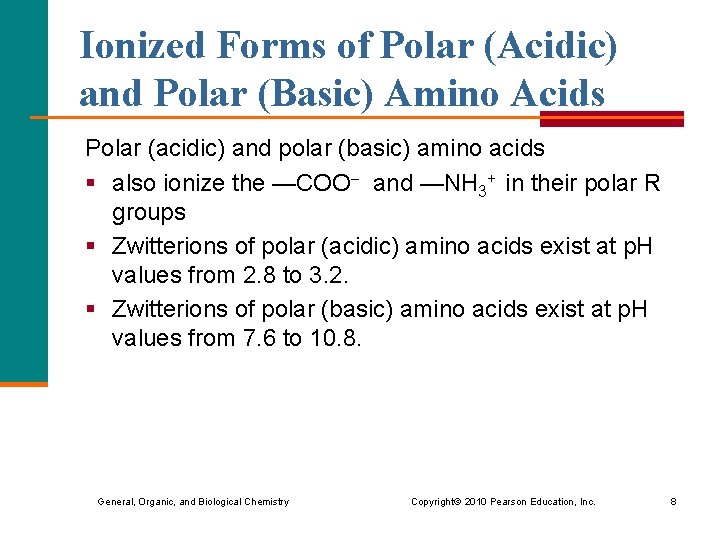
Ionized Forms of Polar (Acidic) and Polar (Basic) Amino Acids Polar (acidic) and polar (basic) amino acids § also ionize the —COO and —NH 3+ in their polar R groups § Zwitterions of polar (acidic) amino acids exist at p. H values from 2. 8 to 3. 2. § Zwitterions of polar (basic) amino acids exist at p. H values from 7. 6 to 10. 8. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

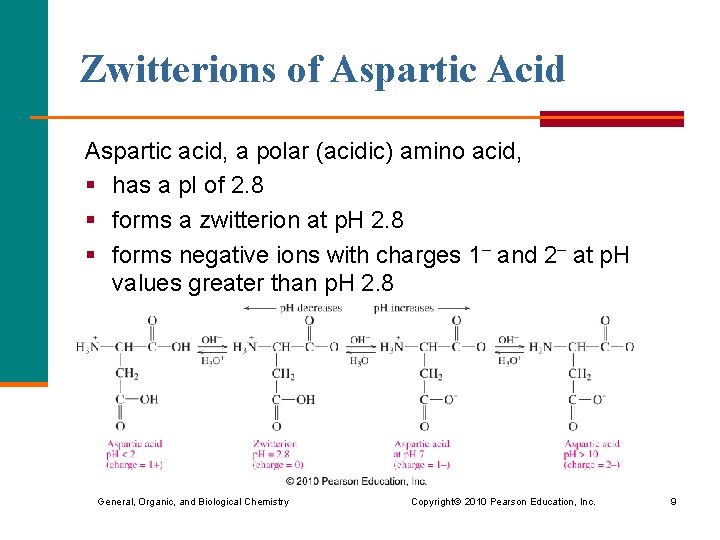
Zwitterions of Aspartic Acid Aspartic acid, a polar (acidic) amino acid, § has a p. I of 2. 8 § forms a zwitterion at p. H 2. 8 § forms negative ions with charges 1– and 2– at p. H values greater than p. H 2. 8 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

Electrophoresis: Separation of Amino Acids In electrophoresis, an electric current is used to separate a mixture of amino acids, and § the positively charged amino acids move toward the negative electrode § the negatively charged amino acids move toward the positive electrode § an amino acid at its p. I does not migrate § the amino acids are identified as separate bands on the filter paper or thin layer plate General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

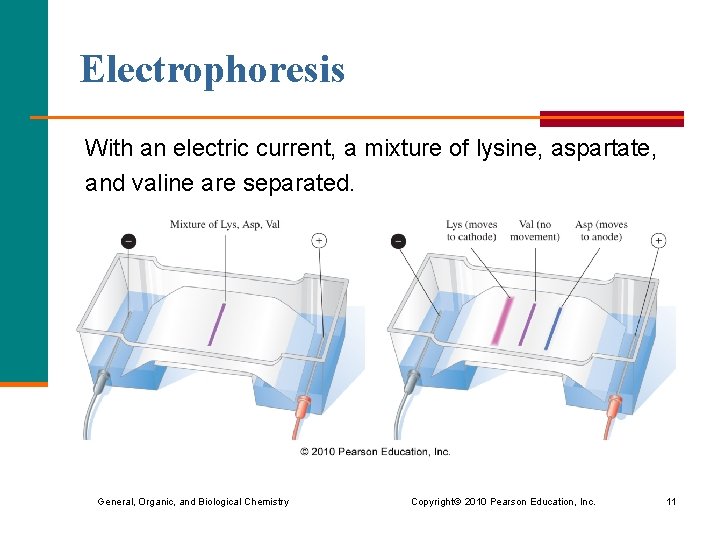
Electrophoresis With an electric current, a mixture of lysine, aspartate, and valine are separated. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

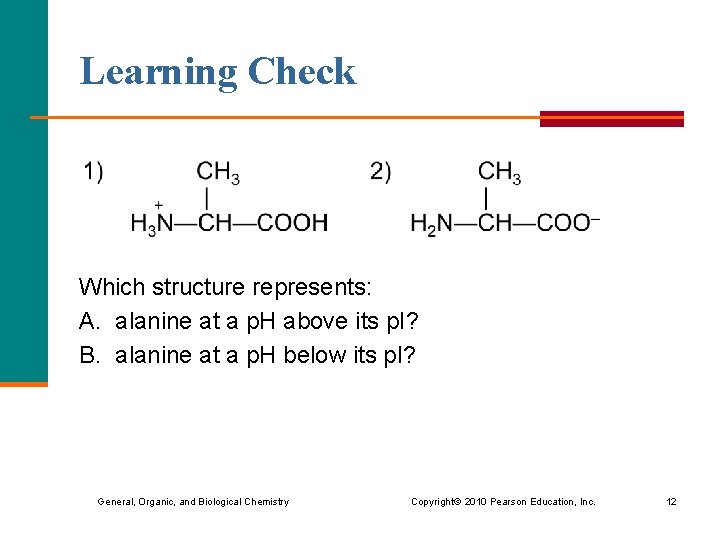
Learning Check Which structure represents: A. alanine at a p. H above its p. I? B. alanine at a p. H below its p. I? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

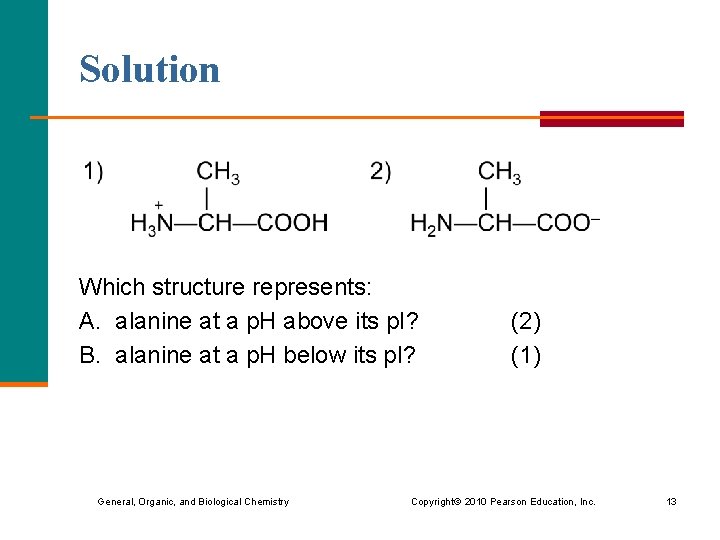
Solution Which structure represents: A. alanine at a p. H above its p. I? B. alanine at a p. H below its p. I? (2) (1) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13