Chapter 19 Amino Acids and Proteins 19 1




































- Slides: 65

Chapter 19 Amino Acids and Proteins 19. 1 Proteins and Amino Acids 19. 2 Amino Acids as Acids and Bases Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

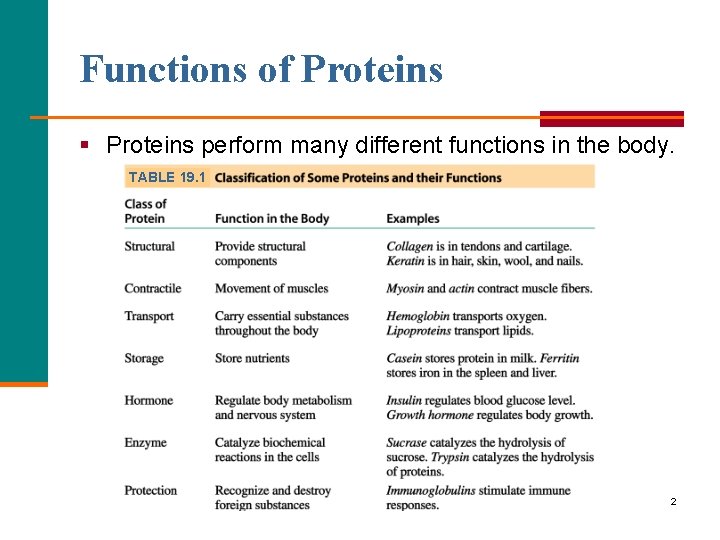
Functions of Proteins § Proteins perform many different functions in the body. TABLE 19. 1 2

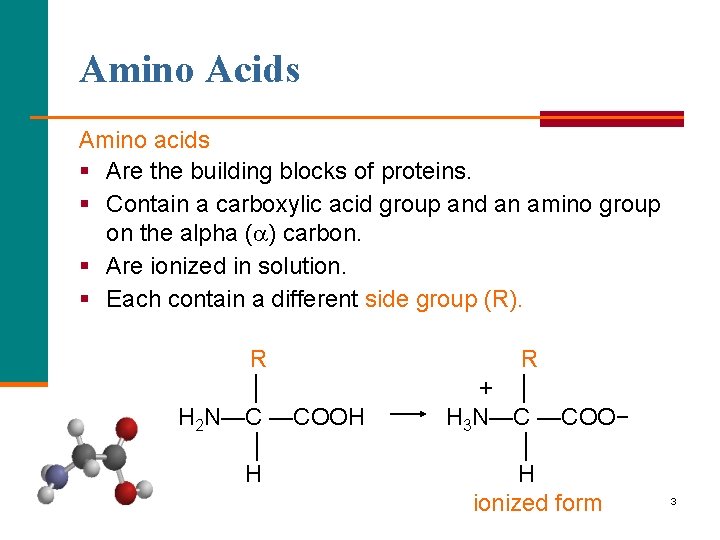
Amino Acids Amino acids § Are the building blocks of proteins. § Contain a carboxylic acid group and an amino group on the alpha ( ) carbon. § Are ionized in solution. § Each contain a different side group (R). R │ H 2 N—C —COOH │ H R + │ H 3 N—C —COO− │ H ionized form 3

Examples of Amino Acids H + │ H 3 N—C—COO− │ H glycine CH 3 + │ H 3 N—C—COO− │ H alanine 4

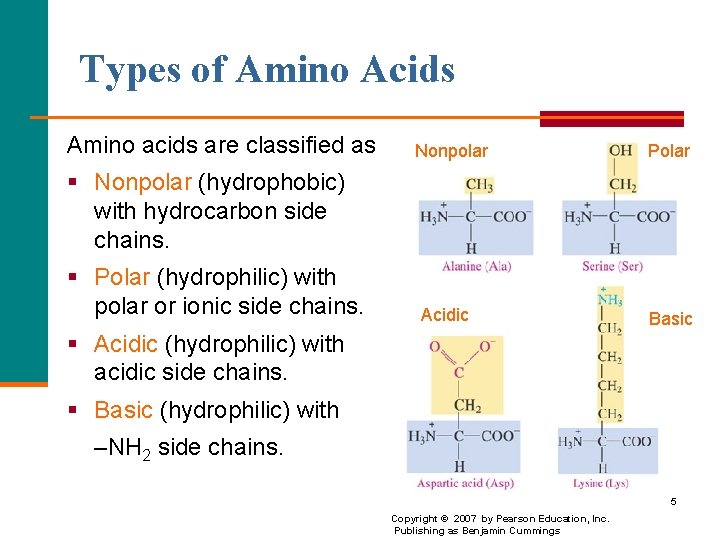
Types of Amino Acids Amino acids are classified as Nonpolar Polar § Nonpolar (hydrophobic) with hydrocarbon side chains. § Polar (hydrophilic) with polar or ionic side chains. Acidic Basic § Acidic (hydrophilic) with acidic side chains. § Basic (hydrophilic) with –NH 2 side chains. 5 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings

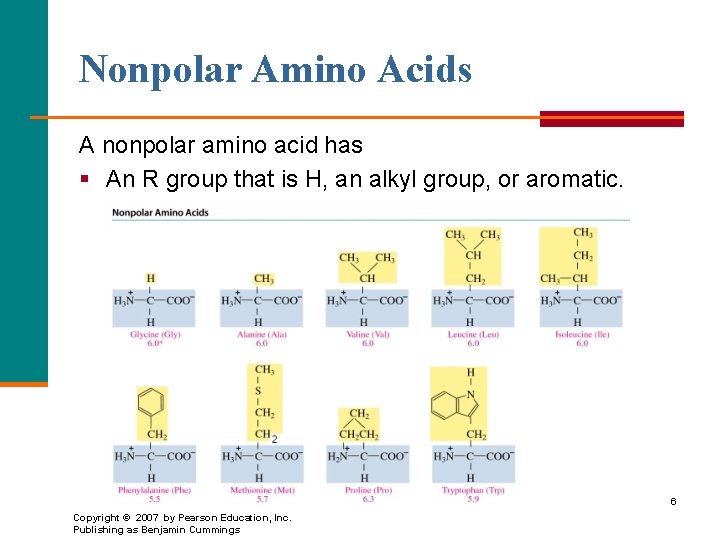
Nonpolar Amino Acids A nonpolar amino acid has § An R group that is H, an alkyl group, or aromatic. 6 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings

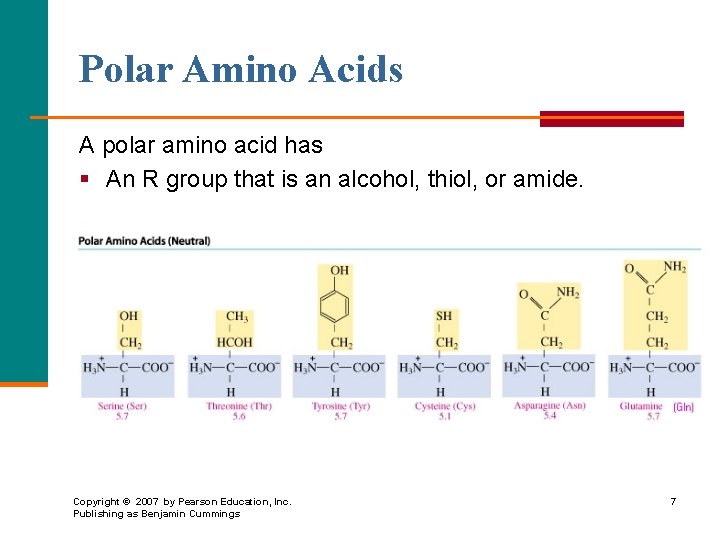
Polar Amino Acids A polar amino acid has § An R group that is an alcohol, thiol, or amide. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

Acidic and Basic Amino Acids An amino acid is § Acidic with a carboxyl R group (COO−). § Basic with an amino R group (NH 3+). Basic Amino Acids Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 8

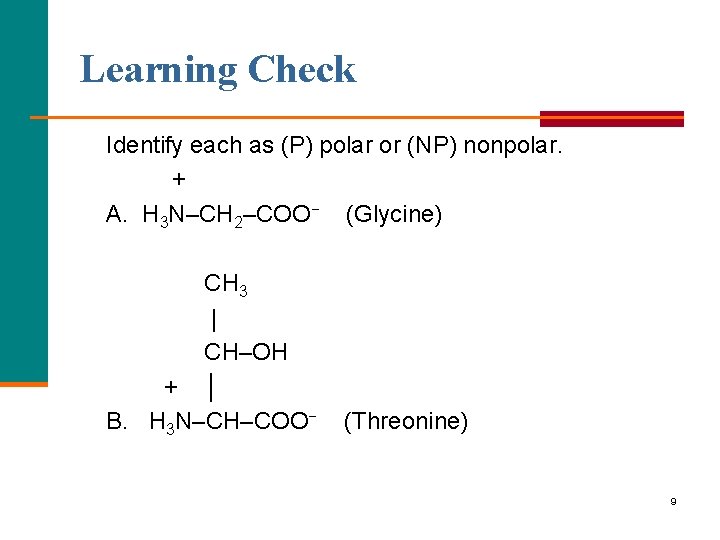
Learning Check Identify each as (P) polar or (NP) nonpolar. + A. H 3 N–CH 2–COO− (Glycine) CH 3 | CH–OH + │ B. H 3 N–CH–COO− (Threonine) 9

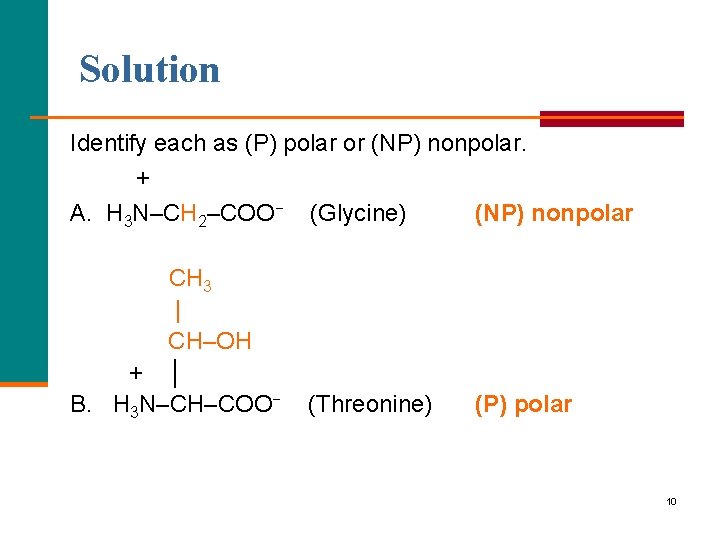
Solution Identify each as (P) polar or (NP) nonpolar. + A. H 3 N–CH 2–COO− (Glycine) (NP) nonpolar CH 3 | CH–OH + │ B. H 3 N–CH–COO− (Threonine) (P) polar 10

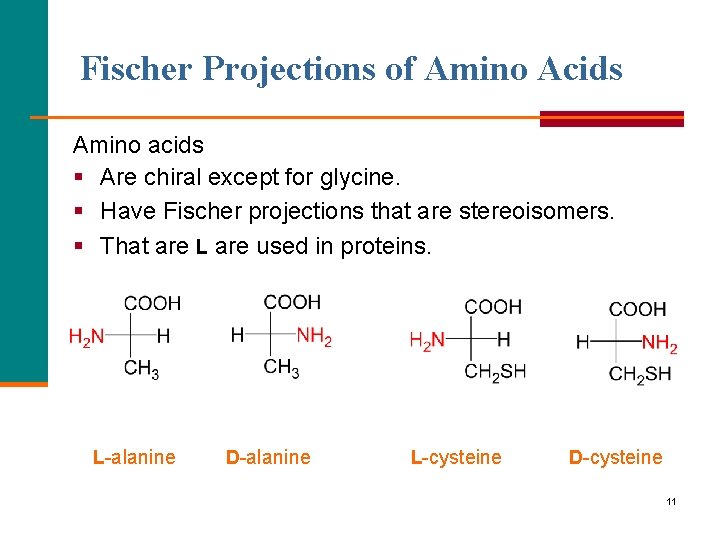
Fischer Projections of Amino Acids Amino acids § Are chiral except for glycine. § Have Fischer projections that are stereoisomers. § That are L are used in proteins. L alanine D alanine L cysteine D cysteine 11

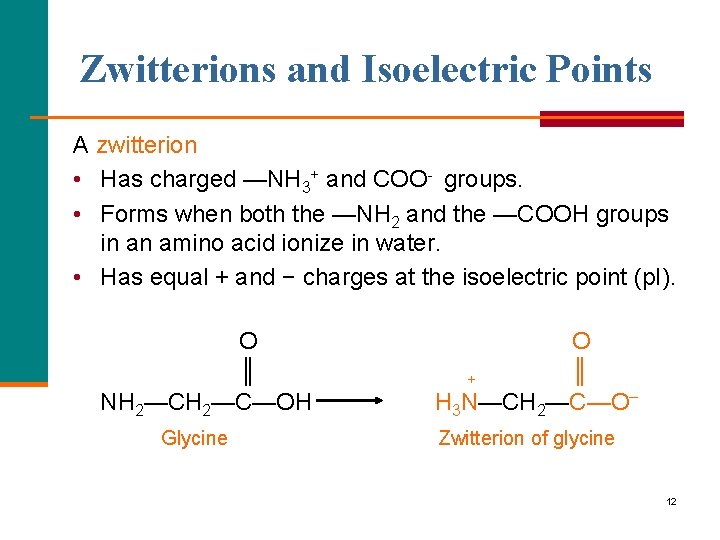
Zwitterions and Isoelectric Points A zwitterion • Has charged —NH 3+ and COO groups. • Forms when both the —NH 2 and the —COOH groups in an amino acid ionize in water. • Has equal + and − charges at the isoelectric point (p. I). O ║ NH 2—C—OH Glycine O ║ + H 3 N—CH 2—C—O– Zwitterion of glycine 12

Amino Acids as Acids In solutions more basic than the p. I, § The —NH 3+ in the amino acid donates a proton. + H 3 N—CH 2—COO– Zwitterion at p. I Charge: 0 OH– H 2 N—CH 2—COO– Negative ion p. H > p. I Charge: 1− 13

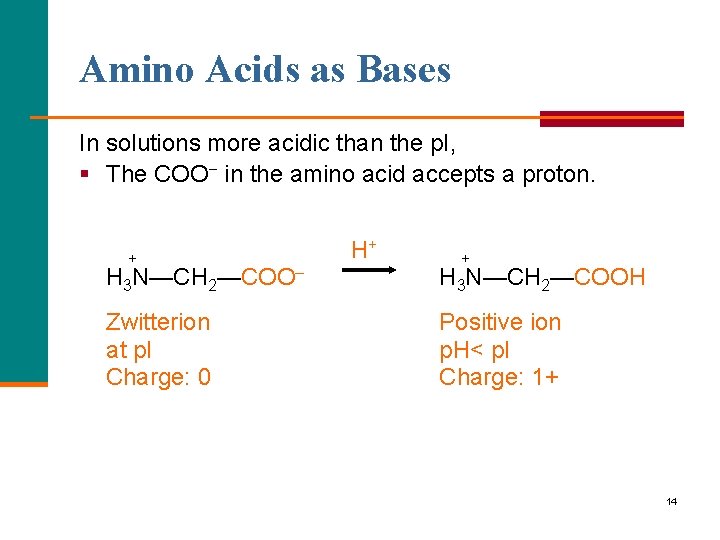
Amino Acids as Bases In solutions more acidic than the p. I, § The COO− in the amino acid accepts a proton. + H+ + H 3 N—CH 2—COO– H 3 N—CH 2—COOH Zwitterion at p. I Charge: 0 Positive ion p. H< p. I Charge: 1+ 14

p. H and Ionization H+ + H 3 N–CH 2–COOH positive ion (at low p. H) OH− + H 3 N–CH 2–COO– zwitterion (at p. I) H 2 N–CH 2–COO– negative ion (at high p. H) 15

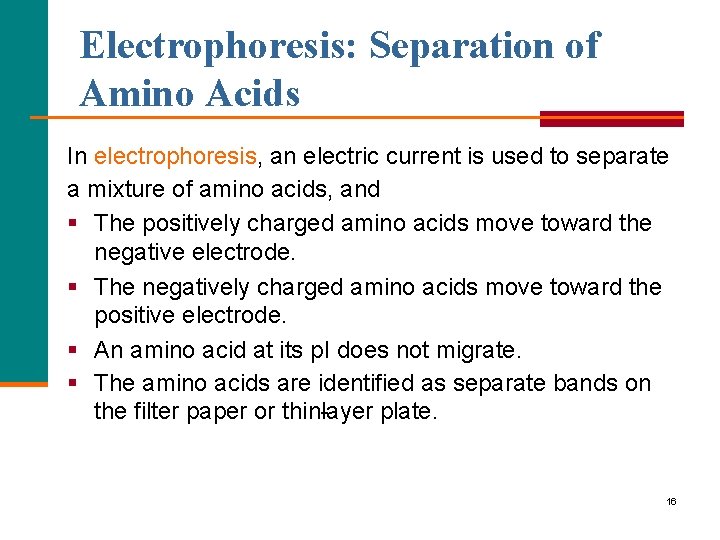
Electrophoresis: Separation of Amino Acids In electrophoresis, an electric current is used to separate a mixture of amino acids, and § The positively charged amino acids move toward the negative electrode. § The negatively charged amino acids move toward the positive electrode. § An amino acid at its p. I does not migrate. § The amino acids are identified as separate bands on the filter paper or thin layer plate. 16

Electrophoresis With an electric current, a mixture of lysine, aspartate, and valine are separated. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 17

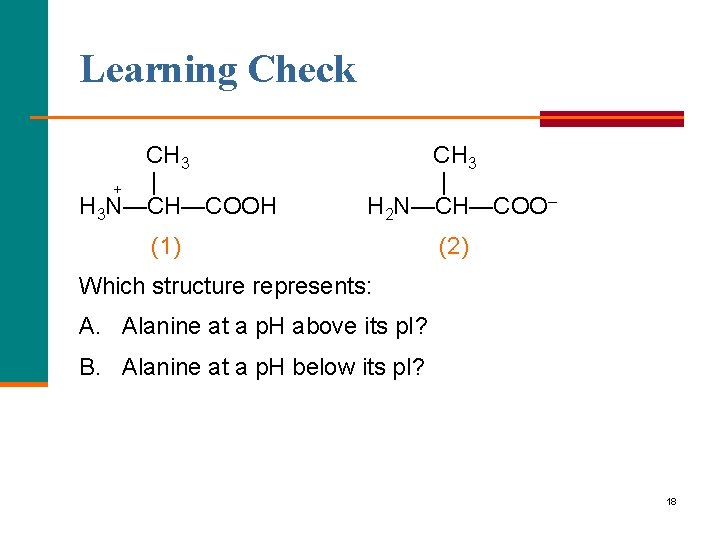
Learning Check CH 3 | + H 3 N—CH—COOH CH 3 | H 2 N—CH—COO– (1) (2) Which structure represents: A. Alanine at a p. H above its p. I? B. Alanine at a p. H below its p. I? 18

Solution CH 3 | + H 3 N—CH—COOH CH 3 | H 2 N—CH—COO– (1) (2) Which structure represents: A. Alanine at a p. H above its p. I? (2) B. Alanine at a p. H below its p. I? (1) 19

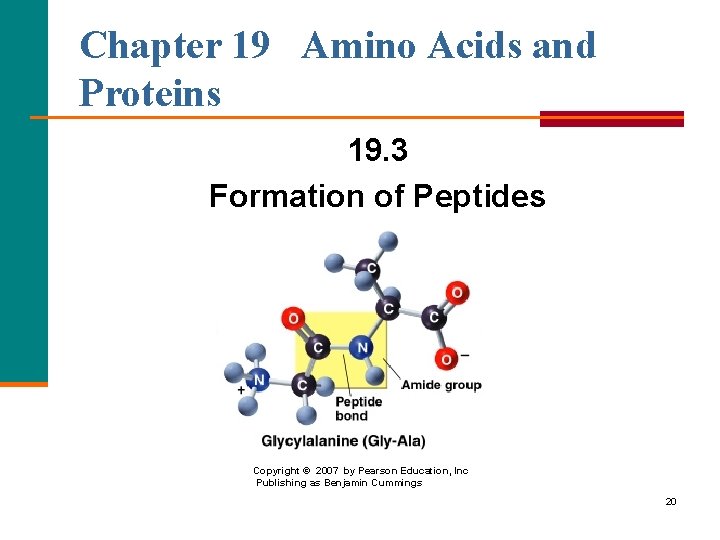
Chapter 19 Amino Acids and Proteins 19. 3 Formation of Peptides Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 20

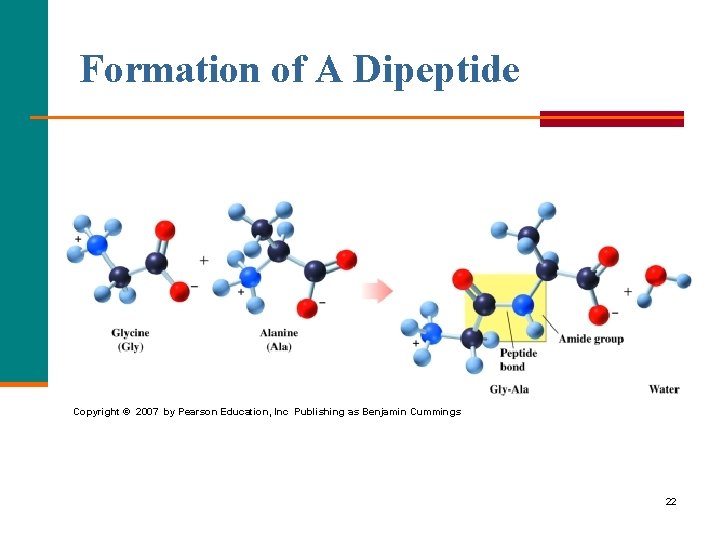
The Peptide Bond A peptide bond § Is an amide bond. § Forms between the carboxyl group of one amino acid and the amino group of the next amino acid. O + || H 3 N—CH 2—C—O– + CH 3 O + | || H 3 N—CH—C—O– O H CH 3 O + || | | || H 3 N—CH 2—C—N—CH—C—O– + H 2 O peptide bond 21

Formation of A Dipeptide Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 22

Learning Check Write the dipeptide Ser Thr. OH CH 3 | | CH 2 O HCOH O + | ║ H 3 N─CH─C─O – + H 3 N─CH─C─O– Ser Thr 23

Solution Write the dipeptide Ser Thr. OH CH 3 | | CH 2 O HCOH O + | ║ H 3 N─CH─C─O – + H 3 N─CH─C─O– Ser peptide Thr OH bond CH 3 | | CH 2 O H HCOH O + | ║ | | ║ NH 3─CH─C─N ─CH─C─O– + H 2 O Ser Thr 24

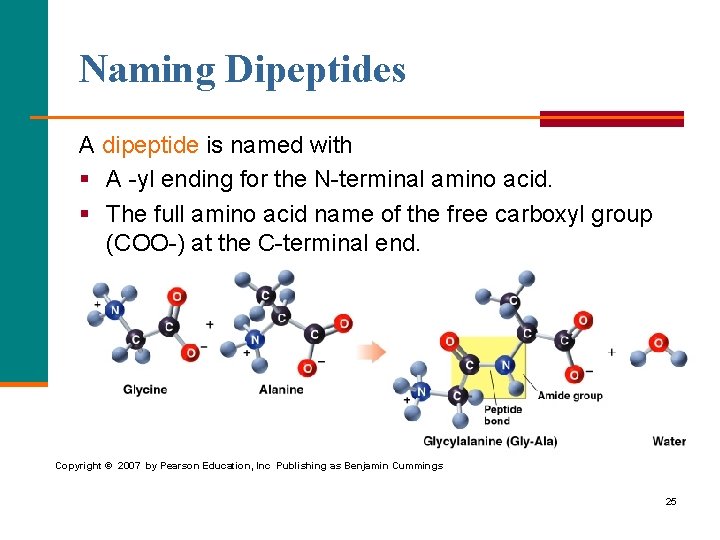
Naming Dipeptides A dipeptide is named with § A yl ending for the N terminal amino acid. § The full amino acid name of the free carboxyl group (COO ) at the C terminal end. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 25

Learning Check Write three letter abbreviations and names of the tripeptides that could form from two glycine and one alanine. 26

Solution Write the names and three letter abbreviations of the tripeptides that could form from two glycine and one alanine. Glycylglycylalanine Glycylalanylglycine Alanylglycine Gly Ala Gly 27

Learning Check What are the possible tripeptides formed from one each of leucine, glycine, and alanine? 28

Solution Tripeptides possible from one each of leucine, glycine, and alanine Leu Gly Ala Leu Ala Gly Ala Leu Gly Ala Gly Leu Gly Ala Leu Gly Leu Ala 29

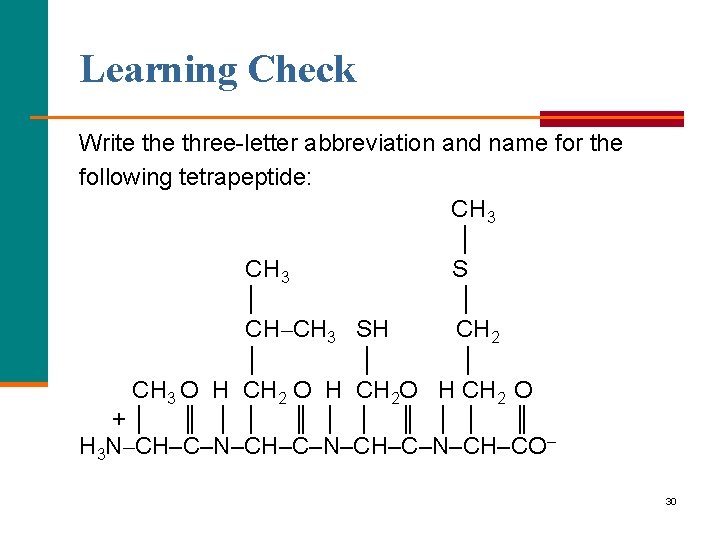
Learning Check Write three letter abbreviation and name for the following tetrapeptide: CH 3 │ CH 3 S │ │ CH–CH 3 SH CH 2 │ │ │ CH 3 O H CH 2 O H CH 2 O +│ ║ │ │ ║ H 3 N–CH–C–N–CH–C–N–CH–CO– 30

Solution Ala Leu Cys Met Alanylleucylcysteylmethionine CH 3 │ CH 3 S │ │ CH–CH 3 SH CH 2 │ │ │ CH 3 O H CH 2 O H CH 2 O +│ ║ │ │ ║ H 3 N–CH–C–N–CH–C–N–CH–CO– Ala Leu Cys Met 31

Chapter 19 Amino Acids and Proteins 19. 4 Protein Structure: Primary and Secondary Levels Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 32

Primary Structure of Proteins The primary structure of a protein is § The particular sequence of amino acids. § The backbone of a peptide chain or protein. Ala─Leu─Cys─Met Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 33

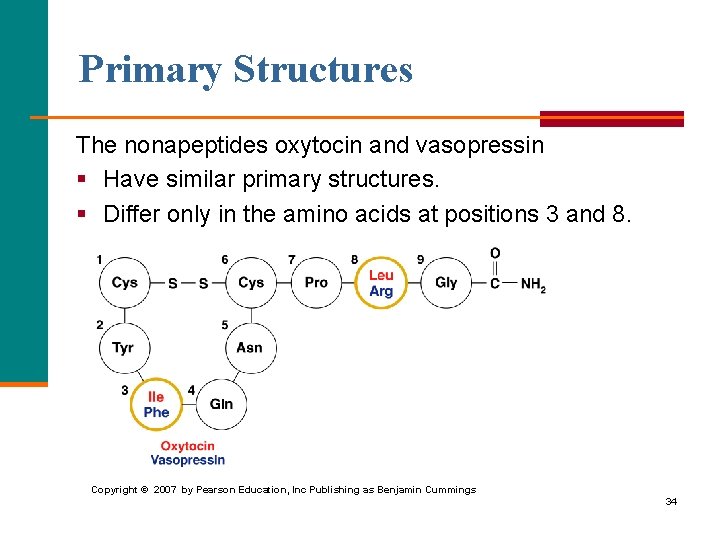
Primary Structures The nonapeptides oxytocin and vasopressin § Have similar primary structures. § Differ only in the amino acids at positions 3 and 8. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 34

Primary Structure of Insulin § Was the first protein to have its primary structure determined. § Has a primary structure of two polypeptide chains linked by disulfide bonds. § Has a chain A with 21 amino acids and a chain B with 30 amino acids. 35 Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings

Secondary Structure – Alpha Helix The secondary structures of proteins indicate three dimensional spatial arrangements of the polypeptide chains. An alpha helix has § A coiled shape held in place by hydrogen bonds between the amide groups and the carbonyl groups of the amino acids along the chain. § Hydrogen bonds between the H of a –N H group and the O of C=O of the fourth amino acid down the chain. 36

Secondary Structure – Alpha Helix Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 37

Secondary Structure – Beta Pleated Sheet A beta pleated sheet is a secondary structure that § Consists of polypeptide chains arranged side by side. § Has hydrogen bonds between chains. § Has R groups above and below the sheet. § Is typical of fibrous proteins such as silk. 38

Secondary Structure: β-Pleated Sheet Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 39

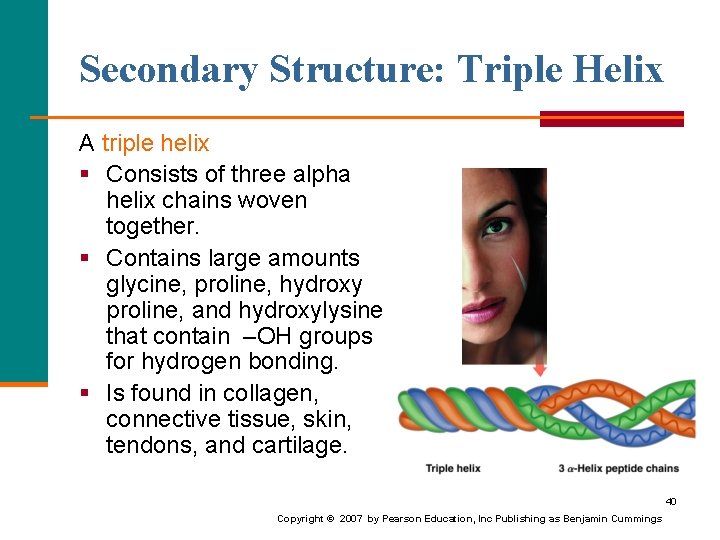
Secondary Structure: Triple Helix A triple helix § Consists of three alpha helix chains woven together. § Contains large amounts glycine, proline, hydroxy proline, and hydroxylysine that contain –OH groups for hydrogen bonding. § Is found in collagen, connective tissue, skin, tendons, and cartilage. 40 Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings

Learning Check Indicate the type of protein structure as 1) primary 2) alpha helix 3) beta pleated sheet 4) triple helix A. Polypeptide chains held side by H bonds. B. Sequence of amino acids in a polypeptide chain. C. Corkscrew shape with H bonds between amino acids. D. Three peptide chains woven like a rope. 41

Solution Indicate the type of protein structure as: 1) primary 2) alpha helix 3) beta pleated sheet 4) triple helix A. 3 Polypeptide chains held side by H bonds. B. 1 Sequence of amino acids in a polypeptide chain. C. 2 Corkscrew shape with H bonds between amino acids. D. 4 Three peptide chains woven like a rope. 42

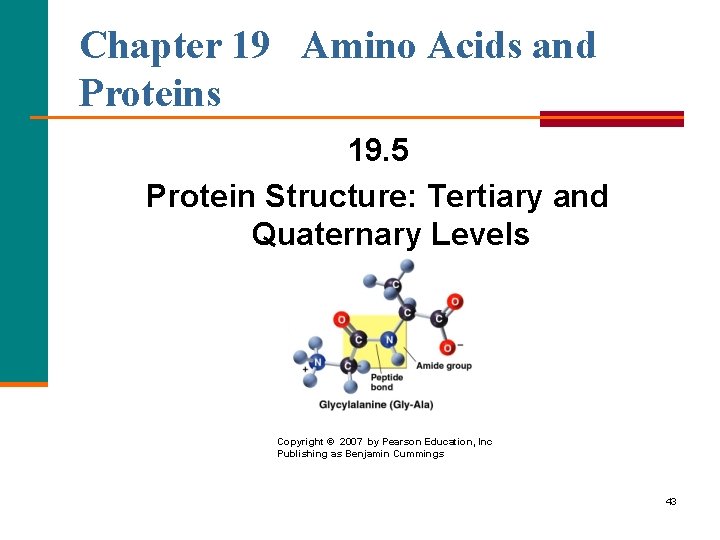
Chapter 19 Amino Acids and Proteins 19. 5 Protein Structure: Tertiary and Quaternary Levels Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 43

Essential Amino Acids Essential amino acids TABLE 19. 3 § Must be obtained from the diet. § Are the ten amino acids not synthesized by the body. § Are in meat and Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings diary products. § Are missing (one or more) in grains and vegetables. 44

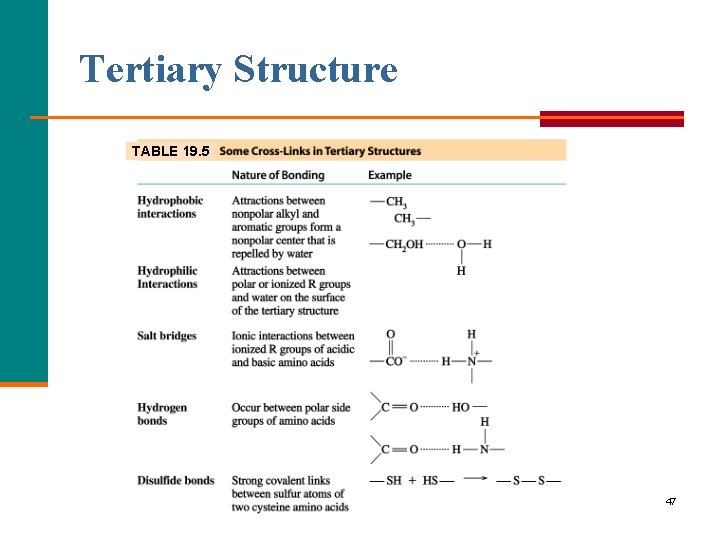
Tertiary Structure The tertiary structure of a protein § Gives a specific three dimensional shape to the polypeptide chain. § Involves interactions and cross links between different parts of the peptide chain. § Is stabilized by Hydrophobic and hydrophilic interactions. Salt bridges. Hydrogen bonds. Disulfide bonds. 45

Tertiary Structure § The interactions of the R groups give a protein its specific three dimensional tertiary structure. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 46

Tertiary Structure TABLE 19. 5 47

Globular Proteins Globular proteins § Have compact, spherical shapes. § Carry out synthesis, transport, and metabolism in the cells. § Such as myoglobin store and transport oxygen in muscle. Myoglobin Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 48

Fibrous Proteins Fibrous proteins § Consist of long, fiber like shapes. § Such as alpha keratins make up hair, wool, skin, and nails. § Such as feathers contain beta keratins with large amounts of beta pleated sheet structures. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 49

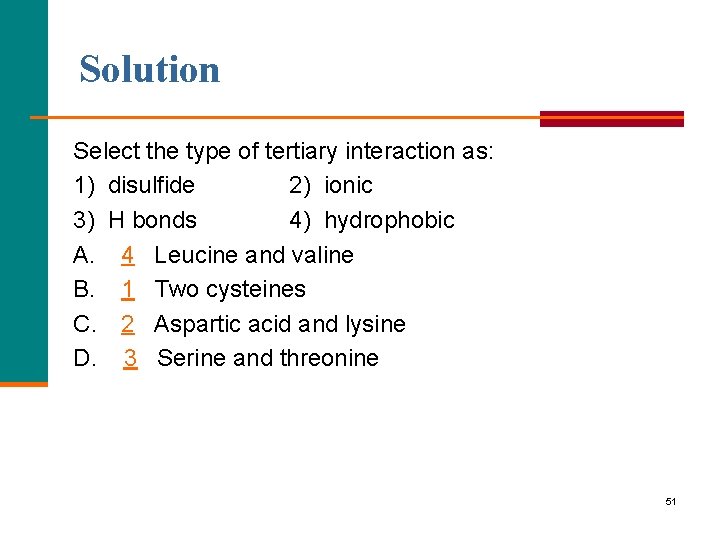
Learning Check Select the type of tertiary interaction 1) disulfide 2) ionic 3) H bonds 4) hydrophobic A. B. C. D. Leucine and valine Two cysteines Aspartic acid and lysine Serine and threonine 50

Solution Select the type of tertiary interaction as: 1) disulfide 2) ionic 3) H bonds 4) hydrophobic A. 4 Leucine and valine B. 1 Two cysteines C. 2 Aspartic acid and lysine D. 3 Serine and threonine 51

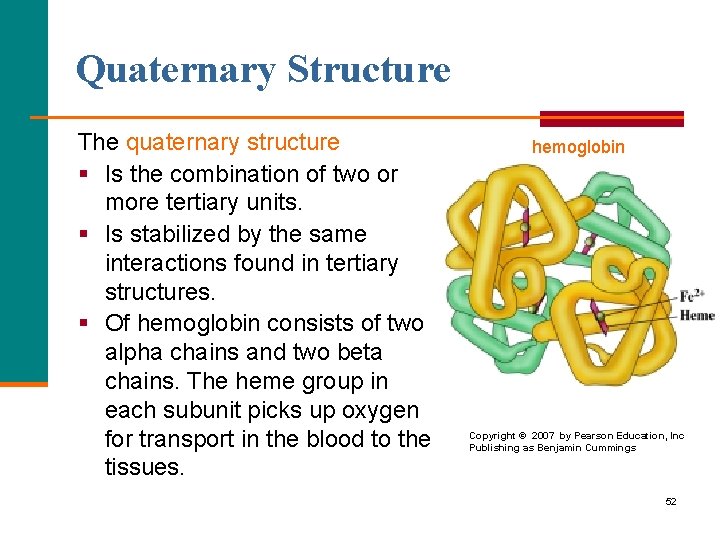
Quaternary Structure The quaternary structure § Is the combination of two or more tertiary units. § Is stabilized by the same interactions found in tertiary structures. § Of hemoglobin consists of two alpha chains and two beta chains. The heme group in each subunit picks up oxygen for transport in the blood to the tissues. hemoglobin Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 52

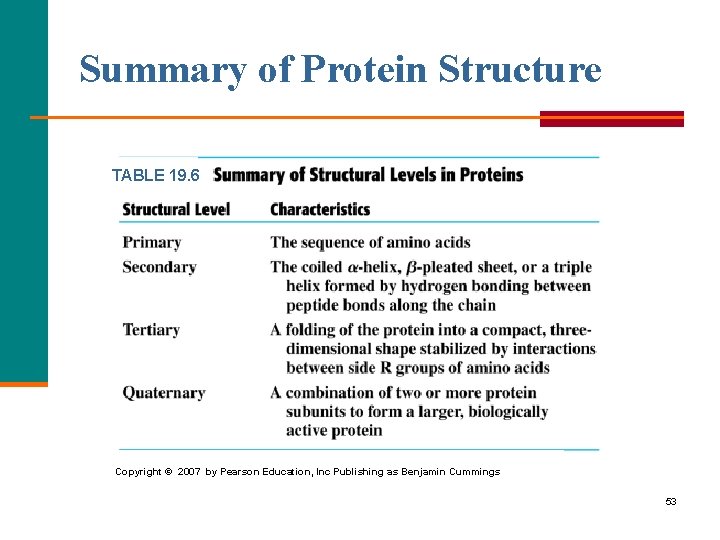
Summary of Protein Structure TABLE 19. 6 Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 53

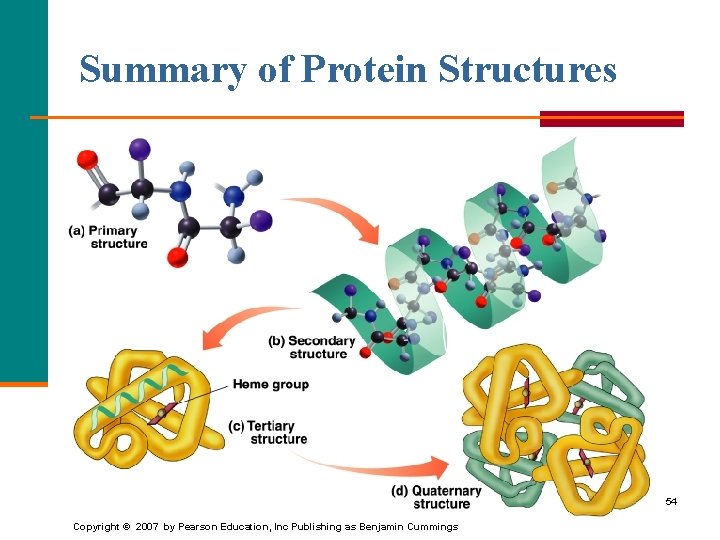
Summary of Protein Structures 54 Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings

Learning Check Identify the level of protein structure as: 1) Primary 2) Secondary 3) Tertiary 4) Quaternary A. B. C. D. E. Beta pleated sheet Order of amino acids in a protein A protein with two or more peptide chains The shape of a globular protein Disulfide bonds between R groups 55

Solution Identify the level of protein structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary A. 2 Beta pleated sheet. B. 1 Order of amino acids in a protein. C. 4 A protein with two or more peptide chains. D. 3 The shape of a globular protein. E. 3 Disulfide bonds between R groups. 56

Chapter 19 Amino Acids and Proteins 19. 6 Protein Hydrolysis and Denaturation Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 57

Protein Hydrolysis Protein hydrolysis § Splits the peptide bonds to give smaller peptides and amino acids. § Occurs in the digestion of proteins. § Occurs in cells when amino acids are needed to synthesize new proteins and repair tissues. 58

Hydrolysis of a Dipeptide § In the lab, the hydrolysis of a peptide requires acid or base, water and heat. § In the body, enzymes catalyze the hydrolysis of proteins. 59

Denaturation involves § The disruption of bonds in the secondary, tertiary and quaternary protein structures. § Heat and organic compounds that break apart H bonds and disrupt hydrophobic interactions. § Acids and bases that break H bonds between polar R groups and disrupt ionic bonds. § Heavy metal ions that react with S S bonds to form solids. § Agitation such as whipping that stretches peptide chains until bonds break. 60

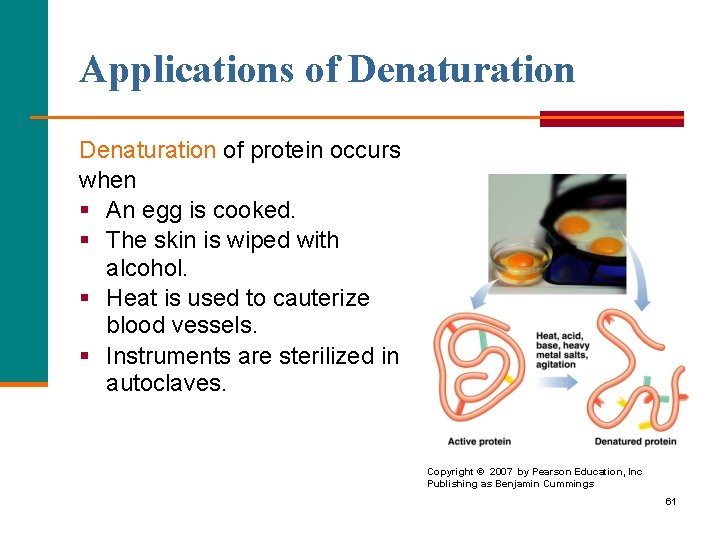
Applications of Denaturation of protein occurs when § An egg is cooked. § The skin is wiped with alcohol. § Heat is used to cauterize blood vessels. § Instruments are sterilized in autoclaves. Copyright © 2007 by Pearson Education, Inc Publishing as Benjamin Cummings 61

Learning Check What are the products of the complete hydrolysis of the peptide Ala Ser Val? 62

Solution The products of the complete hydrolysis of the peptide Ala Ser Val are alanine serine valine 63

Learning Check Tannic acid is used to form a scab on a burn. An egg is hard boiled by placing it in boiling water. What is similar about these two events? 64

Solution Acid and heat cause the denaturation of protein. They both break bonds in the secondary and tertiary structures of proteins. 65