Chapter 18 Section 2 Shifting Equilibrium Preview Lesson

Chapter 18 Section 2 Shifting Equilibrium Preview • • • Lesson Starter Objectives Predicting the Direction of Shift Reactions That Go to Completion Common-Ion Effect

Chapter 18 Section 2 Shifting Equilibrium Lesson Starter • Imagine children playing on a seesaw. • Five boys are sitting on one side and five girls on the other, and the seesaw is just balanced. • Then, one girl gets off, and the system is no longer at equilibrium. • One way to get the seesaw in balance again is for one of the boys to move toward the girls’ side.

Chapter 18 Section 2 Shifting Equilibrium Lesson Starter, continued • When he gets to the middle, the seesaw is again at equilibrium. • The stress of one girl getting off is relieved by having one of the boys move his position. • How would a chemical system in equilibrium respond to removing one of the products?

Chapter 18 Section 2 Shifting Equilibrium Objectives • Discuss the factors that disturb equilibrium. • Discuss conditions under which reactions go to completion. • Describe the common-ion effect.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift • Changes in pressure, concentration, or temperature can alter the equilibrium position and thereby change the relative amounts of reactants and products. • Le Châtelier’s principle states that if a system at equilibrium is subjected to a stress, the equilibrium is shifted in the direction that tends to relieve the stress. • This principle is true for all dynamic equilibria, chemical as well as physical. • Changes in pressure, concentration, and temperature illustrate Le Châtelier’s principle.
Chapter 18 Section 2 Shifting Equilibrium Le Chatelier's Principal Click below to watch the Visual Concept
Chapter 18 Section 2 Shifting Equilibrium Factors Affecting Equilibrium Click below to watch the Visual Concept

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Pressure • A change in pressure affects only equilibrium systems in which gases are involved. • For changes in pressure to affect the system, the total number of moles of gas on the left side of the equation must be different from the total number of moles of gas on the right side of the equation. • An increase in pressure is an applied stress. • It causes an increase in the concentrations of all species. • The system can reduce the total pressure by reducing the number of molecules.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Pressure, continued • the Haber process for the synthesis of ammonia 4 molecules of gas 2 molecules of gas • When pressure is applied, the equilibrium will shift to the right, and produce more NH 3. • By shifting to the right, the system can reduce the total number of molecules. This leads to a decrease in pressure.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Pressure, continued • Even though changes in pressure may shift the equilibrium position, they do not affect the value of the equilibrium constant. • The introduction of an inert gas, such as helium, into the reaction vessel increases the total pressure in the vessel. But it does not change the partial pressures of the reaction gases present. • Increasing pressure by adding a gas that is not a reactant or a product cannot affect the equilibrium position of the reaction system.
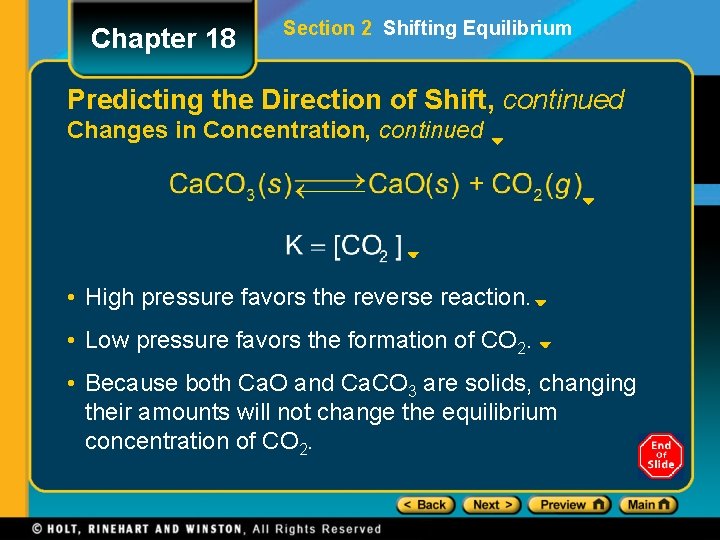
Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Concentration, continued • High pressure favors the reverse reaction. • Low pressure favors the formation of CO 2. • Because both Ca. O and Ca. CO 3 are solids, changing their amounts will not change the equilibrium concentration of CO 2.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Temperature • Reversible reactions are exothermic in one direction and endothermic in the other. • The effect of changing the temperature of an equilibrium mixture depends on which of the opposing reactions is endothermic and which is exothermic. • The addition of energy in the form of heat shifts the equilibrium so that energy is absorbed. This favors the endothermic reaction. • The removal of energy favors the exothermic reaction.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Temperature, continued • A rise in temperature increases the rate of any reaction. • In an equilibrium system, the rates of the opposing reactions are raised unequally. • The value of the equilibrium constant for a given system is affected by the temperature.

Chapter 18 Section 2 Shifting Equilibrium Predicting the Direction of Shift, continued Changes in Temperature, continued • Catalysts have no effect on relative equilibrium amounts. • They only affect the rates at which equilibrium is reached. • Catalysts increase the rates of forward and reverse reactions in a system by equal factors. Therefore, they do not affect K.

End of Chapter 18 Section 2 Show
- Slides: 15