CHAPTER 18 NUCLEAR DECAY Nuclear Chemistry The study


































- Slides: 62

CHAPTER 18 NUCLEAR DECAY

Nuclear Chemistry The study of processes that cause nuclear changes in the form of: Radioactive decay Isotopes Nuclear fusion Nuclear fission

Chapter 18 Visual Concepts Isotopes and Nuclides

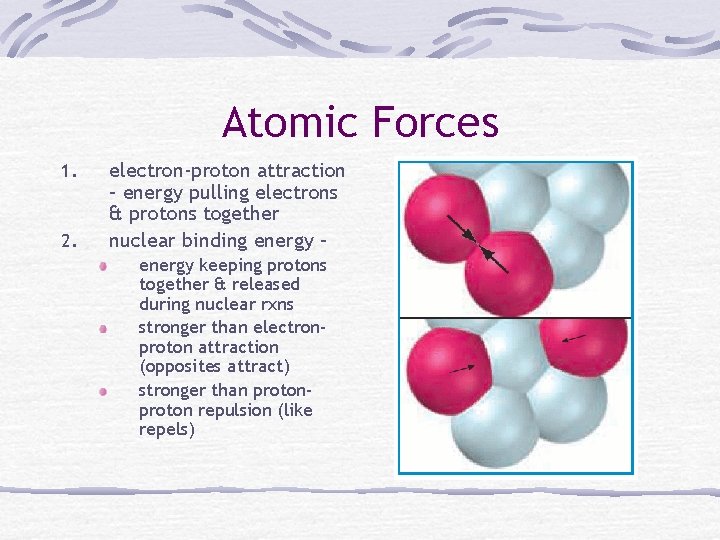
Atomic Forces 1. 2. electron-proton attraction – energy pulling electrons & protons together nuclear binding energy – energy keeping protons together & released during nuclear rxns stronger than electronproton attraction (opposites attract) stronger than proton repulsion (like repels)

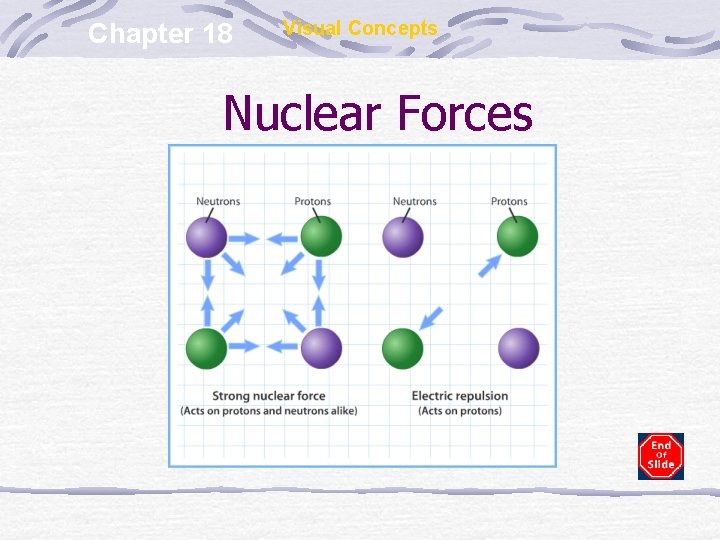
Chapter 18 Visual Concepts Nuclear Forces

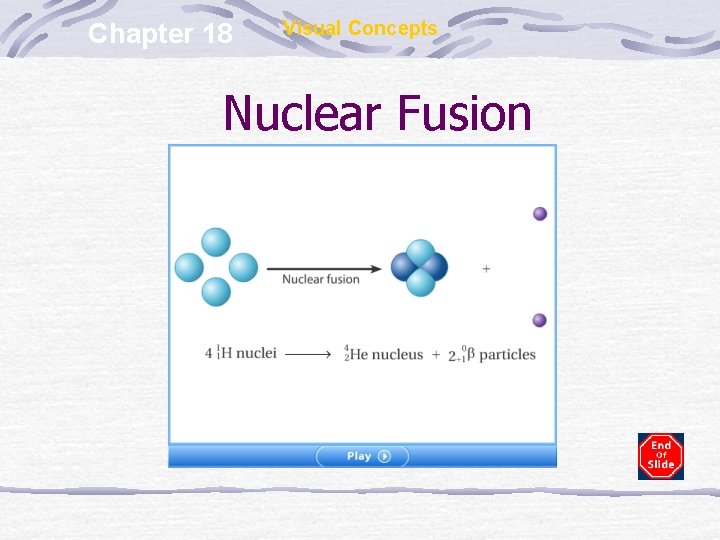
Chapter 18 Visual Concepts Nuclear Fusion

Nuclear Fusion Small nuclei combine to form larger, more stable nuclei More energetic than fission reactions Source of energy of the hydrogen bomb Could be utilized for human purposes if the energy could be contained

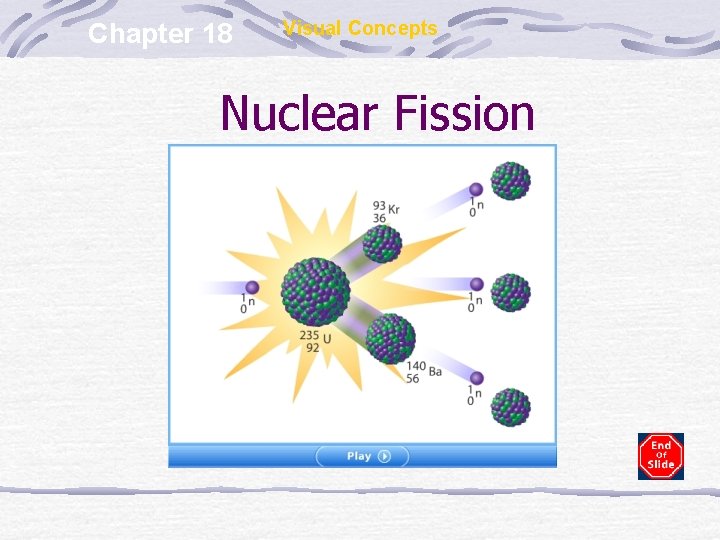
Chapter 18 Visual Concepts Nuclear Fission

Nuclear Fission Large nuclei break down to form smaller, more stable nuclei Mass of products < mass of reactants Missing mass is found in the form of energy (E = mc 2) Used in nuclear chain reactions

Chapter 18 Visual Concepts Nuclear Chain Reaction

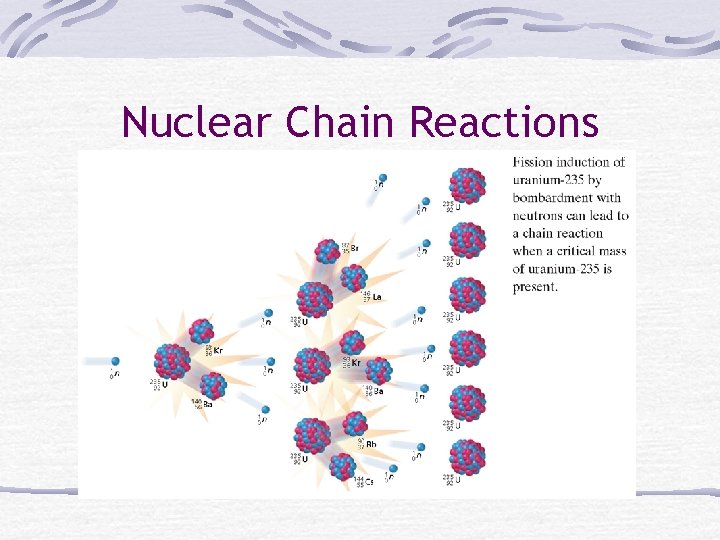
Nuclear Chain Reactions A rxn in which a neutron is used to start one rxn The rxn then starts another rxn, etc.

Nuclear Chain Reactions

Critical Mass The minimum amount of nuclides (atoms) needed to start & continue the chain rxn

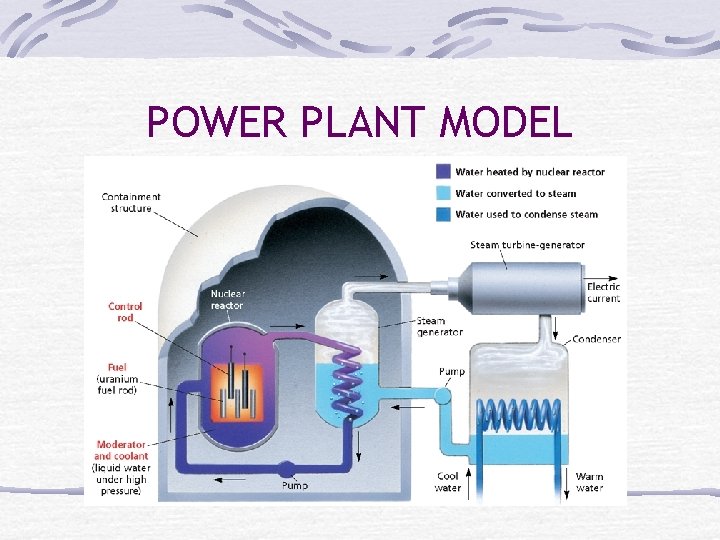
POWER PLANT MODEL

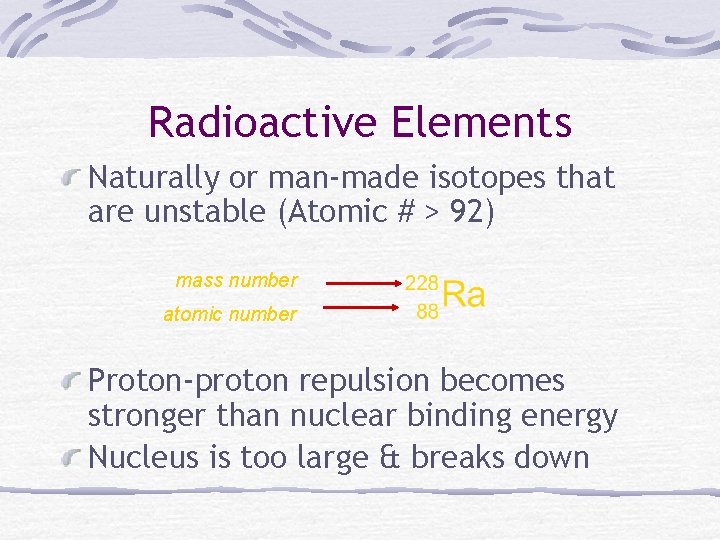
Radioactive Elements Naturally or man-made isotopes that are unstable (Atomic # > 92) mass number atomic number Proton-proton repulsion becomes stronger than nuclear binding energy Nucleus is too large & breaks down

RADIOACTIVE DECAY spontaneous break down of atom

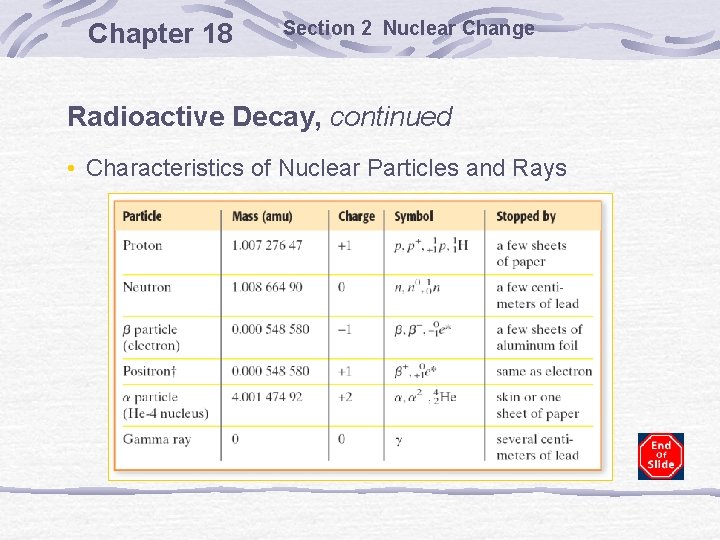
Chapter 18 Section 2 Nuclear Change Radioactive Decay, continued • Characteristics of Nuclear Particles and Rays

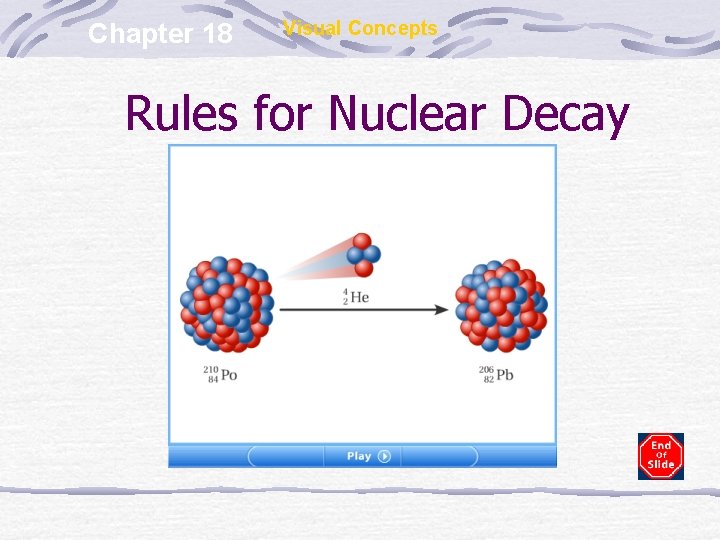
Chapter 18 Visual Concepts Rules for Nuclear Decay

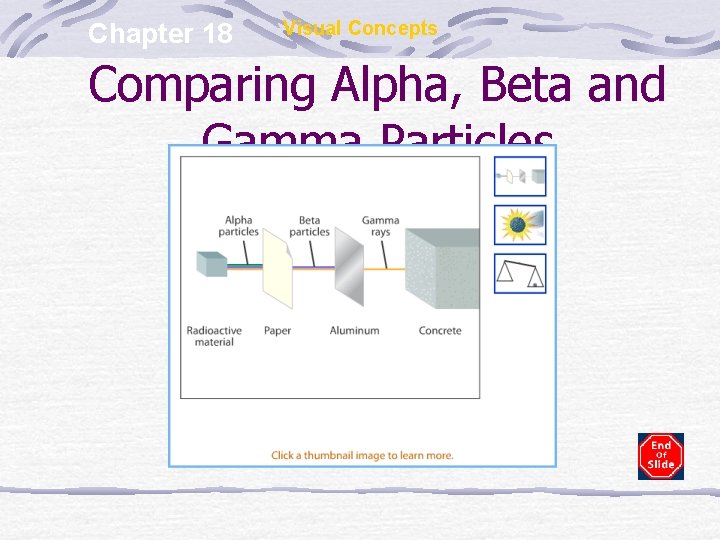
Alpha Emission Alpha particle (α) is a helium nucleus (42 He) Restrict mostly to very heavy nuclei Weak penetrating ability – cannot pass through skin or paper Causes harm through ingestion or inhalation

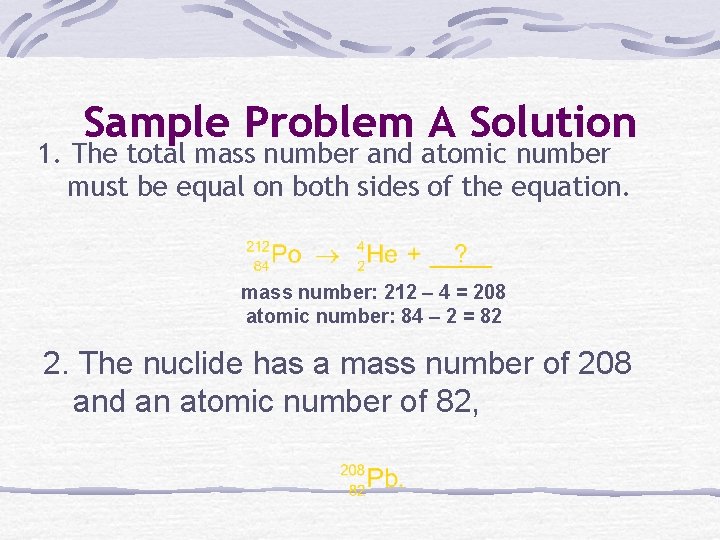
Sample Problem A Identify the product that balances the following nuclear reaction:

Sample Problem A Solution 1. The total mass number and atomic number must be equal on both sides of the equation. mass number: 212 4 = 208 atomic number: 84 2 = 82 2. The nuclide has a mass number of 208 and an atomic number of 82,

SAMPLE PROBLEM A SOLUTION 3. The balanced nuclear Equation is

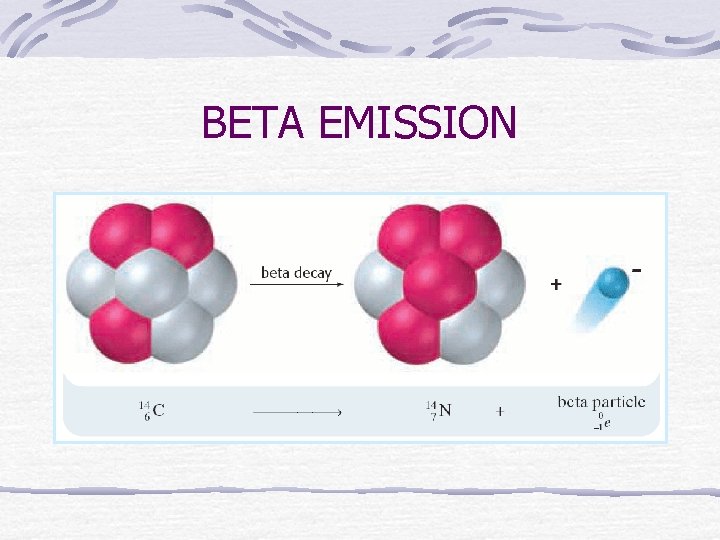
BETA EMISSION

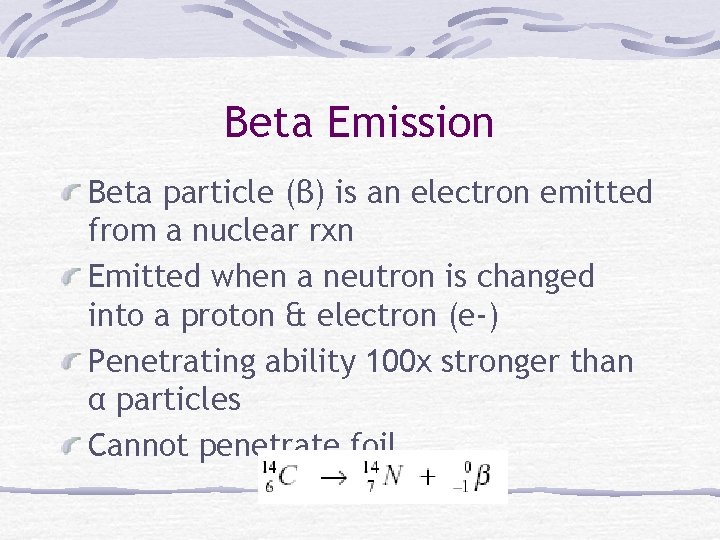
Beta Emission Beta particle (β) is an electron emitted from a nuclear rxn Emitted when a neutron is changed into a proton & electron (e-) Penetrating ability 100 x stronger than α particles Cannot penetrate foil

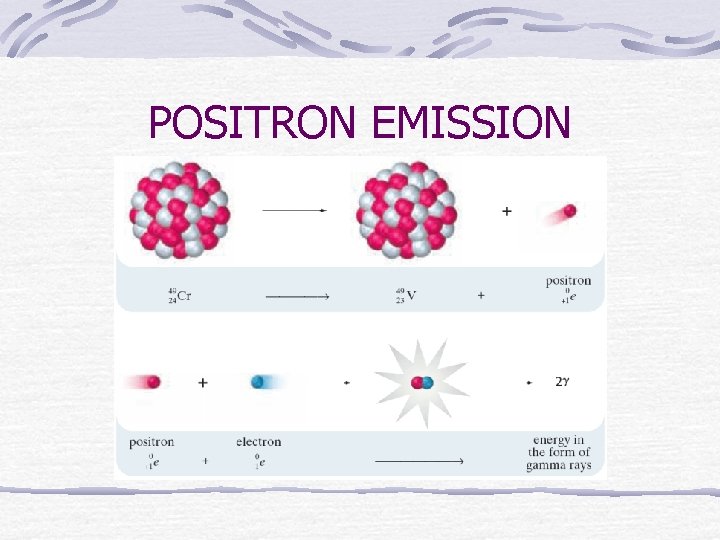
POSITRON EMISSION

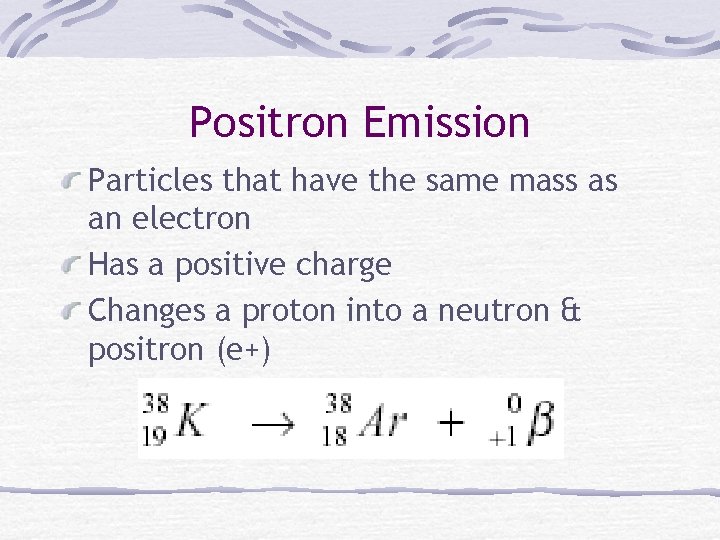
Positron Emission Particles that have the same mass as an electron Has a positive charge Changes a proton into a neutron & positron (e+)

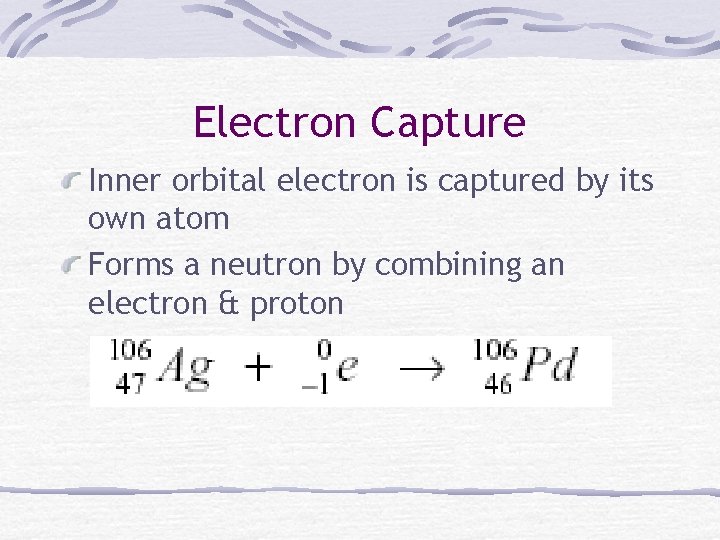
Electron Capture Inner orbital electron is captured by its own atom Forms a neutron by combining an electron & proton

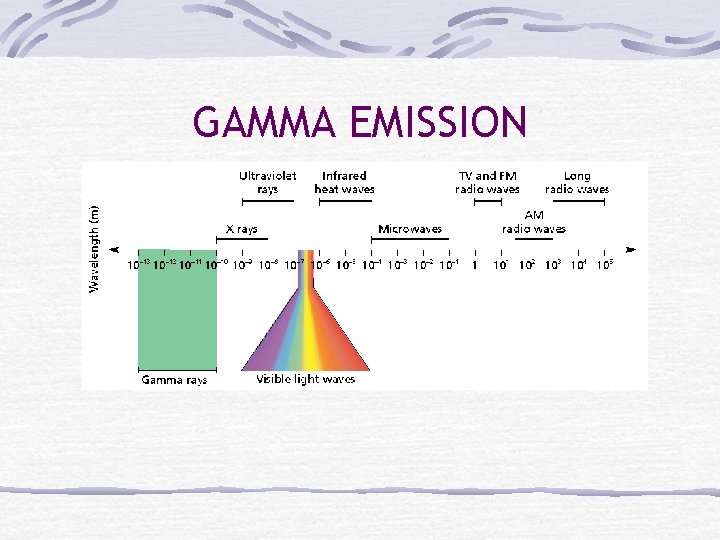
Gamma Emission Gamma rays (γ) are high energy EM waves released Produced when nuclear particles change energy levels Usually follows other types of decay Does not change the identity of the atom as in other decays Greatest penetrating ability Cannot penetrate thick layers of lead, cement or both

GAMMA EMISSION

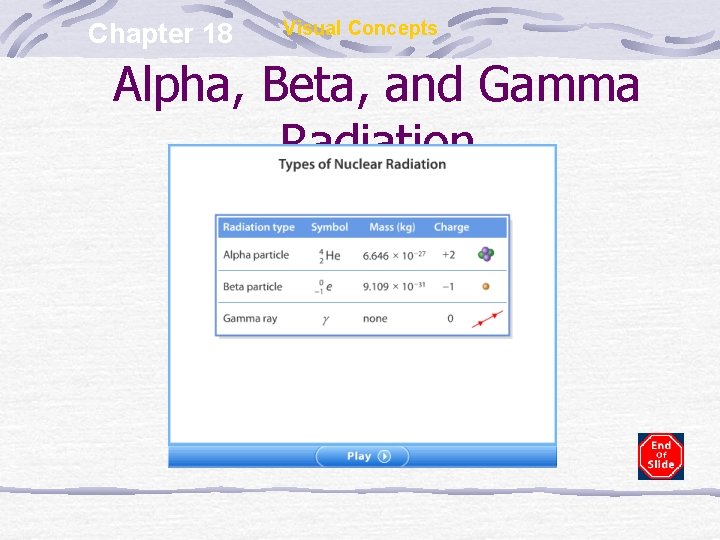
Chapter 18 Visual Concepts Alpha, Beta, and Gamma Radiation

Chapter 18 Visual Concepts Comparing Alpha, Beta and Gamma Particles

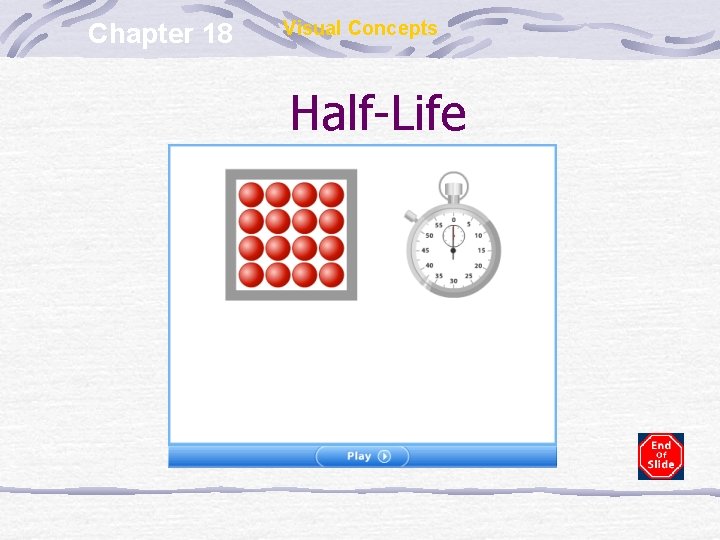
Chapter 18 Visual Concepts Half-Life

Half Life (t 1/2) The time it takes for ½ of the atoms to decay Stable nuclei have longer half lives (decay slowly) Unstable nuclei have shorter half lives (decay quickly)

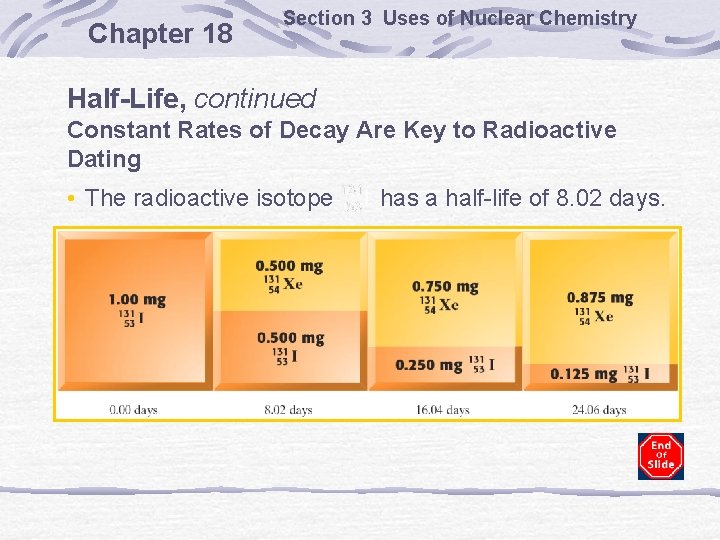
Chapter 18 Section 3 Uses of Nuclear Chemistry Half-Life, continued Constant Rates of Decay Are Key to Radioactive Dating • The radioactive isotope has a half-life of 8. 02 days.

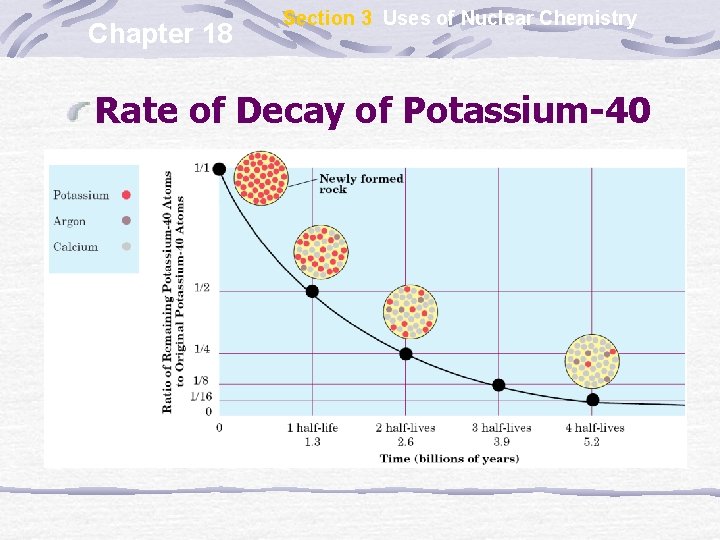
Chapter 18 Section 3 Uses of Nuclear Chemistry Rate of Decay of Potassium-40

When do we use this?

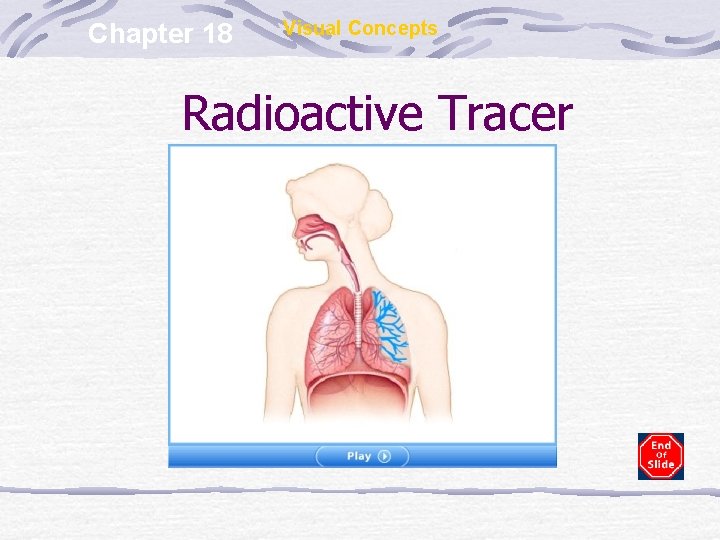
Chapter 18 Visual Concepts Radioactive Tracer

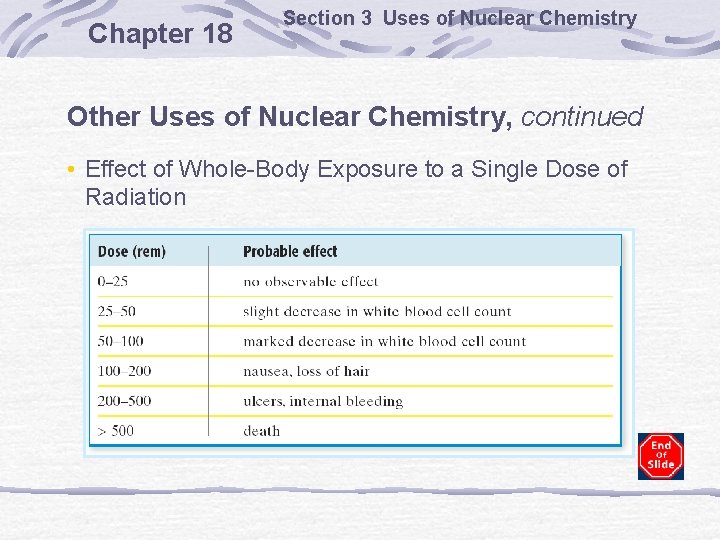
Chapter 18 Section 3 Uses of Nuclear Chemistry Other Uses of Nuclear Chemistry, continued • Effect of Whole-Body Exposure to a Single Dose of Radiation

Multiple Choice 1. Complete the following nuclear equation: A. B. C. D.

Multiple Choice 1. Complete the following nuclear equation: A. B. C. D.

Multiple Choice 2. The mass of the nucleus is A. greater than the mass of the protons and neutrons that make up the nucleus. B. equal to the mass of the protons and neutrons that make up the nucleus. C. less than the mass of the protons and neutrons that make up the nucleus. D. converted to energy.

Multiple Choice 2. The mass of the nucleus is A. greater than the mass of the protons and neutrons that make up the nucleus. B. equal to the mass of the protons and neutrons that make up the nucleus. C. less than the mass of the protons and neutrons that make up the nucleus. D. converted to energy.

Multiple Choice 3. Which type of radiation has the most penetrating ability? A. an alpha particle B. a beta particle C. a gamma ray D. a neutron

Multiple Choice 3. Which type of radiation has the most penetrating ability? A. an alpha particle B. a beta particle C. a gamma ray D. a neutron

Multiple Choice 4. Which two particles have the same mass but opposite charge? A. a beta particle and a positron B. a neutron and a proton C. a proton and an electron D. an alpha particle and a proton

Multiple Choice 4. Which two particles have the same mass but opposite charge? A. a beta particle and a positron B. a neutron and a proton C. a proton and an electron D. an alpha particle and a proton

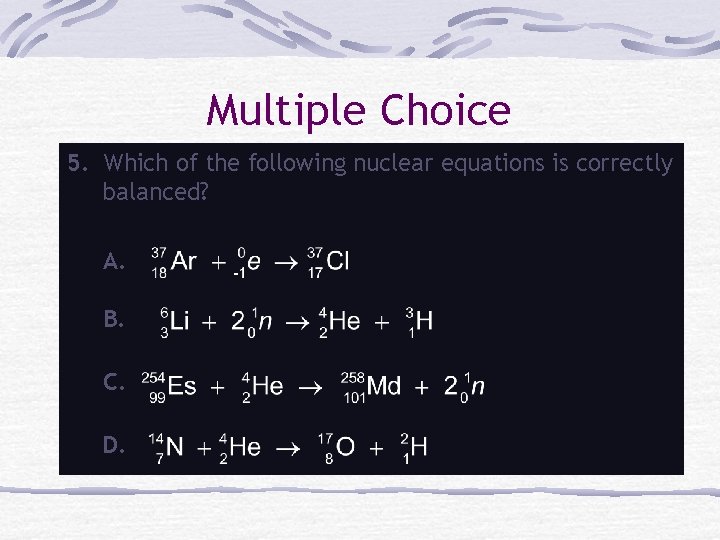
Multiple Choice 5. Which of the following nuclear equations is correctly balanced? A. B. C. D.

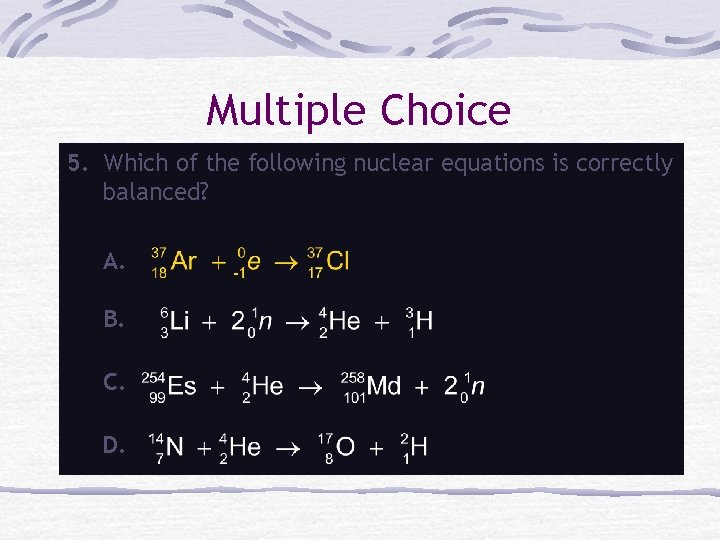
Multiple Choice 5. Which of the following nuclear equations is correctly balanced? A. B. C. D.

Multiple Choice 6. Gamma rays A. have the same energy as beta particles do. B. are visible light. C. have no charge and no mass. D. are not a form of electromagnetic radiation.

Multiple Choice 6. Gamma rays A. have the same energy as beta particles do. B. are visible light. C. have no charge and no mass. D. are not a form of electromagnetic radiation.

Multiple Choice 7. Which of the following nuclides is radioactive? A. B. C. D.

Multiple Choice 7. Which of the following nuclides is radioactive? A. B. C. D.

Multiple Choice 8. The half-life of thorium-234 is 24 days. If you have a 42. 0 g sample of thorium-24, how much will remain after 72 days? A. 42. 0 g B. 21. 0 g C. 10. 5 g D. 5. 25 g

Multiple Choice 8. The half-life of thorium-234 is 24 days. If you have a 42. 0 g sample of thorium-234, how much will remain after 72 days? A. 42. 0 g B. 21. 0 g C. 10. 5 g D. 5. 25 g

Chapter 21 Multiple Choice 9. It takes 5. 2 min for a 4. 0 g sample of francium-210 to decay until only 1. 0 g is left. What is the half-life of francium-210? A. 1. 3 min B. 2. 6 min C. 5. 2 min D. 7. 8 min

Multiple Choice 9. It takes 5. 2 min for a 4. 0 g sample of francium-210 to decay until only 1. 0 g is left. What is the half-life of francium-210? A. 1. 3 min B. 2. 6 min C. 5. 2 min D. 7. 8 min

Short Answer 10. Write the nuclear equation that represents the process in which a neutron in the nucleus is changed to a proton with the emission of a beta particle.

Short Answer 10. Write the nuclear equation that represents the process in which a neutron in the nucleus is changed to a proton with the emission of a beta particle. Answer:

Short Answer 11. Describe a positron, and write its nuclear symbol.

Short Answer 11. Describe a positron, and write its nuclear symbol. Answer: A positron is a positively charged particle with the same mass as a beta particle.

Short Answer 12. Explain the difference between nuclear fission and nuclear fusion, and explain the energy changes that accompany each process.

Short Answer 12. Explain the difference between nuclear fission and nuclear fusion, and explain the energy changes that accompany each process. Answer: Fission occurs when a heavy nuclide is bombarded with nucleons, such as neutrons and disintegrates into two smaller nuclides producing more neutrons and energy. Fusion occurs when two light nuclides combine to form a heavier nuclide and energy.