Chapter 18 Nuclear Chemistry Review Mass number A

Chapter 18 Nuclear Chemistry
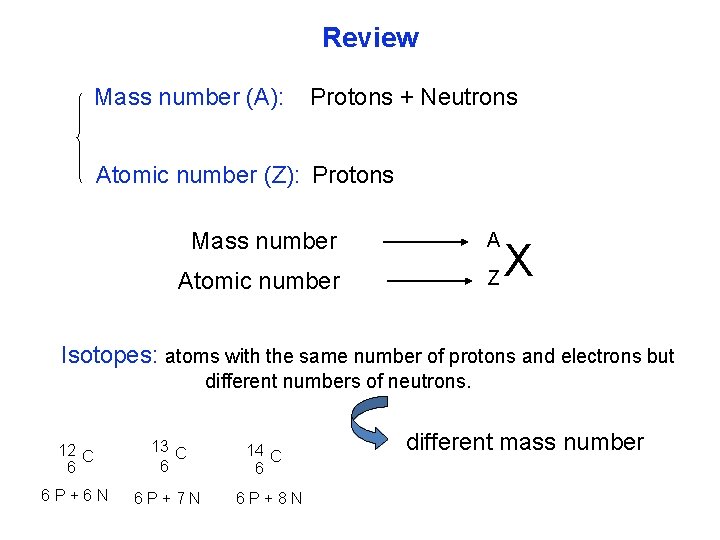
Review Mass number (A): Protons + Neutrons Atomic number (Z): Protons Mass number A Atomic number Z X Isotopes: atoms with the same number of protons and electrons but different numbers of neutrons. 12 C 6 13 C 6 6 P+6 N 6 P+7 N 14 C 6 6 P+8 N different mass number
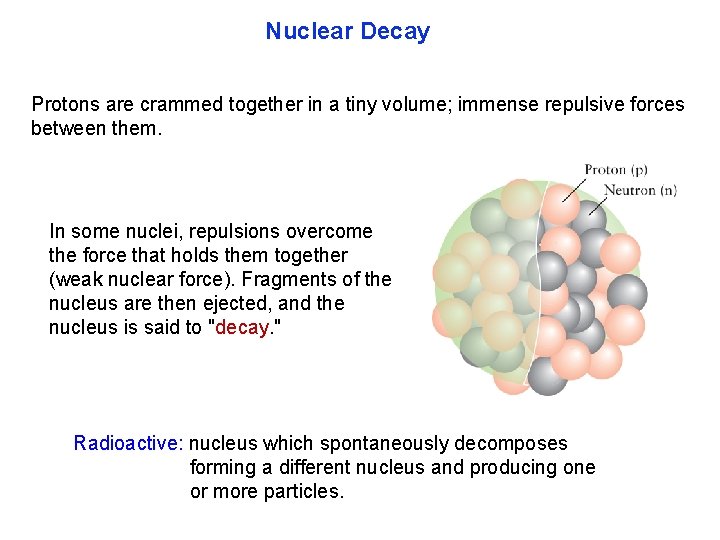
Nuclear Decay Protons are crammed together in a tiny volume; immense repulsive forces between them. In some nuclei, repulsions overcome the force that holds them together (weak nuclear force). Fragments of the nucleus are then ejected, and the nucleus is said to "decay. " Radioactive: nucleus which spontaneously decomposes forming a different nucleus and producing one or more particles.

Evidence for Spontaneous Decay Marie Sklodowska Curie, a young Polish doctoral student, showed that the radiation, which she called radioactivity, was emitted by uranium regardless of the compound in which it was found. Together with her husband, Pierre, she went on to show that uranium atoms, thorium, radium, and polonium are also radioactive.
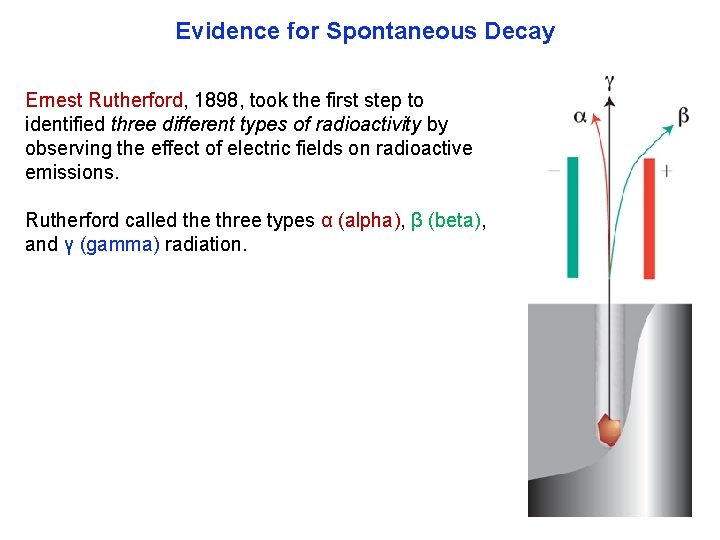
Evidence for Spontaneous Decay Ernest Rutherford, 1898, took the first step to identified three different types of radioactivity by observing the effect of electric fields on radioactive emissions. Rutherford called the three types α (alpha), β (beta), and γ (gamma) radiation.
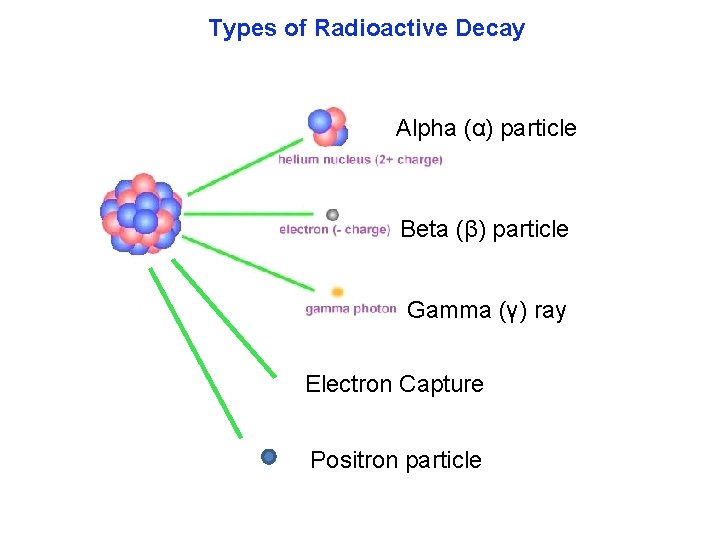
Types of Radioactive Decay Alpha (α) particle Beta (β) particle Gamma (γ) ray Electron Capture Positron particle
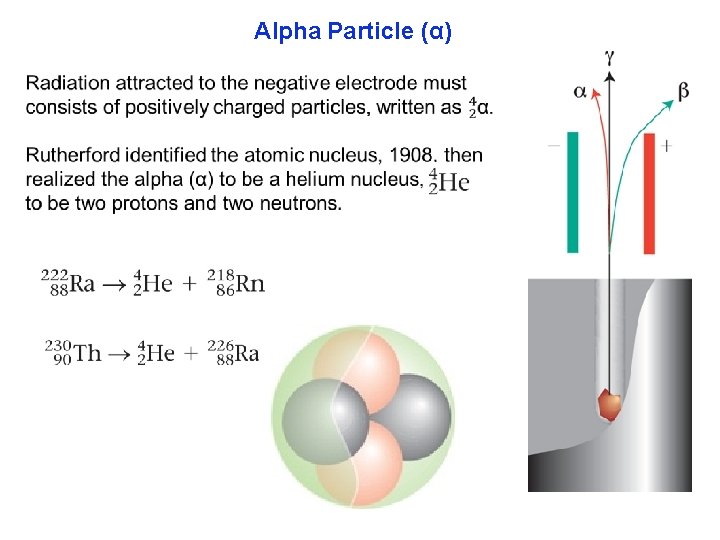
Alpha Particle (α)
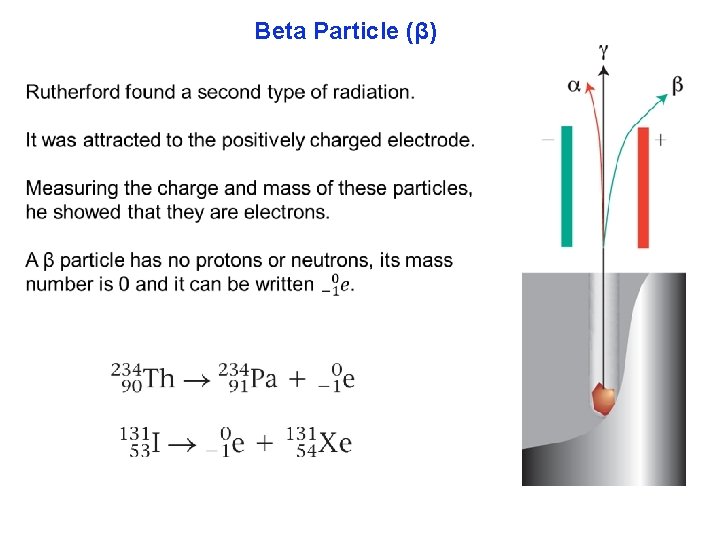
Beta Particle (β)
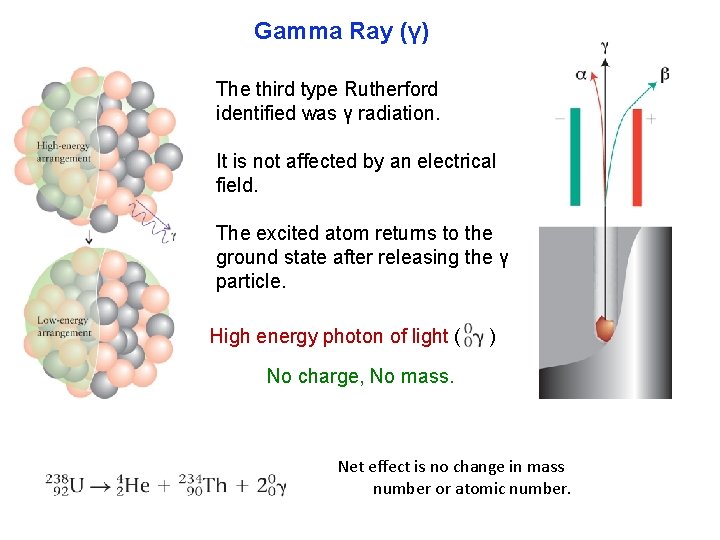
Gamma Ray (γ) The third type Rutherford identified was γ radiation. It is not affected by an electrical field. The excited atom returns to the ground state after releasing the γ particle. High energy photon of light ( ) No charge, No mass. Net effect is no change in mass number or atomic number.
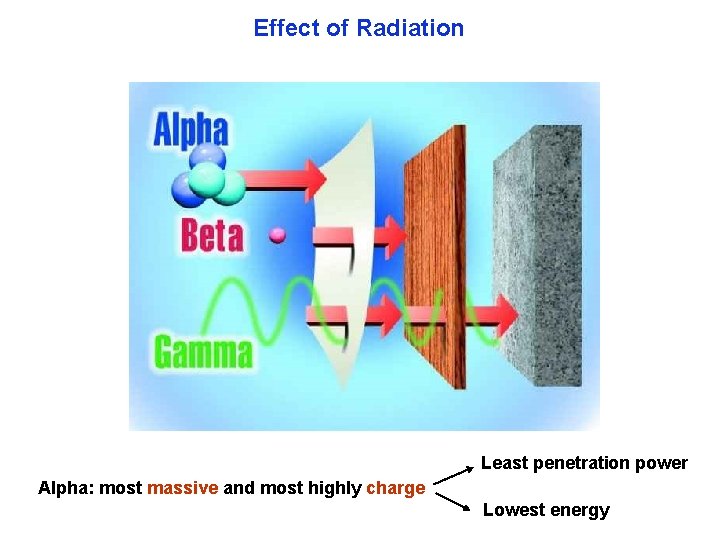
Effect of Radiation Least penetration power Alpha: most massive and most highly charge Lowest energy
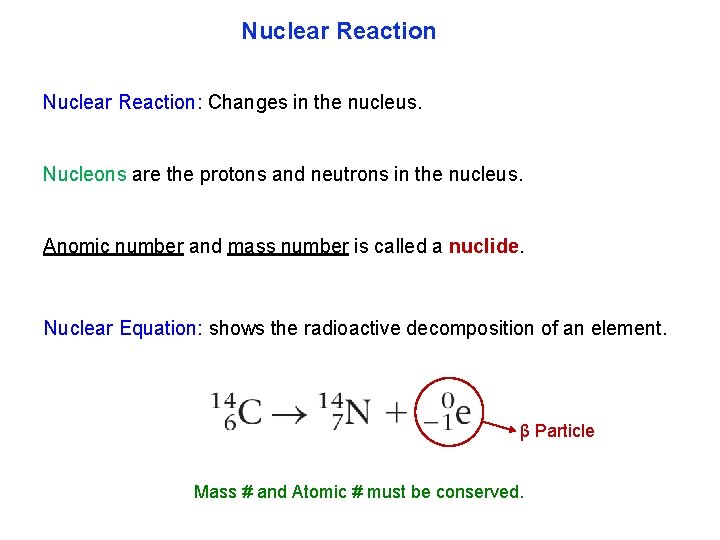
Nuclear Reaction: Changes in the nucleus. Nucleons are the protons and neutrons in the nucleus. Anomic number and mass number is called a nuclide. Nuclear Equation: shows the radioactive decomposition of an element. β Particle Mass # and Atomic # must be conserved.
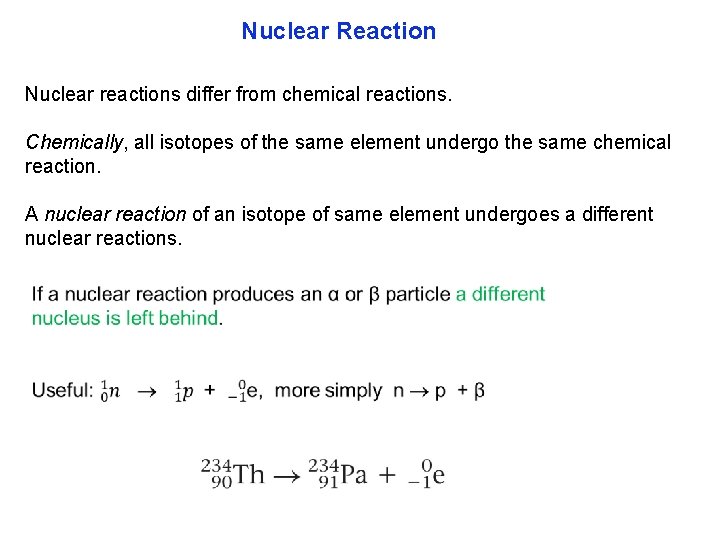
Nuclear Reaction Nuclear reactions differ from chemical reactions. Chemically, all isotopes of the same element undergo the same chemical reaction. A nuclear reaction of an isotope of same element undergoes a different nuclear reactions.
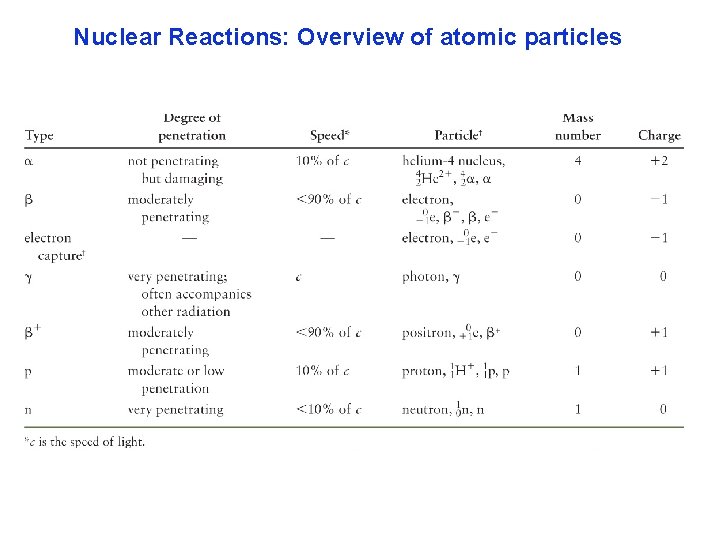
Nuclear Reactions: Overview of atomic particles
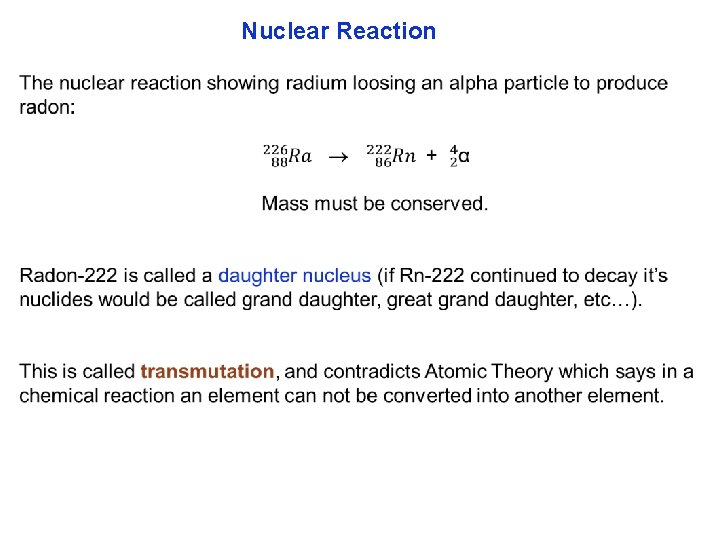
Nuclear Reaction
Nuclear Reaction Another important distinction between nuclear and chemical reactions is energy. • Combusting 1. 0 g of methane produces 52 k. J of energy as heat. • A nuclear reaction of 1. 0 g of uranium 235 produces 8. 2× 107 k. J of energy, a million times more.
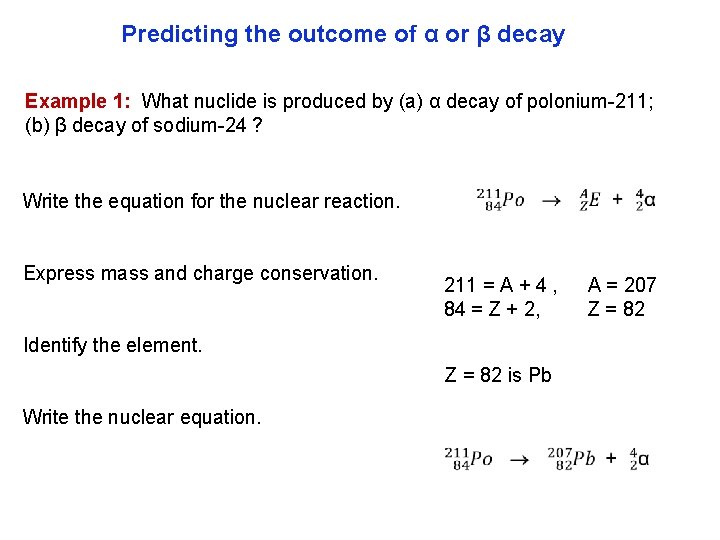
Predicting the outcome of α or β decay Example 1: What nuclide is produced by (a) α decay of polonium 211; (b) β decay of sodium 24 ? Write the equation for the nuclear reaction. Express mass and charge conservation. 211 = A + 4 , 84 = Z + 2, Identify the element. Z = 82 is Pb Write the nuclear equation. A = 207 Z = 82

Predicting the outcome of α or β decay Example 1: What nuclide is produced by (a) α decay of polonium 211; (b) β decay of sodium 24 ? Write the equation for the nuclear reaction. Express mass and charge conservation. (Note the “ ” for a gain of a proton) 24 = A + 0 , 11 = Z 1, Identify the element. Z = 12 is Mg Write the nuclear equation. A = 24 Z = 12
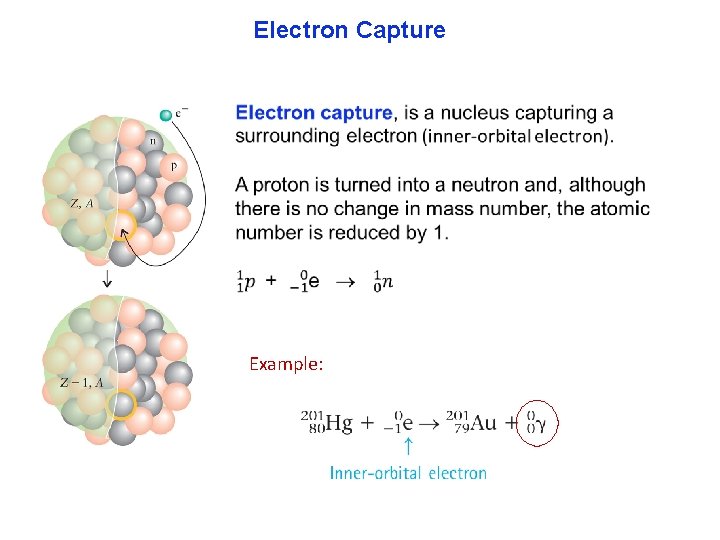
Electron Capture Example:
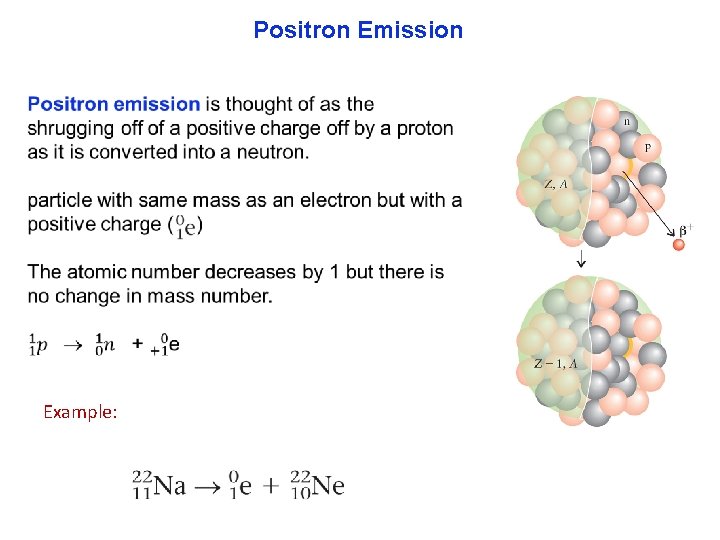
Positron Emission Example:
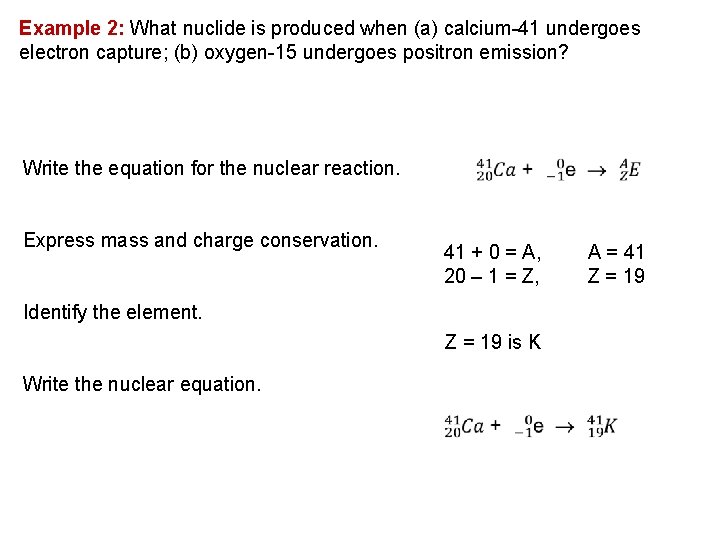
Example 2: What nuclide is produced when (a) calcium 41 undergoes electron capture; (b) oxygen 15 undergoes positron emission? Write the equation for the nuclear reaction. Express mass and charge conservation. 41 + 0 = A, 20 – 1 = Z, Identify the element. Z = 19 is K Write the nuclear equation. A = 41 Z = 19
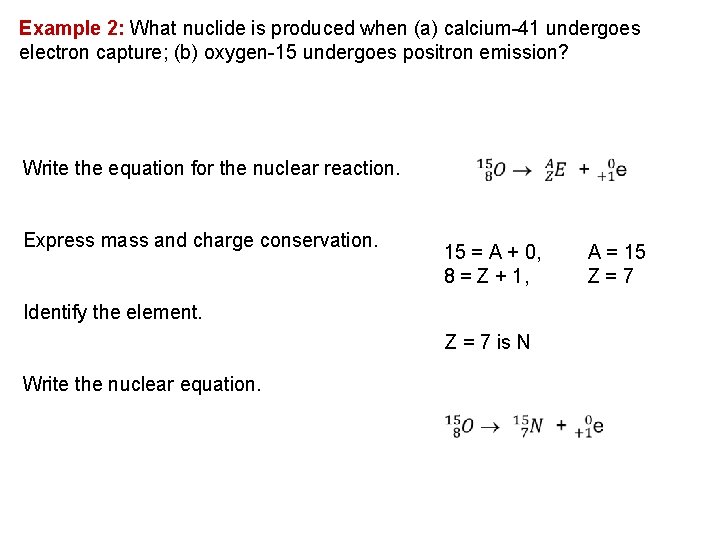
Example 2: What nuclide is produced when (a) calcium 41 undergoes electron capture; (b) oxygen 15 undergoes positron emission? Write the equation for the nuclear reaction. Express mass and charge conservation. 15 = A + 0, 8 = Z + 1, Identify the element. Z = 7 is N Write the nuclear equation. A = 15 Z=7
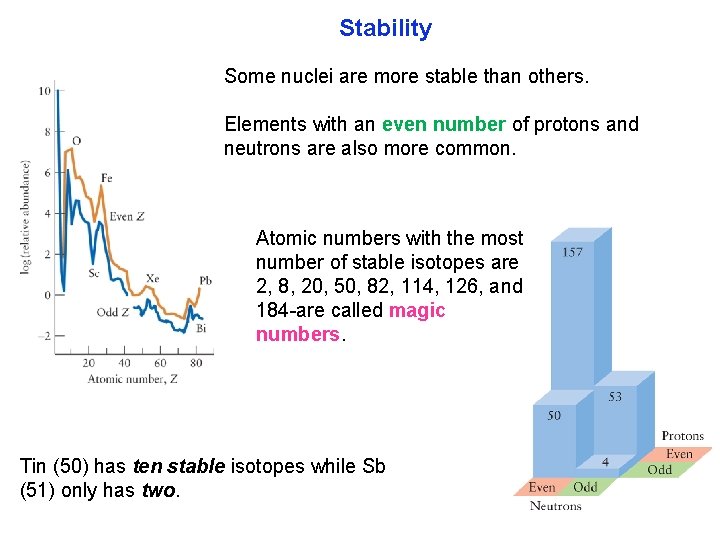
Stability Some nuclei are more stable than others. Elements with an even number of protons and neutrons are also more common. Atomic numbers with the most number of stable isotopes are 2, 8, 20, 50, 82, 114, 126, and 184 are called magic numbers. Tin (50) has ten stable isotopes while Sb (51) only has two.
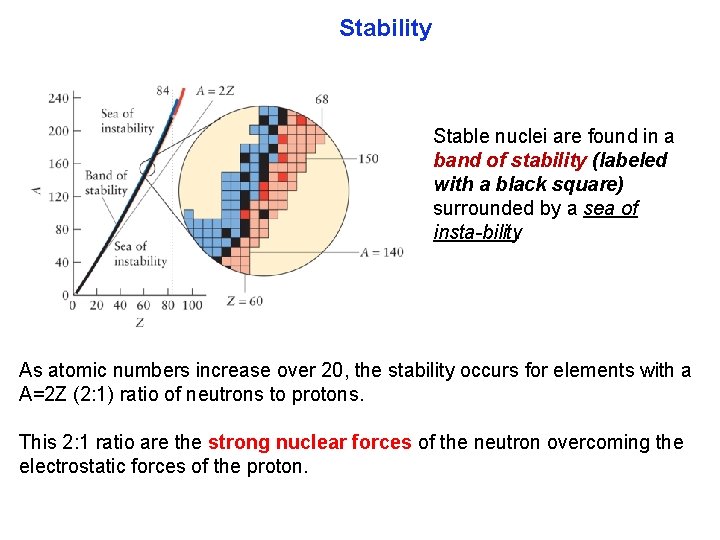
Stability Stable nuclei are found in a band of stability (labeled with a black square) surrounded by a sea of insta bility. As atomic numbers increase over 20, the stability occurs for elements with a A=2 Z (2: 1) ratio of neutrons to protons. This 2: 1 ratio are the strong nuclear forces of the neutron overcoming the electrostatic forces of the proton.
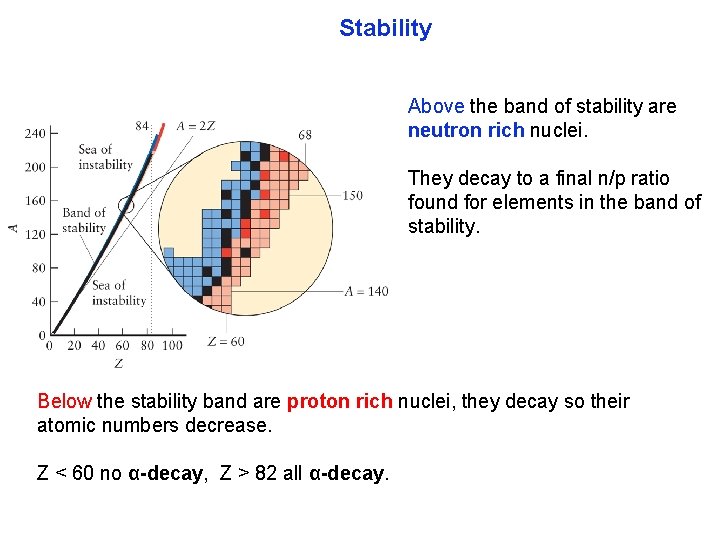
Stability Above the band of stability are neutron rich nuclei. They decay to a final n/p ratio found for elements in the band of stability. Below the stability band are proton rich nuclei, they decay so their atomic numbers decrease. Z < 60 no α-decay, Z > 82 all α-decay.
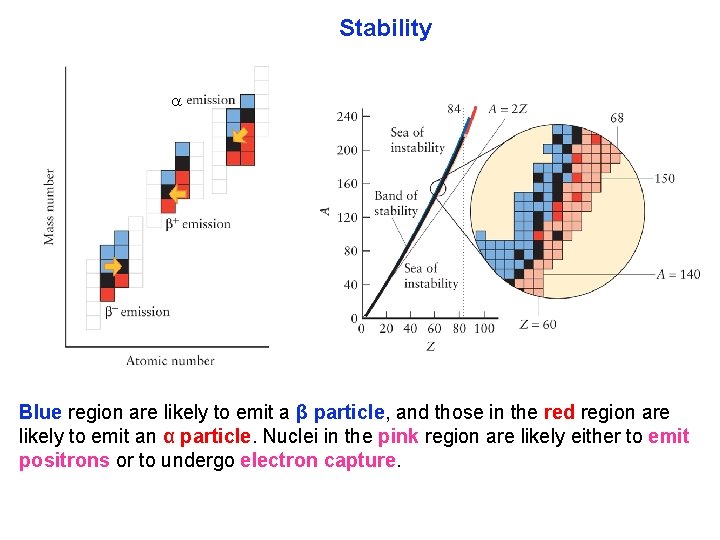
Stability Blue region are likely to emit a β particle, and those in the red region are likely to emit an α particle. Nuclei in the pink region are likely either to emit positrons or to undergo electron capture.
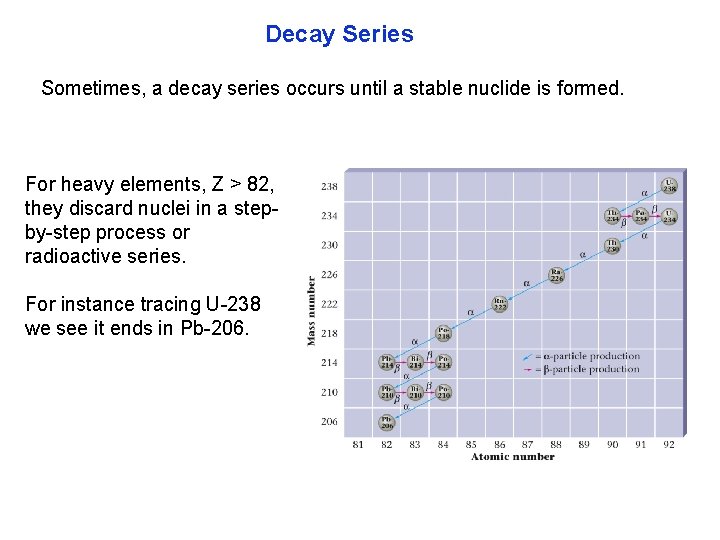
Decay Series Sometimes, a decay series occurs until a stable nuclide is formed. For heavy elements, Z > 82, they discard nuclei in a step by step process or radioactive series. For instance tracing U 238 we see it ends in Pb 206.
Nucleosynthesis is the formation of elements. Transmutation: converting one element into another.
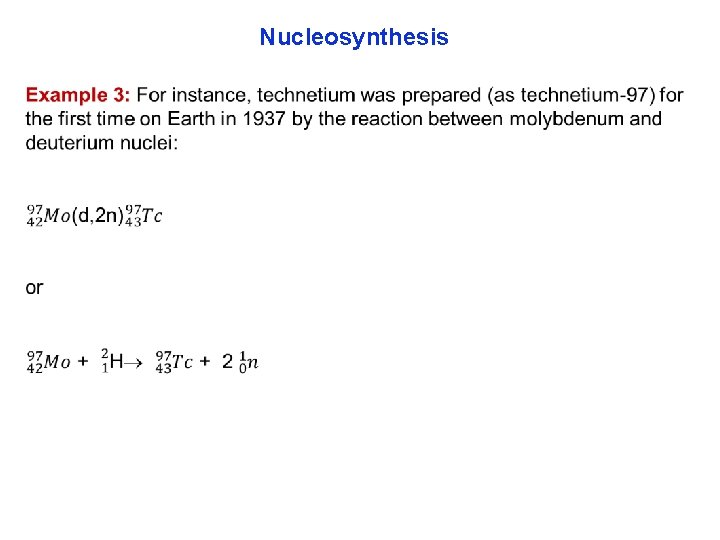
Nucleosynthesis
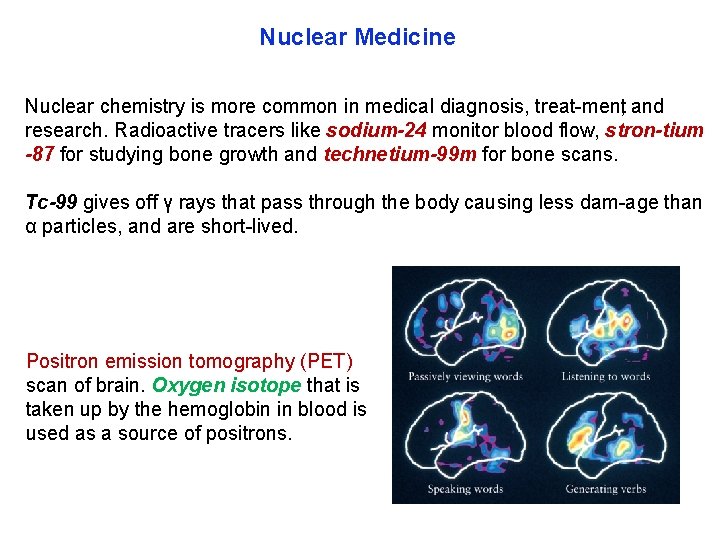
Nuclear Medicine Nuclear chemistry is more common in medical diagnosis, treat ment, and research. Radioactive tracers like sodium 24 monitor blood flow, stron tium 87 for studying bone growth and technetium 99 m for bone scans. Tc 99 gives off γ rays that pass through the body causing less dam age than α particles, and are short lived. Positron emission tomography (PET) scan of brain. Oxygen isotope that is taken up by the hemoglobin in blood is used as a source of positrons.
Nuclear Medicine Positron emission tomography (PET) uses short lived positron emitter, fluorine 18, to image human tissue. When injecting fluorine 18 labeled estrogen into a cancer patients, the fluorine bearing compound is preferentially absorbed by tumor. Boron neutron capture therapy is unusual in that Boron 10, the isotope injected, is not radioactive. How ever, when boron 10 is bombarded with neutrons, it gives off highly destructive α particles. Boron 10 is preferentially absorbed by tumors. The patient is briefly bombarded by neutrons. As soon as the bombardment ceases, the boron 10 stops generating α particles.

Nuclear Medicine Nuclear radiation is sometimes called Ionizing Radiation, because it is energetic enough to eject electrons. Massive, highly charged α particles interact strongly with matter, slowing down, capturing electrons, and change into bulky helium atoms before traveling very far. α particles are absorbed into the top layers of dead skin so they do little damage, but are dangerous when inhaled or swallowed leading to serious illness and even death. Plutonium (IV), a strong α emitter, similar to Fe 3+ found in our blood, interacts with blood cells causing sickness, cancer and death.
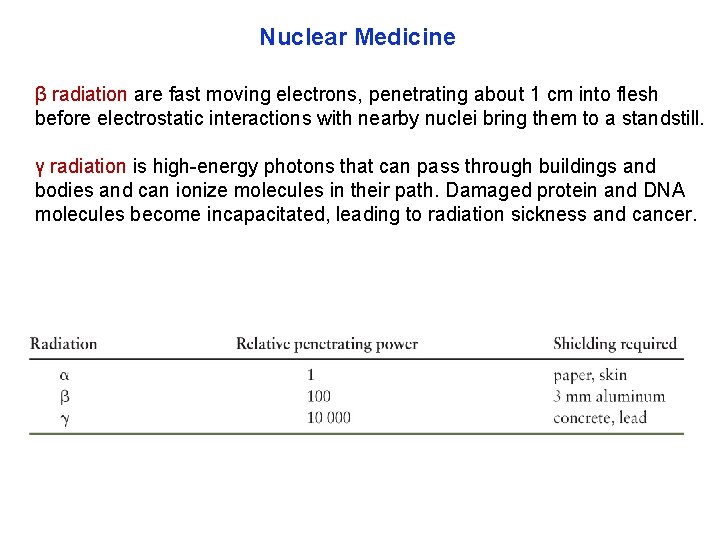
Nuclear Medicine β radiation are fast moving electrons, penetrating about 1 cm into flesh before electrostatic interactions with nearby nuclei bring them to a standstill. γ radiation is high energy photons that can pass through buildings and bodies and can ionize molecules in their path. Damaged protein and DNA molecules become incapacitated, leading to radiation sickness and cancer.
Measuring the Rate of Nuclear Decay Nuclear decays, are unimolecular chemical reaction, so are first order. The law of radioactive decay: Activity = rate of decay = k × N k is called the decay constant, and N is the number of nuclei. It follows that the number N of nuclei remaining after a time t is first order rate expression: N = N 0 e kt N 0 is the initial number of radioactive nuclei.
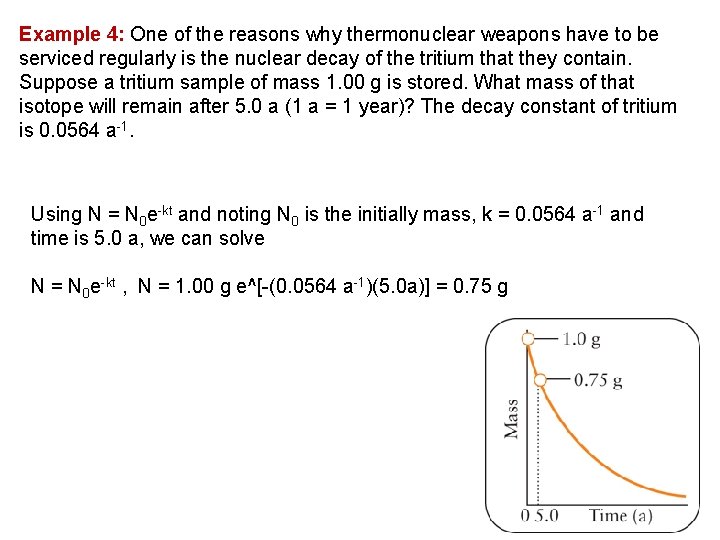
Example 4: One of the reasons why thermonuclear weapons have to be serviced regularly is the nuclear decay of the tritium that they contain. Suppose a tritium sample of mass 1. 00 g is stored. What mass of that isotope will remain after 5. 0 a (1 a = 1 year)? The decay constant of tritium is 0. 0564 a 1. Using N = N 0 e kt and noting N 0 is the initially mass, k = 0. 0564 a 1 and time is 5. 0 a, we can solve N = N 0 e kt , N = 1. 00 g e^[ (0. 0564 a 1)(5. 0 a)] = 0. 75 g 34
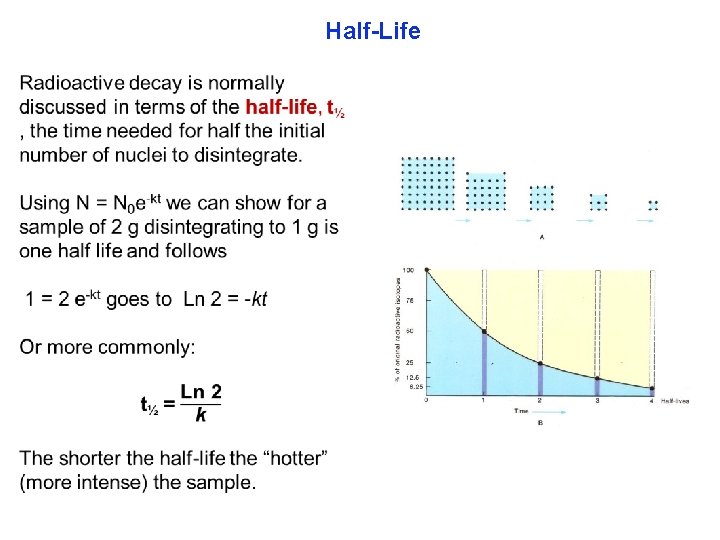
Half-Life
Use of Radioisotopes are used for curing disease, preserve food, to trace mechanisms of reactions, and to power spacecraft. Radioactive tracers, water with O 18, shows where the atoms end up in a sample of sugar; 6 CO 2(g) + 6 H 218 O(l) C 6 H 12 O 6 (s, glucose) + 6 18 O 2 (g) Labeled fertilizers follow the mechanism of plant growth and the passage of these elements through the environment. Ameri cium 241 is used insmoke detectors. Voy ager 2, is powered by radiation from plutonium.

Nuclear Energy Nuclear reactions can release huge amounts of energy. The benefits include the large quantity of energy obtained from a small mass of fuel and the absence of chemical pollution of the kind associated with fossil fuels. However, like other powerful resources, nuclear energy presents us with great technical challenges and hazards

Energy-Mass Conversion Einstein's theory of relativity tells us that the mass of an object is a measure of its energy content. Specifi cally, the total energy, E, and the mass, m, and c is the speed of light (3. 00 × 108 m·s 1), are related by Einstein's famous equation: E = mc 2 Strongly exothermic chemical reaction releasing 103 k. J of energy, has a mass difference of only 10 8 g between products and reactants. Nuclear reactions have a much greater mass loss.
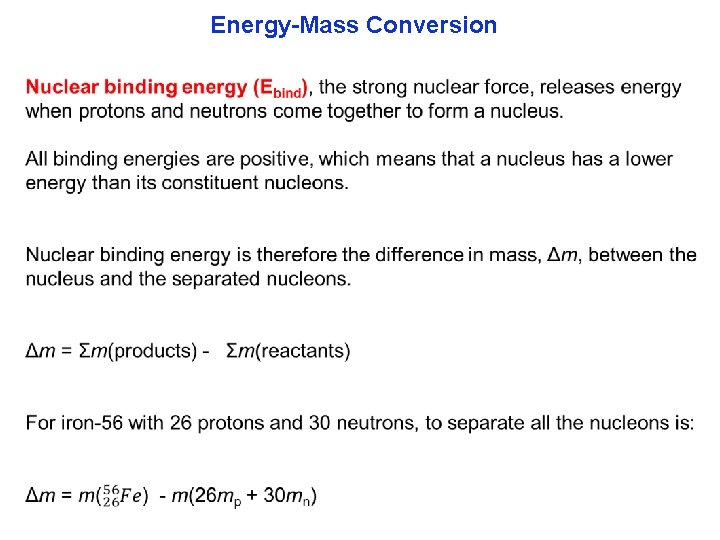
Energy-Mass Conversion
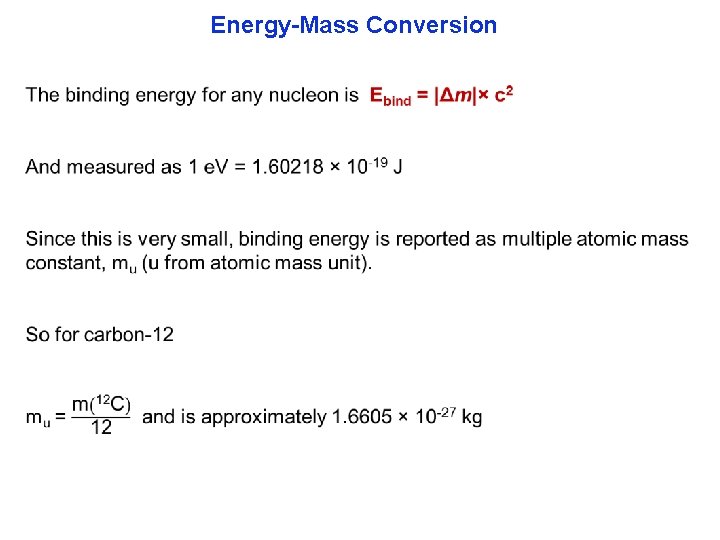
Energy-Mass Conversion
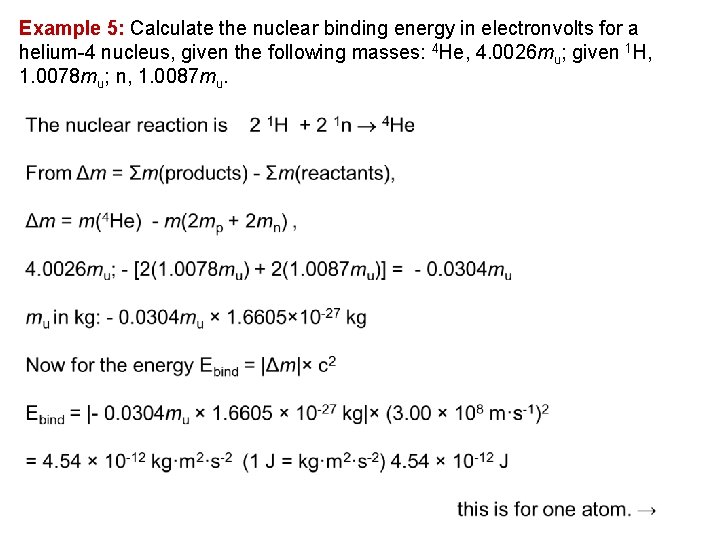
Example 5: Calculate the nuclear binding energy in electronvolts for a helium 4 nucleus, given the following masses: 4 He, 4. 0026 mu; given 1 H, 1. 0078 mu; n, 1. 0087 mu.
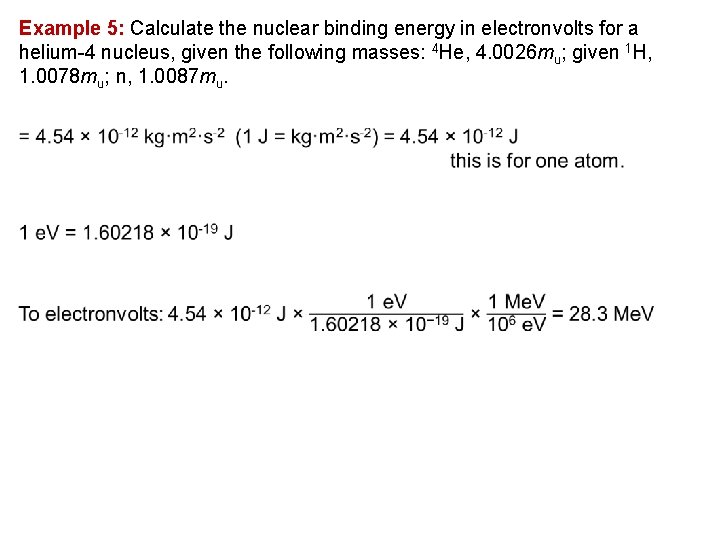
Example 5: Calculate the nuclear binding energy in electronvolts for a helium 4 nucleus, given the following masses: 4 He, 4. 0026 mu; given 1 H, 1. 0078 mu; n, 1. 0087 mu.
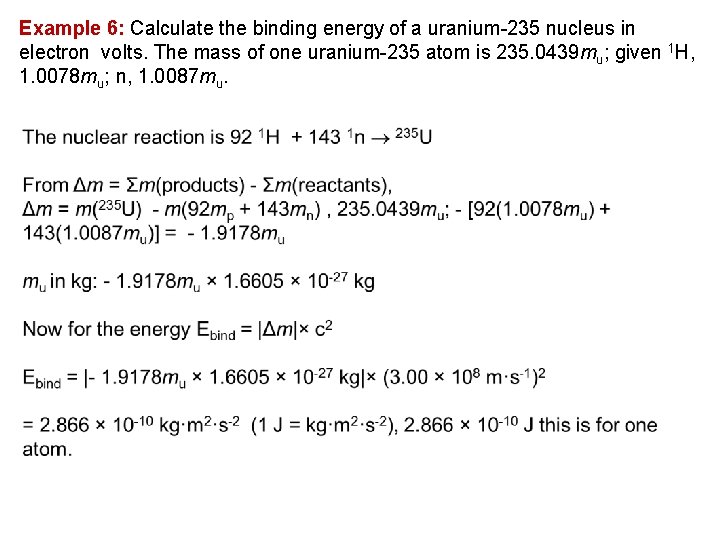
Example 6: Calculate the binding energy of a uranium 235 nucleus in electron volts. The mass of one uranium 235 atom is 235. 0439 mu; given 1 H, 1. 0078 mu; n, 1. 0087 mu.
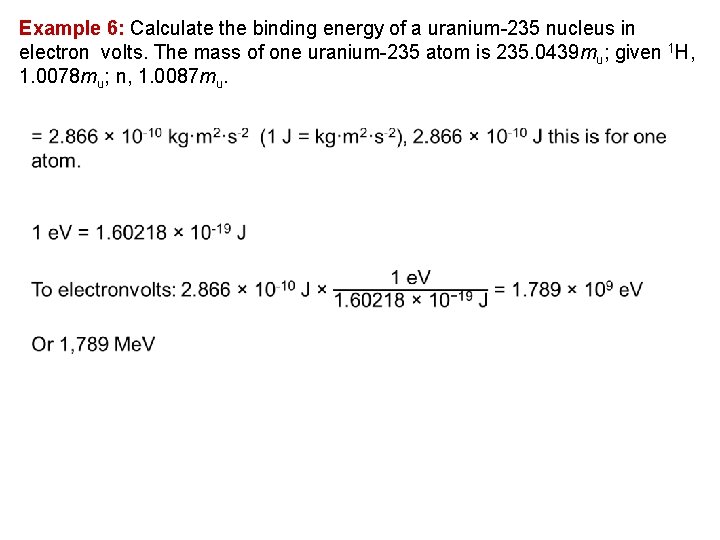
Example 6: Calculate the binding energy of a uranium 235 nucleus in electron volts. The mass of one uranium 235 atom is 235. 0439 mu; given 1 H, 1. 0078 mu; n, 1. 0087 mu.
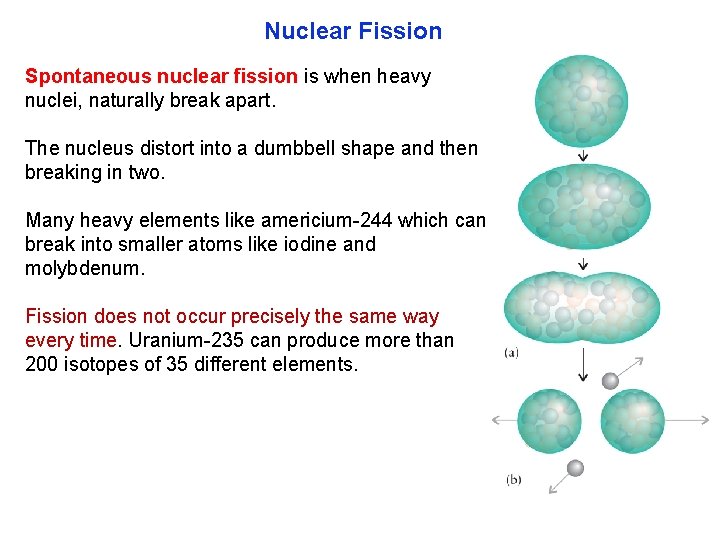
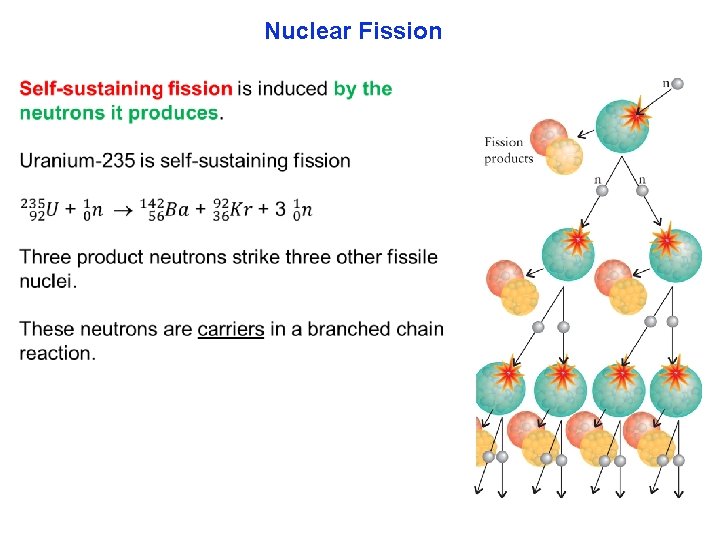
Nuclear Fission Spontaneous nuclear fission is when heavy nuclei, naturally break apart. The nucleus distort into a dumbbell shape and then breaking in two. Many heavy elements like americium 244 which can break into smaller atoms like iodine and molybdenum. Fission does not occur precisely the same way every time. Uranium 235 can produce more than 200 isotopes of 35 different elements.
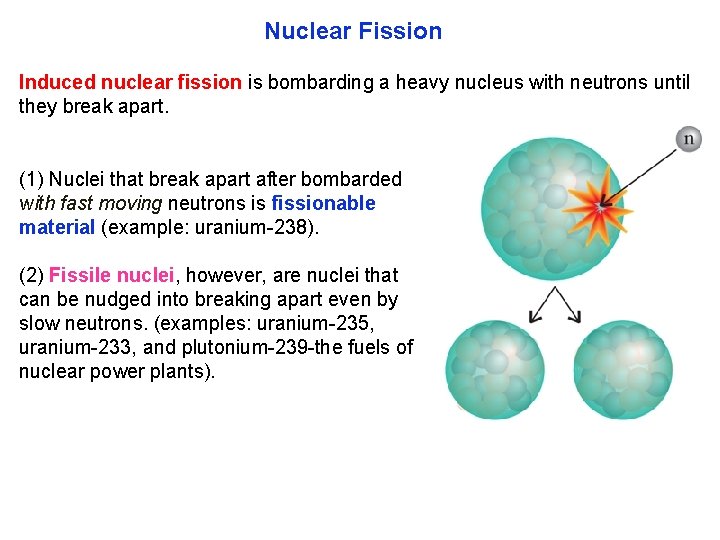
Nuclear Fission Induced nuclear fission is bombarding a heavy nucleus with neutrons until they break apart. (1) Nuclei that break apart after bombarded with fast moving neutrons is fissionable material (example: uranium 238). (2) Fissile nuclei, however, are nuclei that can be nudged into breaking apart even by slow neutrons. (examples: uranium 235, uranium 233, and plutonium 239 the fuels of nuclear power plants).
Nuclear Fission
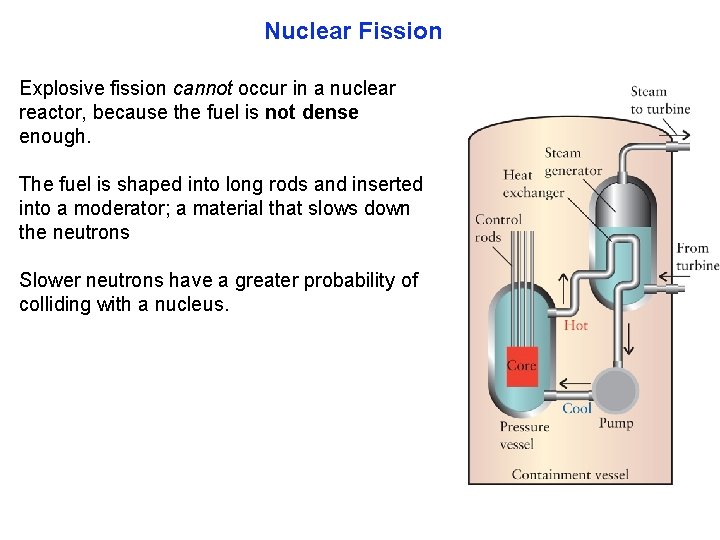
Nuclear Fission Explosive fission cannot occur in a nuclear reactor, because the fuel is not dense enough. The fuel is shaped into long rods and inserted into a moderator; a material that slows down the neutrons Slower neutrons have a greater probability of colliding with a nucleus.
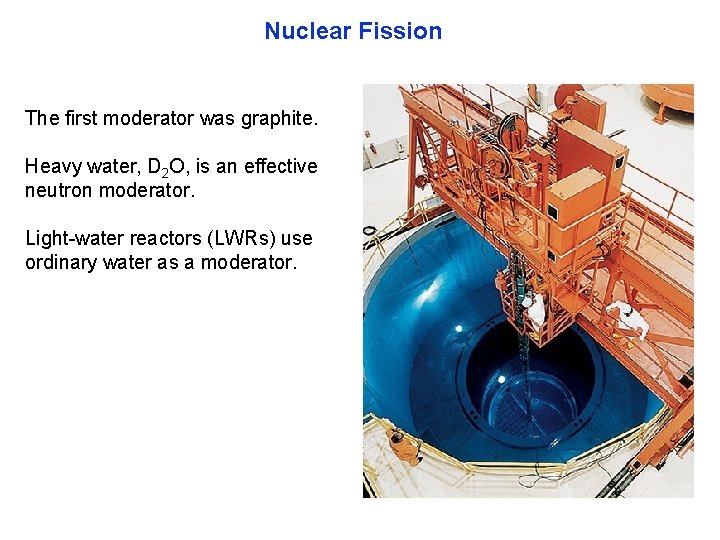
Nuclear Fission The first moderator was graphite. Heavy water, D 2 O, is an effective neutron moderator. Light water reactors (LWRs) use ordinary water as a moderator.

Nuclear Fission One of the many problems of nuclear power is the availability of fuel. Ura nium 235 reserves are only about 0. 7% those of the nonfissile uranium 238, and the separation of the isotopes is costly. One solution is to synthe sizefissile nuclides from other elements in a breeder reactor. However, breeder reactors are more haz ardousto operate than nuclear power plants. They run very hot, so require more control than nuclear power generation. The "hydrogen bomb, " a fission bomb (using uranium or plutonium).

Nuclear Fusion: Combining two light nuclei to form a heavier nucleus. Fission reactors do not generate highly hazardous radioactive waste. One area of research is the fusion of hydrogen nuclei to form helium nuclei from seawater. In light elements strong electrical repulsions between protons make it difficult for protons to approach each other. A fusion bombs can have 200 times the destruc tive capacity of the fission bombs dropped on Hiroshima and Nagasaki in World War II.
Nuclear Fusion One fusion scheme uses deuterium (D) and tritium (T) : D + D 3 He + n D+D T+p D + T 4 He + n D + 3 He 4 He + p The overall reaction releases 3 × 108 k. J per gram of deuterium. That’s the same amount of energy the Hoover Dam processes, at full capacity, every hour.
- Slides: 52