Chapter 18 Electrochemistry Homework 9 11 12 13































- Slides: 99

Chapter 18 - Electrochemistry Homework: 9, 11, 12, 13, 14, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 31, 33, 34, 36, 37, 39, 41, 42, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57, 58, 60, 61, 63, 64, 83, 85, 87, 88, 89

Oxidation # Redux n n Any ion will have an oxidation number equal to its charge Oxygen in a compound (except in peroxide, then it is -1) will have an oxidation number of -2 Hydrogen will have an oxidation number of +1 or -1, depending on what it is bonded to Everything else s oxidation number is whatever is necessary to have the sum of the oxidation numbers of the compound add up to the charge on the compound.

18. 2 – Balancing Oxidation. Reduction Equations n How do we tell whether a given reaction is an oxidation-reduction reaction? n n We do so by keeping track of the oxidation states/number of all the elements involved in the reaction This procedure tells us which elements (if any) are changing oxidation state

Oxidized vs. Reduced n Oxidized n n An atom/element is oxidized when it loses electrons Reduced n An atom/element is reduced when it gains electrons

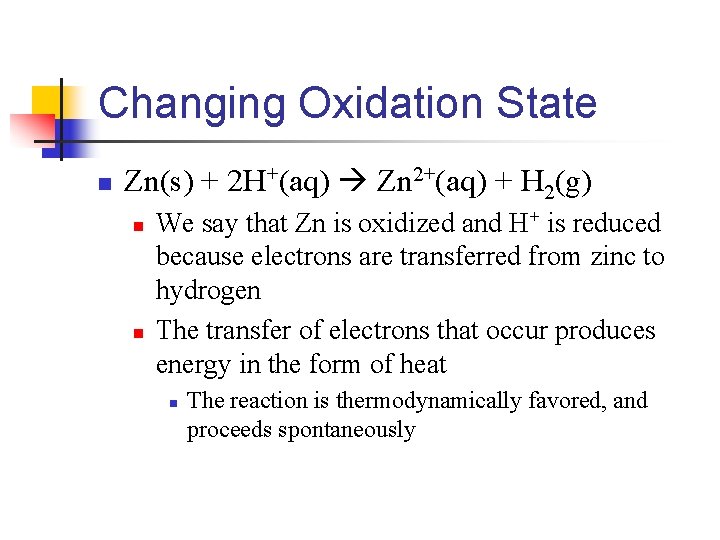
Changing Oxidation State n Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) n n We say that Zn is oxidized and H+ is reduced because electrons are transferred from zinc to hydrogen The transfer of electrons that occur produces energy in the form of heat n The reaction is thermodynamically favored, and proceeds spontaneously

Changing Oxidation? We can write the previous reaction in terms of oxidation numbers n Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) 0 +1 +2 0 § By writing the oxidation state for each element above or below the equation, we can see the oxidation state changes that happen n

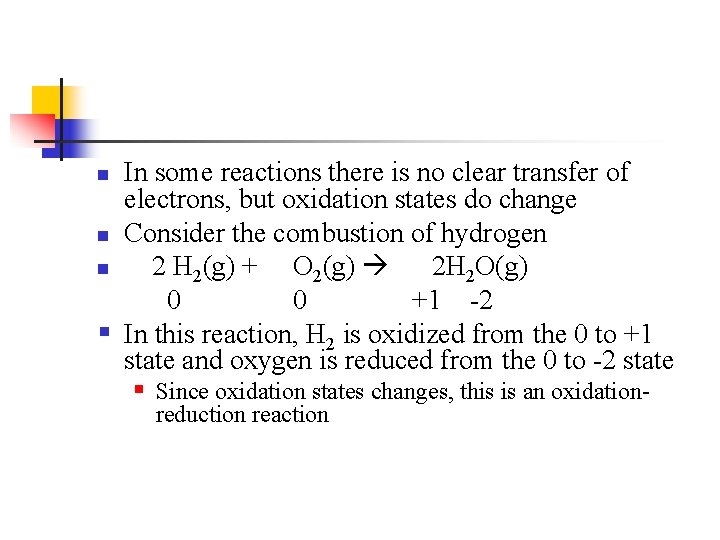
In some reactions there is no clear transfer of electrons, but oxidation states do change n Consider the combustion of hydrogen n 2 H 2(g) + O 2(g) 2 H 2 O(g) 0 0 +1 -2 § In this reaction, H 2 is oxidized from the 0 to +1 state and oxygen is reduced from the 0 to -2 state § Since oxidation states changes, this is an oxidationn reduction reaction

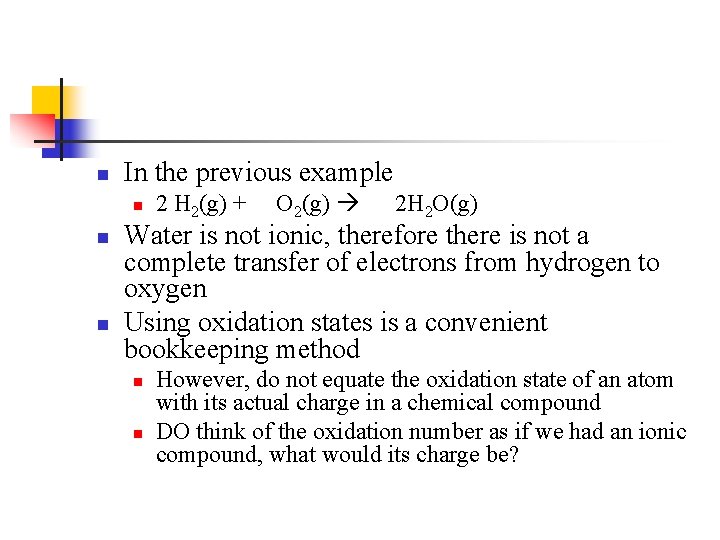
n In the previous example n n n 2 H 2(g) + O 2(g) 2 H 2 O(g) Water is not ionic, therefore there is not a complete transfer of electrons from hydrogen to oxygen Using oxidation states is a convenient bookkeeping method n n However, do not equate the oxidation state of an atom with its actual charge in a chemical compound DO think of the oxidation number as if we had an ionic compound, what would its charge be?

n In any redox reaction, both oxidation and reduction must occur n n In other words: If one substance is oxidized, then another must be reduced The electrons MUST go from one place to another

n n Whenever we balance an equation, we MUST obey the law of conservation of mass As we balance an oxidation-reduction reaction, we add another rule n n The gains and losses of electrons must be balanced This means if a substance loses a certain number of electrons during a reaction, some other substance must gain that same number of electrons Some redox reactions do this pretty much automatically Others do not.

Half-Reactions n n Although oxidation and reduction take place at the same time, often easier to consider them separate processes Sn 2+(aq) + 2 Fe 3+(aq) Sn 4+(aq) + 2 Fe 2+(aq) n n n We can think of this as two separate process The oxidation of Sn 2+ and the reduction of Fe 3+ Oxidation: n n Reduction: n n Sn 2+(aq) Sn 4+(aq) + 2 e 2 Fe 3+(aq) + 2 e- 2 Fe 2+(aq) Note: During oxidation electrons are a product, while during reduction electrons are a reactant

n Equations that show either oxidation or reduction alone are called half-reactions n n In the overall redox reaction, the number of electrons lost in the oxidation half-reaction must equal the number of electrons gained in the reduction halfreaction When this condition is met and each half-reaction is balanced, the electrons on the two sides cancel when the two half-reactions are then added to give the overall, balanced equation

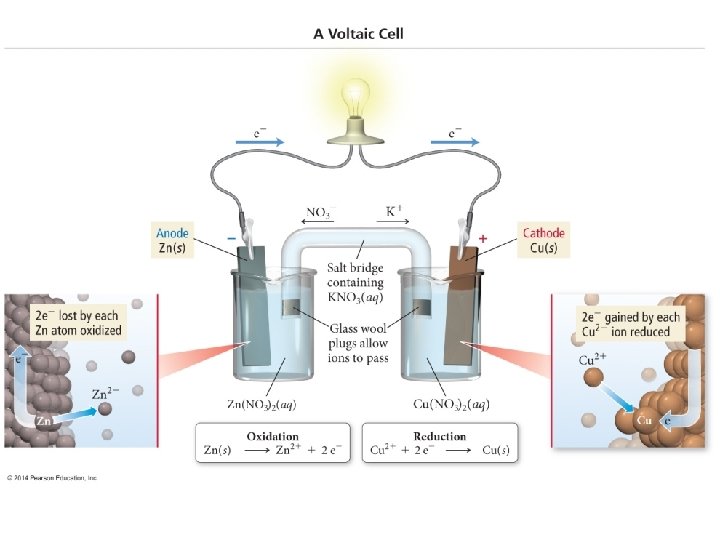
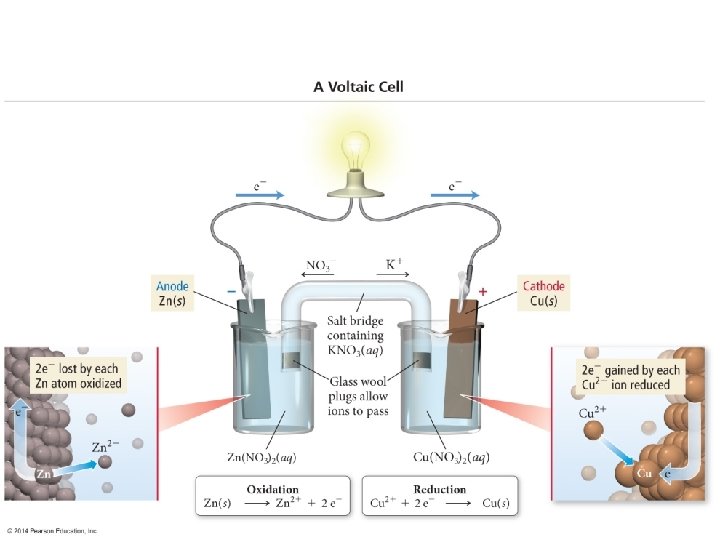
18. 3 - Voltaic Cells** n The energy released in a spontaneous redox reaction can be used to perform electrical work n This is done through a voltaic (or galvanic) cell n n A device in which the transfer of electrons takes place through an external pathway Rather than directly between reactants

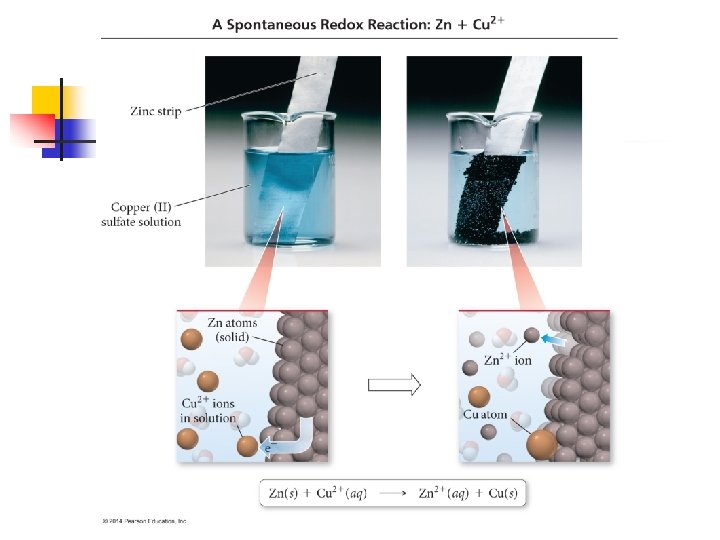
n An example of this is when a strip of zinc is placed in contact with a solution containing Cu 2+ n n n As reaction proceeds, the blue color of the Cu 2+ ions fade and the copper metal deposits onto the zinc. At the same time, the zinc dissolves Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)


n Same basic principle as last n n n But this time the Zn(s) and Cu 2+ are not in direct contact The Zn is placed in contact with Zn 2+ in one part of the cell The Cu is placed in contact with the Cu 2+ in another cell

How Does this Work? n The reduction of the Cu 2+ can occur only by the flow of electrons through an external circuit n n The wire connecting the Zn and Cu strips By physically separating the reduction half of the reaction from the oxidation half n n We create a flow of electrons through an external circuit We have electricity!

n The two solid metals that are connected by the external circuit are called electrodes n n The electrode at which oxidation occurs is called the anode The electrode at which reduction occurs is called the cathode n n Remember that oxidation and anode both start with a vowel While reduction and cathode both start with a consonant


Or This is my cat Matthew. • He is fat, and must go on a diet. • Because cats must be reduced. • Cathode is where reduction takes place

n As in this example, the electrodes can be made of materials that participate in the reaction n n But typically the electrodes are made of a conducting material that does not gain or lose mass during the reaction Just acts as a surface at which electrons are transferred n Such as platinum or graphite

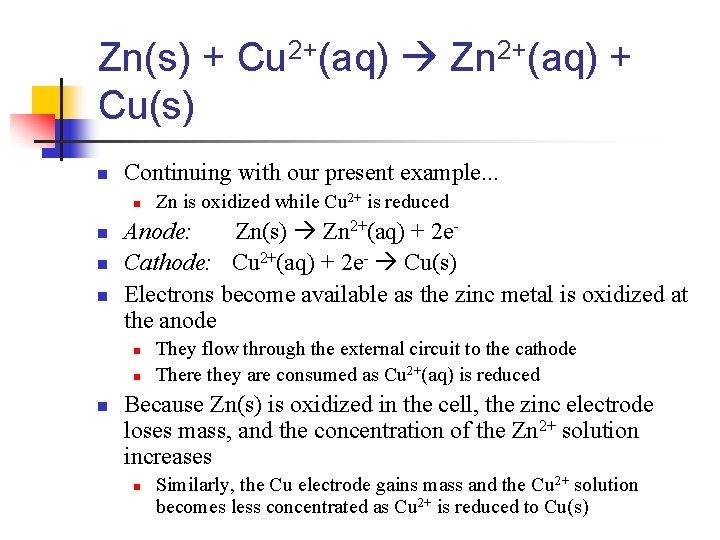
n Each compartment in a voltaic cell is called a half-cell. n n One half-cell is where the oxidation halfreaction takes place One half-cell is where the reduction halfreaction takes place

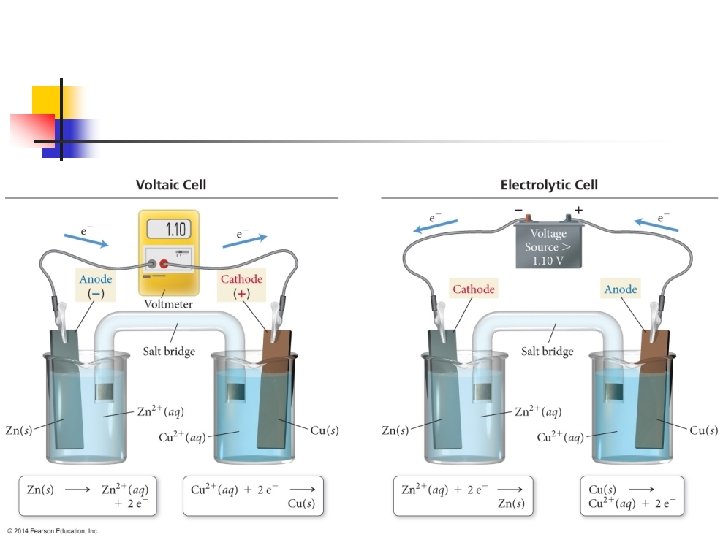
Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) n Continuing with our present example. . . n n Anode: Zn(s) Zn 2+(aq) + 2 e. Cathode: Cu 2+(aq) + 2 e- Cu(s) Electrons become available as the zinc metal is oxidized at the anode n n n Zn is oxidized while Cu 2+ is reduced They flow through the external circuit to the cathode There they are consumed as Cu 2+(aq) is reduced Because Zn(s) is oxidized in the cell, the zinc electrode loses mass, and the concentration of the Zn 2+ solution increases n Similarly, the Cu electrode gains mass and the Cu 2+ solution becomes less concentrated as Cu 2+ is reduced to Cu(s)

n For a voltaic cell to work n n The solutions in the two half-cells must remain electrically neutral As Zn is oxidized, Zn 2+ ions enter the solution n So we need a way to for the positive ions to leave the anode compartment or for negative ions to enter n To keep solution electrically neutral

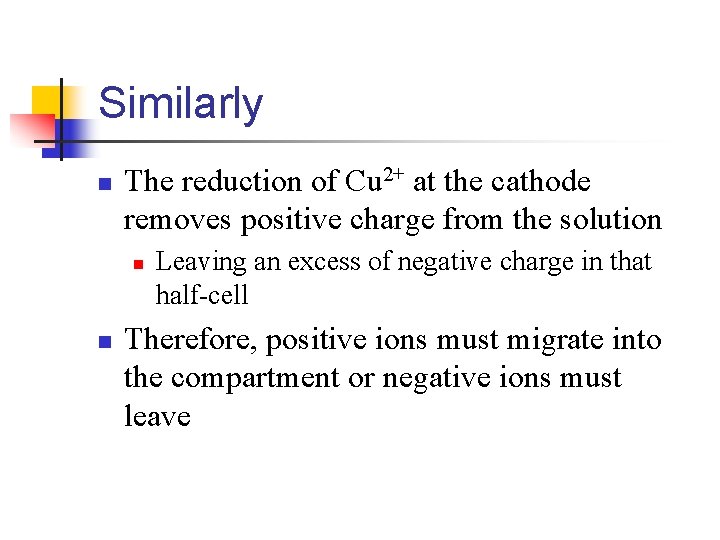
Similarly n The reduction of Cu 2+ at the cathode removes positive charge from the solution n n Leaving an excess of negative charge in that half-cell Therefore, positive ions must migrate into the compartment or negative ions must leave

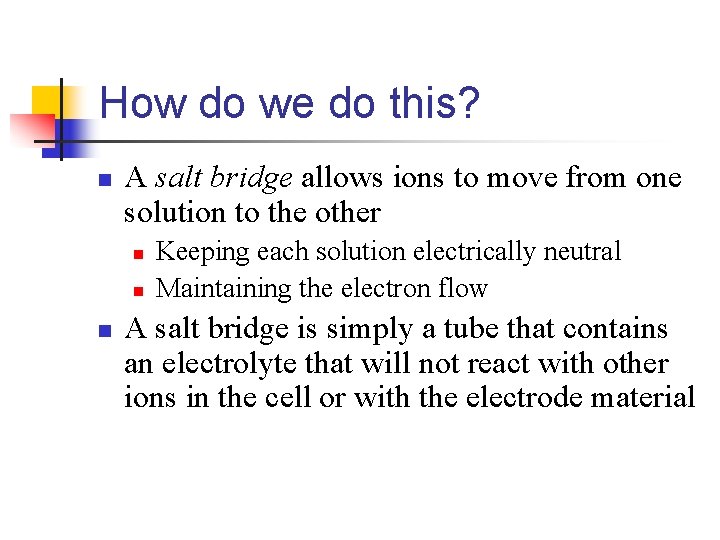
How do we do this? n A salt bridge allows ions to move from one solution to the other n n n Keeping each solution electrically neutral Maintaining the electron flow A salt bridge is simply a tube that contains an electrolyte that will not react with other ions in the cell or with the electrode material

n As oxidation and reduction proceed at the electrodes, ions from the salt bridge migrate to neutralize the charge in each cell n No matter how this works, anions will always migrate toward the anode and cations toward the cathode


n Note the direction of ions in the solution


n Notice also that in any voltaic cell, the electrons will flow from the anode through the external circuit to the cathode. n n Because the negative charged electrons flow from the anode to the cathode, the anode in a voltaic cell is labeled with a negative sign And the cathode is labeled with a positive sign n Think of the electrons as being attracted to the positive cathode from the negative anode through the external circuit

18. 4 - Cell EMF Under Standard Conditions n Why do electrons transfer spontaneously from a Zn atom to a Cu 2+ ion? n n In this section we will look at the main driving force that pushes the electrons through the external circuit in a voltaic cell The chemical processes that make up any voltaic cell are spontaneous n n Electrons flow from the anode of a voltaic cell to the cathode because of a difference in potential energy The potential energy of the electrons is higher in the anode than in the cathode n So they spontaneously move to lower their energy

n The difference in potential energy per electrical charge (called potential difference) between two electrodes is measured in volts n One volt (V) is the potential energy required to impart 1 J of energy to a charge of 1 coulomb (C) n Remember that an electron has a charge of 1. 60 x 1019 C.

n The potential difference between the two electrodes in a voltaic cell provides the driving force to push the electrons through the external circuit n We will therefore call this potential difference the electromotive force, or emf n n electromotive means to “cause electron motion” The emf of a cell (Ecell) is also called the cell potential. n n Because Ecell is measured in volts, we often refer to this as the cell voltage. For any cell reaction that proceeds spontaneously (like in a voltaic cell), the cell potential will be positive.

n The actual emf of a particular cell depends on the n n n specific reactions that take place at the cathode and anode concentrations of the reactants and products temperature (which we will generally assume to be 25ºC) unless told otherwise n We will focus on this temperature, and refer to this as standard conditions

At standard conditions n If we have 1 M concentrations for reactants and products and 1 atm pressure for gases and 25ºC n Then the emf for these standard conditions is called the standard emf or the standard cell potential n n Eºcell For example, for the Zn-Cu voltaic cell seen earlier, the standard cell potential is +1. 10 V

Standard Reduction (Half-Cell) Potentials n The emf of a voltaic cell depends on the particular cathode and anode half-cell involved n n n We COULD tabulate the standard potentials for all possible combinations However, that is not necessary We assign a standard potential to each individual half-cell, and use these half-cells to determine Eºcell

n The cell potential is the difference between the two electrode potentials n n One from the cathode, one from the anode Due to convention, the potential associated with each electrode is the potential for reduction to occur at that electrode n n Thus, standard electrode potentials are found for reduction reactions Called standard reduction potentials, Eºred

Calculating Eºcell n The cell potential (Eºcell) is given by the standard reduction potential of the cathode, Eºred(cathode), minus the standard reduction potential of the anode, Eºred(anode) n Eºcell = Eºred(cathode) - Eºred(anode)

n Since every voltaic cell involves two halfcells, it is impossible to measure the standard reduction potential directly n n All based off of a certain reference half-reaction The reference half-reaction is the reduction of H+(aq) to H 2(g) under standard conditions n n 2 H+(aq, 1 M) + 2 e- H 2(g, 1 atm) Eºred = 0 V The standard reduction potential of this reaction is defined to be 0 V

n Various standard reduction potentials for half-reactions are found on pg. 873 (table 18. 1) or in Appendix II (Table D)

n Because electrical potential measures potential energy per electrical charge, standard reduction potentials are intensive properties n n n This means that if we increased the amount of chemicals in the redox reaction, we would increase both energy and charges involved But the ratio of the energy and charge would remain constant Therefore, changing the stoichiometric coefficients in a half-reaction does not affect the value of the standard reduction potential

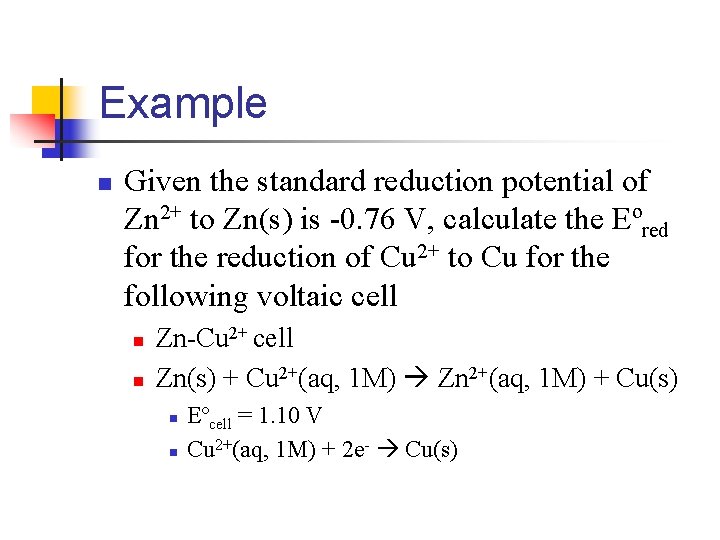
Example n Given the standard reduction potential of Zn 2+ to Zn(s) is -0. 76 V, calculate the Eºred for the reduction of Cu 2+ to Cu for the following voltaic cell n n Zn-Cu 2+ cell Zn(s) + Cu 2+(aq, 1 M) Zn 2+(aq, 1 M) + Cu(s) n n Eºcell = 1. 10 V Cu 2+(aq, 1 M) + 2 e- Cu(s)

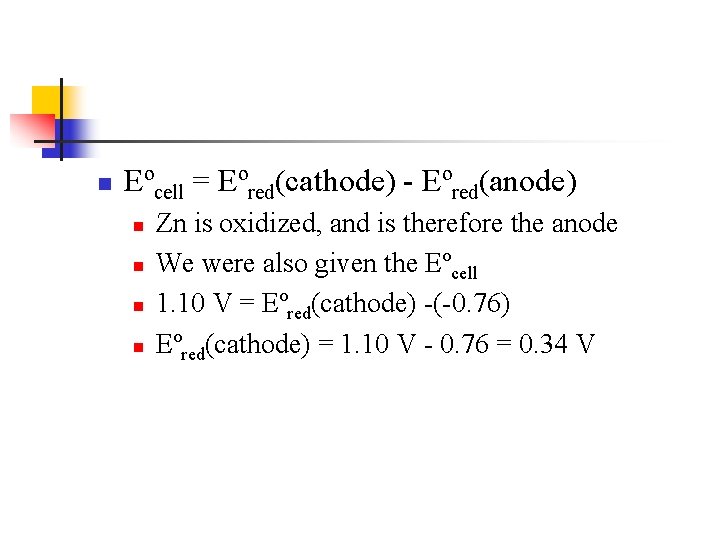
n Eºcell = Eºred(cathode) - Eºred(anode) n n Zn is oxidized, and is therefore the anode We were also given the Eºcell 1. 10 V = Eºred(cathode) -(-0. 76) Eºred(cathode) = 1. 10 V - 0. 76 = 0. 34 V

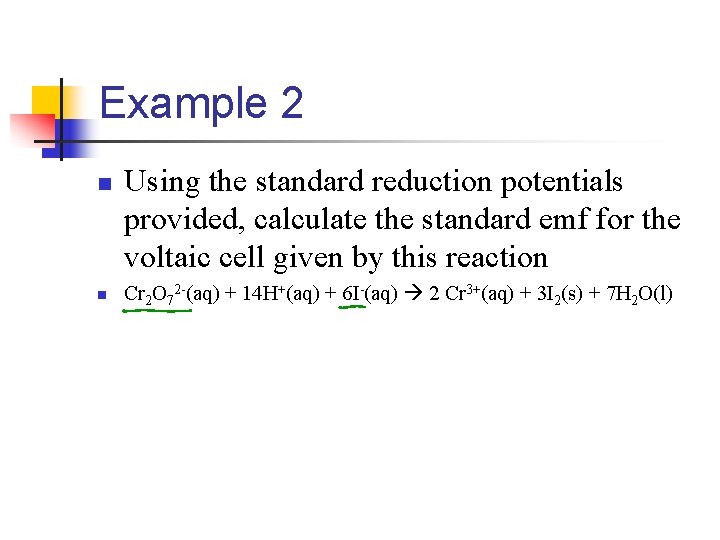
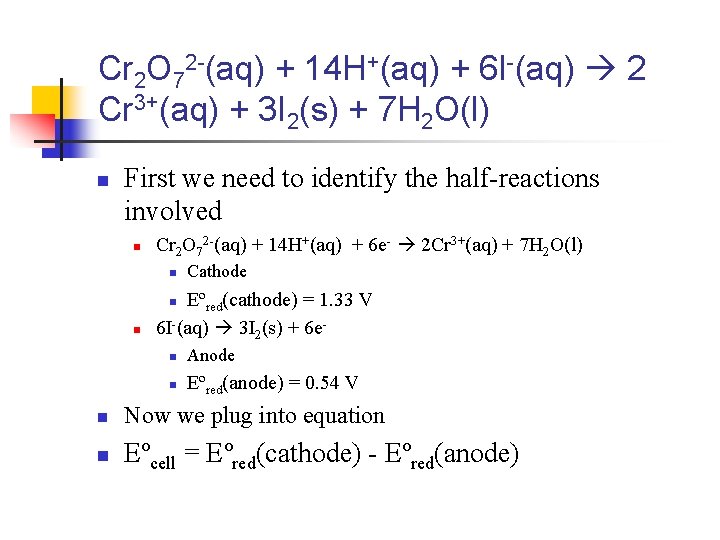
Example 2 n n Using the standard reduction potentials provided, calculate the standard emf for the voltaic cell given by this reaction Cr 2 O 72 -(aq) + 14 H+(aq) + 6 I-(aq) 2 Cr 3+(aq) + 3 I 2(s) + 7 H 2 O(l)

Cr 2 O 72 -(aq) + 14 H+(aq) + 6 I-(aq) 2 Cr 3+(aq) + 3 I 2(s) + 7 H 2 O(l) n First we need to identify the half-reactions involved n Cr 2 O 72 -(aq) + 14 H+(aq) + 6 e- 2 Cr 3+(aq) + 7 H 2 O(l) n Cathode Eºred(cathode) = 1. 33 V 6 I-(aq) 3 I 2(s) + 6 en n n Anode n Eºred(anode) = 0. 54 V n Now we plug into equation n Eºcell = Eºred(cathode) - Eºred(anode)

Eºcell = Eºred(cathode) Eºred(anode) n Eºcell = 1. 33 V - 0. 54 V = 0. 79 V

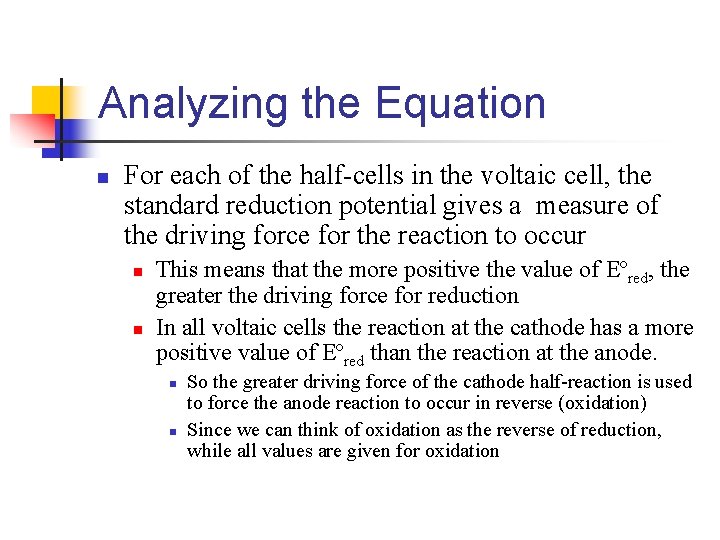
Analyzing the Equation n For each of the half-cells in the voltaic cell, the standard reduction potential gives a measure of the driving force for the reaction to occur n n This means that the more positive the value of Eºred, the greater the driving force for reduction In all voltaic cells the reaction at the cathode has a more positive value of Eºred than the reaction at the anode. n n So the greater driving force of the cathode half-reaction is used to force the anode reaction to occur in reverse (oxidation) Since we can think of oxidation as the reverse of reduction, while all values are given for oxidation

n We know that Eºcell is the difference between the standard reduction potential of the cathode reaction and the standard reduction potential of the anode n We can think of Eºcell as the net driving force that pushes the electrons through the external circuit

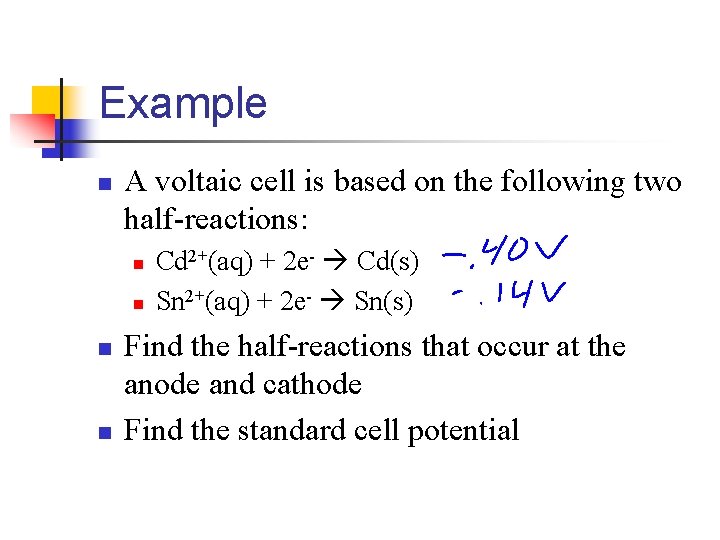
Example n A voltaic cell is based on the following two half-reactions: n n Cd 2+(aq) + 2 e- Cd(s) Sn 2+(aq) + 2 e- Sn(s) Find the half-reactions that occur at the anode and cathode Find the standard cell potential

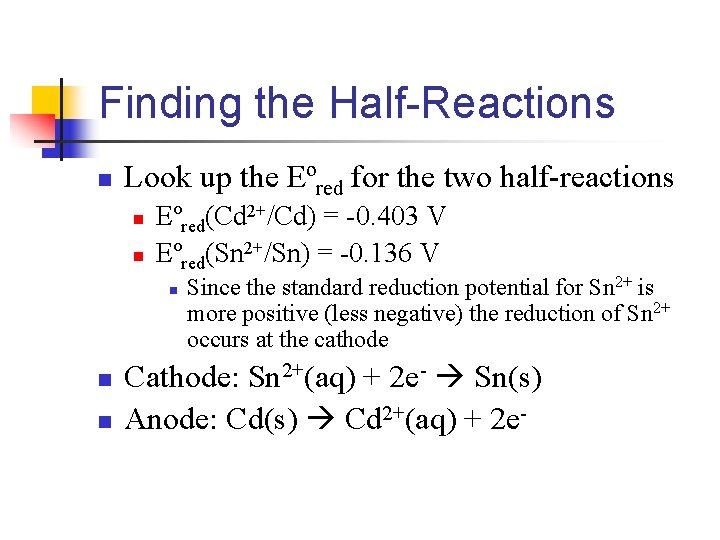
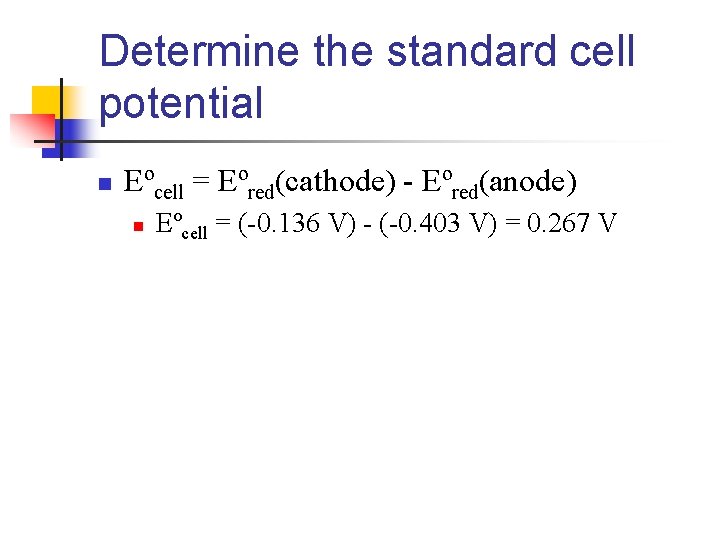
Finding the Half-Reactions n Look up the Eºred for the two half-reactions n n Eºred(Cd 2+/Cd) = -0. 403 V Eºred(Sn 2+/Sn) = -0. 136 V n n n Since the standard reduction potential for Sn 2+ is more positive (less negative) the reduction of Sn 2+ occurs at the cathode Cathode: Sn 2+(aq) + 2 e- Sn(s) Anode: Cd(s) Cd 2+(aq) + 2 e-

Determine the standard cell potential n Eºcell = Eºred(cathode) - Eºred(anode) n Eºcell = (-0. 136 V) - (-0. 403 V) = 0. 267 V

How to tell how easily something is reduced/oxidized n Generally, the more positive the Eºred value for a half-reaction, the greater the tendency for the reactant of the half-reaction to be reduced n Which means a greater chance of it to oxidize another species

Easily reduced n Among the most easily reduced we find the halogens, O 2 and oxyanions n n Such as Mn. O 4 -, Cr 2 O 72 - and NO 3 All have a central atom with a large, positive oxidation state

Easily oxidized n Like acid-base strength, the strongest oxidizers are the worst at being reduced n n n So the half-reaction with the smallest reduction potential is the most easily reversed Means they most easily give up electrons to other species So they are easily oxidized

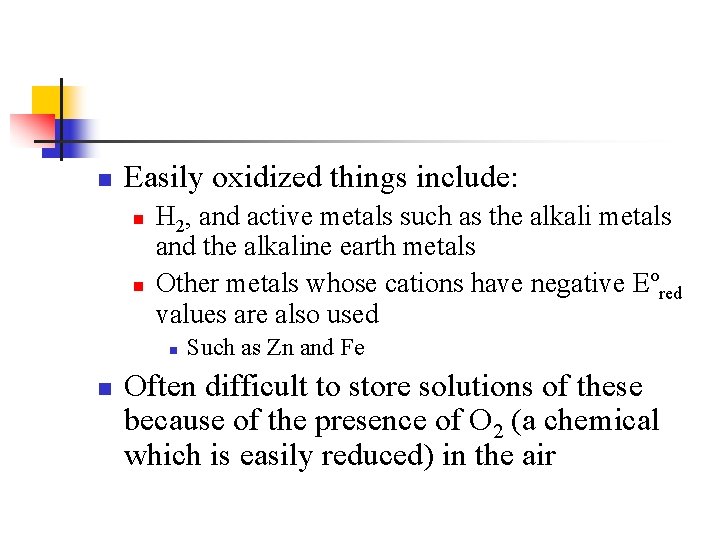
n Easily oxidized things include: n n H 2, and active metals such as the alkali metals and the alkaline earth metals Other metals whose cations have negative Eºred values are also used n n Such as Zn and Fe Often difficult to store solutions of these because of the presence of O 2 (a chemical which is easily reduced) in the air

Table 18. 1, pg. 873 n The list orders the ability of substances to act as an oxidizing or reducing agent n Remember, the more positive the value of Eºred, the more it will tend to be reduced

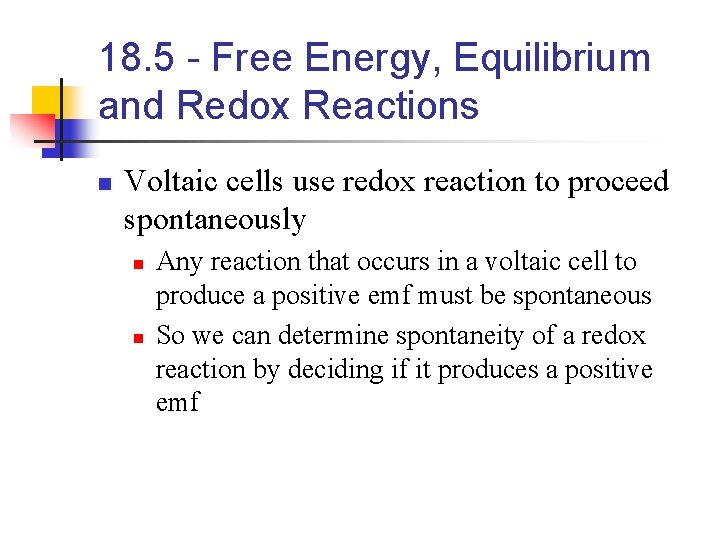
18. 5 - Free Energy, Equilibrium and Redox Reactions n Voltaic cells use redox reaction to proceed spontaneously n n Any reaction that occurs in a voltaic cell to produce a positive emf must be spontaneous So we can determine spontaneity of a redox reaction by deciding if it produces a positive emf

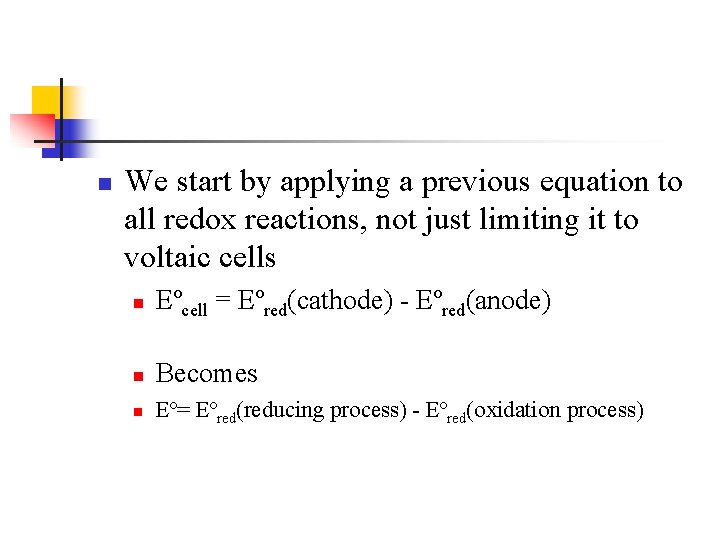
n We start by applying a previous equation to all redox reactions, not just limiting it to voltaic cells n Eºcell = Eºred(cathode) - Eºred(anode) n Becomes n Eº= Eºred(reducing process) - Eºred(oxidation process)

n Eº= Eºred(reducing process) - Eºred(oxidation process) n n A positive value of E tells us this is a spontaneous process While a negative value of E indicates a nonspontaneous one Use E to represent emf under nonstandard conditions Use Eº to represent emf under standard conditions

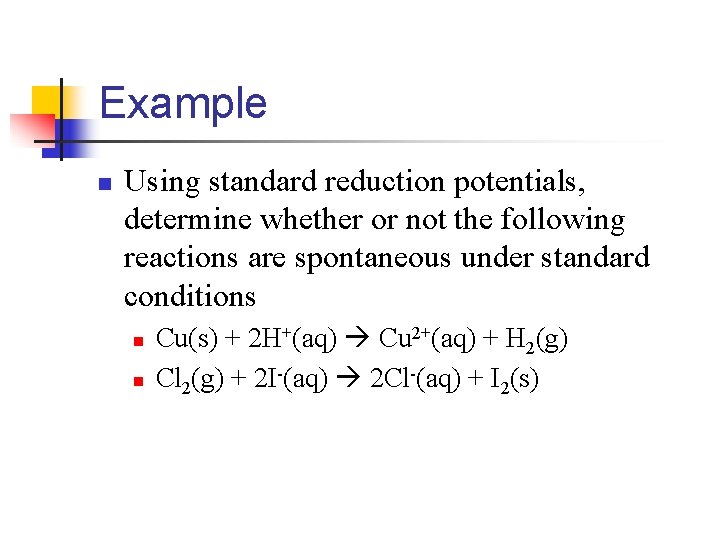
Example n Using standard reduction potentials, determine whether or not the following reactions are spontaneous under standard conditions n n Cu(s) + 2 H+(aq) Cu 2+(aq) + H 2(g) Cl 2(g) + 2 I-(aq) 2 Cl-(aq) + I 2(s)

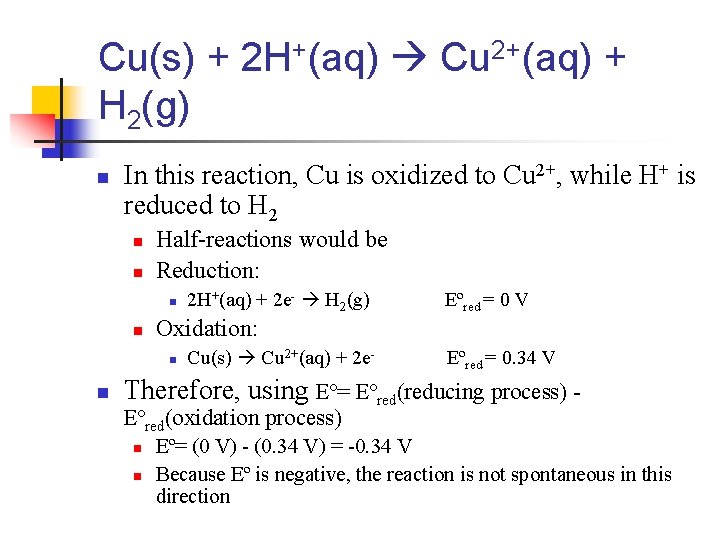
Cu(s) + 2 H+(aq) Cu 2+(aq) + H 2(g) n In this reaction, Cu is oxidized to Cu 2+, while H+ is reduced to H 2 n n Half-reactions would be Reduction: n n Eºred = 0 V Oxidation: n n 2 H+(aq) + 2 e- H 2(g) Cu(s) Cu 2+(aq) + 2 e- Eºred = 0. 34 V Therefore, using Eº= Eºred(reducing process) Eºred(oxidation process) n n Eº= (0 V) - (0. 34 V) = -0. 34 V Because Eº is negative, the reaction is not spontaneous in this direction

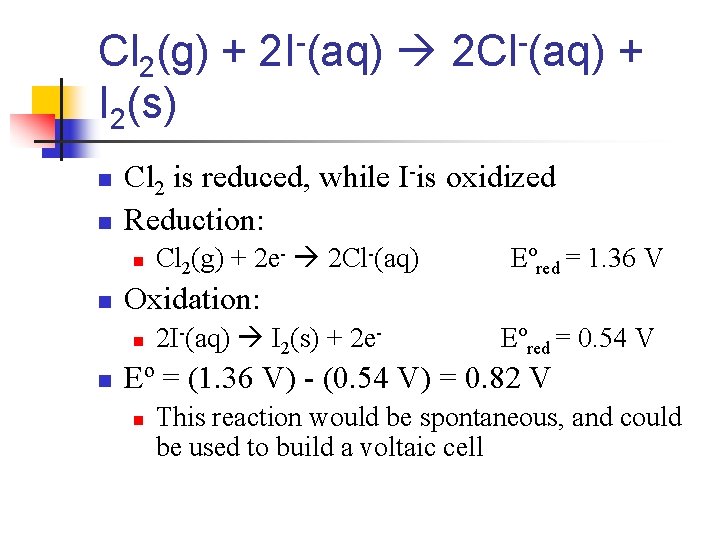
Cl 2(g) + 2 I-(aq) 2 Cl-(aq) + I 2(s) n n Cl 2 is reduced, while I-is oxidized Reduction: n n Eºred = 1. 36 V Oxidation: n n Cl 2(g) + 2 e- 2 Cl-(aq) 2 I-(aq) I 2(s) + 2 e- Eºred = 0. 54 V Eº = (1. 36 V) - (0. 54 V) = 0. 82 V n This reaction would be spontaneous, and could be used to build a voltaic cell

Activity Series of Metals n Remember, when dealing with single and double replacement reactions that you consult the activity series to determine if one metal will replace another n n If metal A is above metal B on the table, then A will replace B Any metal in the activity series will be oxidized by the ions of any metal below it n n n Activity series really shows the oxidation reactions of the metals, ordered from strongest reducing agent at the top to the weakest reducing agent at the bottom So we can use the relative strength of oxidizers to predict the results of displacement reactions with metals In other words, activity series is the OPPOSITE of the reduction potential series.

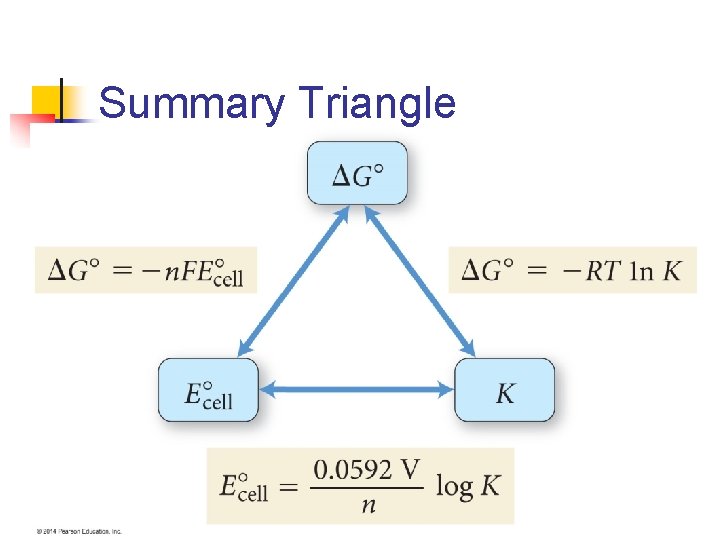
EMF and ΔG n The change in Gibbs free energy, ΔG measures the spontaneity of a process at constant temperature and pressure n Because EMF, E, of a redox reaction is spontaneous, there is a relationship between these two terms

Relationship n ΔG = -n. FE n n is a positive number without units n n F is called Faraday’s constant n n Represents the number of electrons transferred in the reaction Equal to 96, 485 J/V-mol The units of ΔG is J/mol n The /mol means per mole of reaction as written

ΔG = -n. FE n Both n and F are positive numbers n So a positive value of E means a negative ΔG n n So a positive value of E and a negative value for ΔG both indicate a spontaneous reaction Now, since ΔGº = -RT(ln. K) n We can now relate the standard emf to the equilibrium constant for the reaction

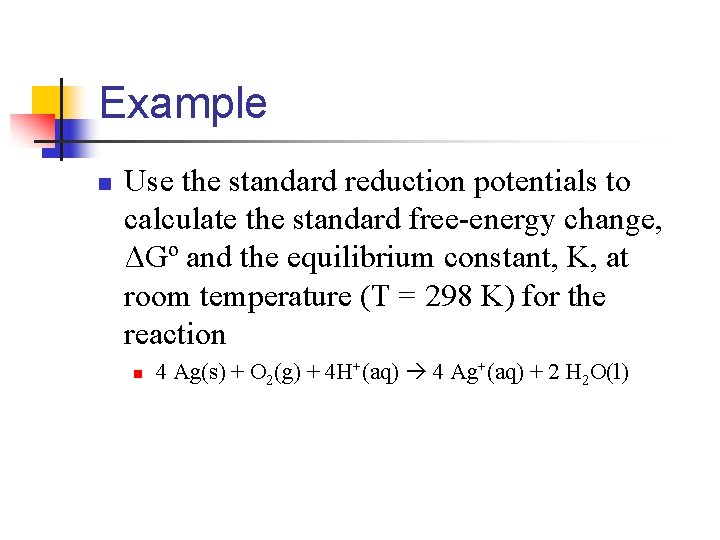
Example n Use the standard reduction potentials to calculate the standard free-energy change, ΔGº and the equilibrium constant, K, at room temperature (T = 298 K) for the reaction n 4 Ag(s) + O 2(g) + 4 H+(aq) 4 Ag+(aq) + 2 H 2 O(l)

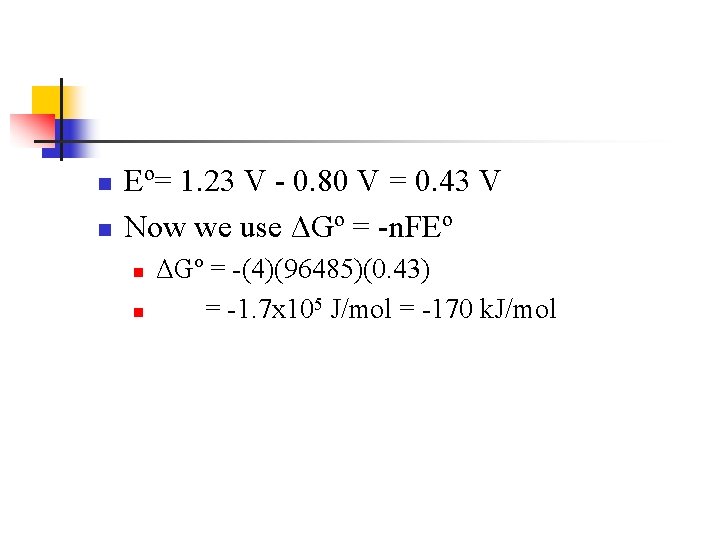
Find Eº First n To find Eº we need to break into halfreactions and obtain Eºred values n Reduction: n n n O 2(g) + 4 H+(aq) + 4 e- 2 H 2 O(l) Eºred = +1. 23 V Oxidation: n n 4 Ag(s) 4 Ag+(aq) + 4 e. Eºred = +0. 80 V

n n Eº= 1. 23 V - 0. 80 V = 0. 43 V Now we use ΔGº = -n. FEº n n ΔGº = -(4)(96485)(0. 43) = -1. 7 x 105 J/mol = -170 k. J/mol

Now solve for K n n n ΔGº = -RT(ln. K) K = e-ΔGº/RT = e-1. 7 x 10^5/(8. 314 x 298) K = 9 x 1029 n n n Why would we do this? Such a large K value very hard to measure However, the voltage measured is easy to measure.

Summary Triangle

18. 6 - Cell Potential and Concentration n As a voltaic cell is discharged, the reactants are consumed and products generated n n Which changes the concentrations The emf keeps dropping until E = 0, at which point we say the cell is “dead” n n n At this point the concentrations of products and reactants will cease to change They are at equilibrium This section will look at how emf changes with concentration, which will show nonstandard conditions

In General n If the concentrations of reactants increase relative to the concentrations of the products, the emf increases n Whereas if the concentrations of products increase relative to the reactants, the emf decreases n n Which is what happens when a batter goes dead! Think of this as this: The more reactants you have, the stronger the cell. The more product you have, the weaker.

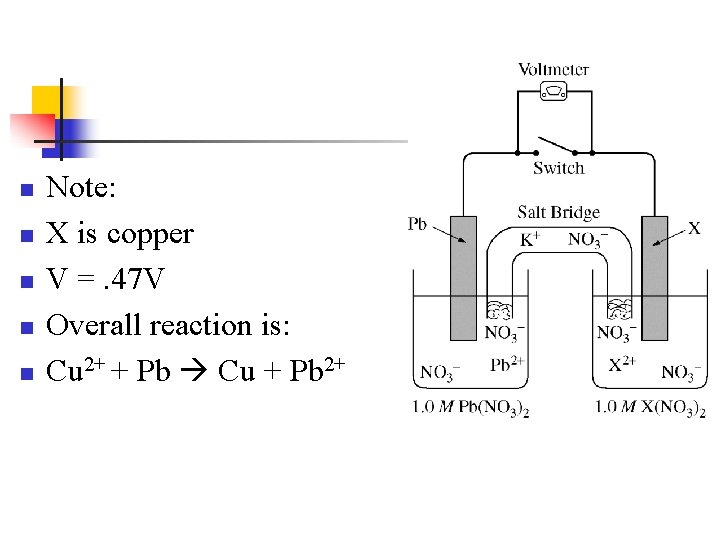
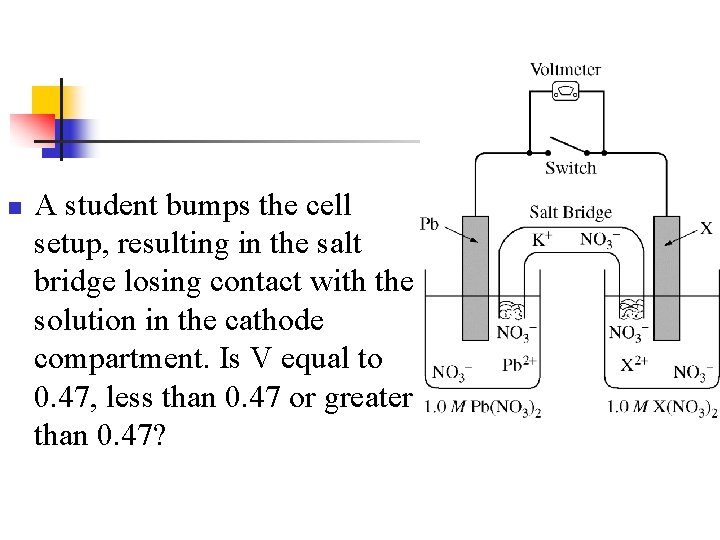
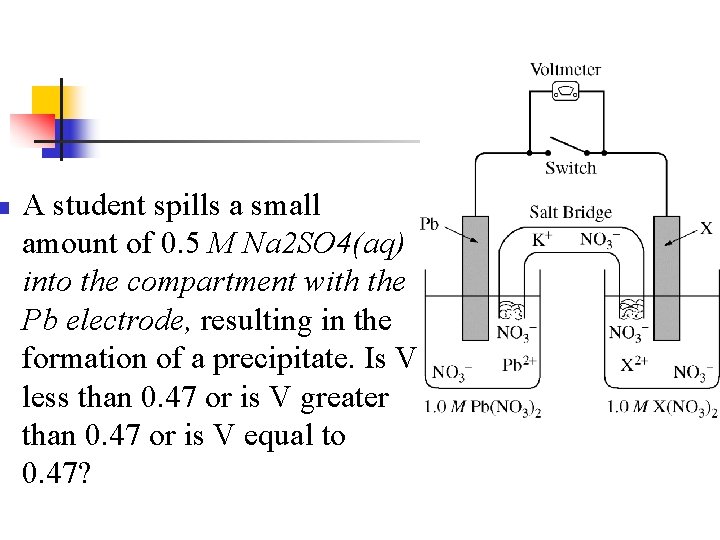
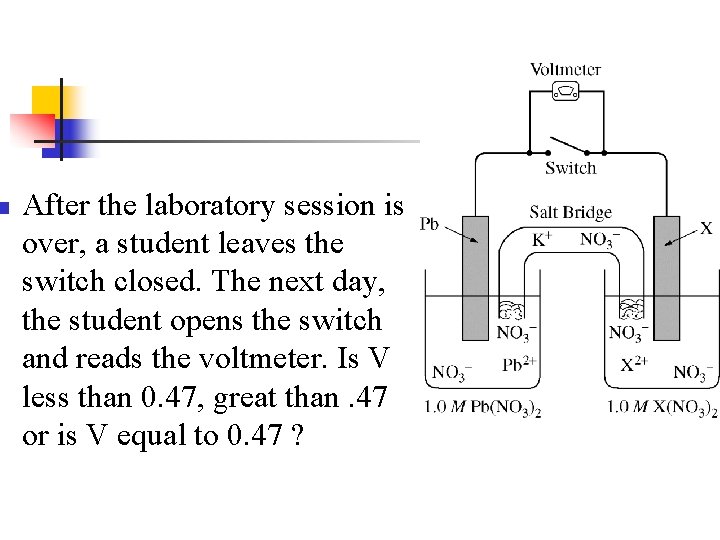
n n n Note: X is copper V =. 47 V Overall reaction is: Cu 2+ + Pb Cu + Pb 2+

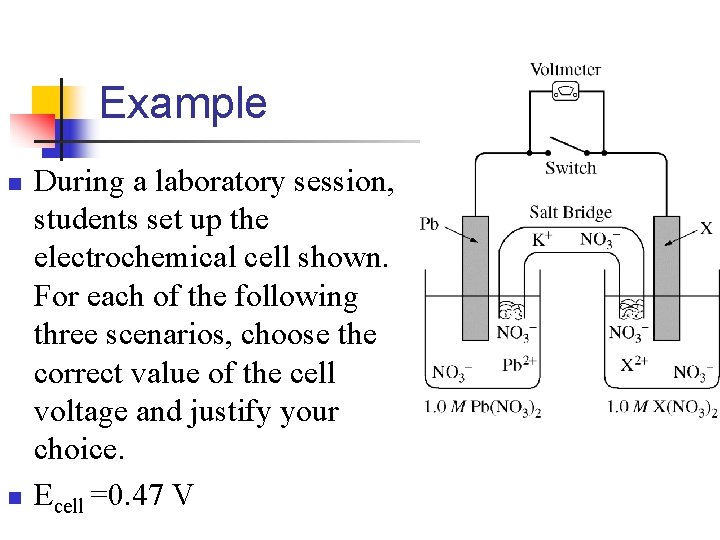
Example n n During a laboratory session, students set up the electrochemical cell shown. For each of the following three scenarios, choose the correct value of the cell voltage and justify your choice. Ecell =0. 47 V

n A student bumps the cell setup, resulting in the salt bridge losing contact with the solution in the cathode compartment. Is V equal to 0. 47, less than 0. 47 or greater than 0. 47?

n A student spills a small amount of 0. 5 M Na 2 SO 4(aq) into the compartment with the Pb electrode, resulting in the formation of a precipitate. Is V less than 0. 47 or is V greater than 0. 47 or is V equal to 0. 47?

n After the laboratory session is over, a student leaves the switch closed. The next day, the student opens the switch and reads the voltmeter. Is V less than 0. 47, great than. 47 or is V equal to 0. 47 ?

Concentration Cells n In each of the voltaic cells we have seen, the reactive species at the anode has been difference from the one at the cathode n n Cell emf depends on concentration Which means that a voltaic cell can be made using the same species at both the anode and cathode n n As long a the concentrations are different A cell based only on the emf generated because of difference in concentration is called a concentration cell

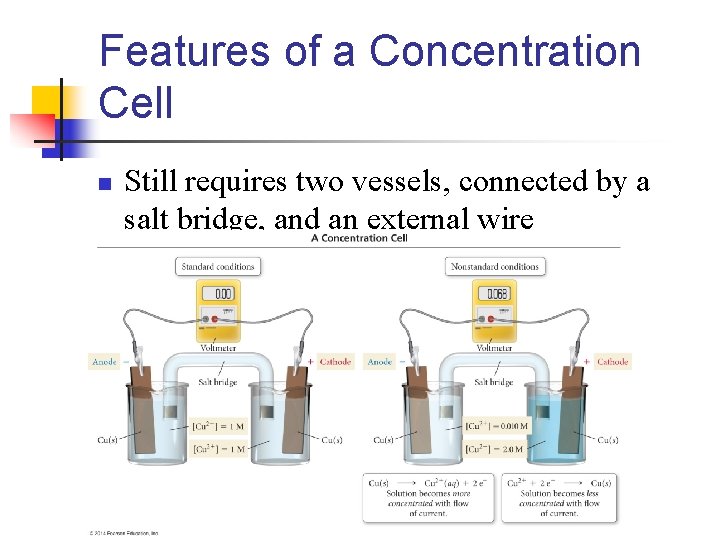
Features of a Concentration Cell n Still requires two vessels, connected by a salt bridge, and an external wire

Overview n n In a concentration cell, the more dilute halfcell will seek to be more concentrated (increasing # of ions) The more concentrated half cell will seek to become more dilute (decreasing # of ions)

n So with this cell n The. 01 M half-cell is more dilute. So it will create ions. n n n Cu 2++2 e. Oxidation taking place in this cell The 2 M half-cell is more concentrated. So it will lose ions. n n Cu 2++2 e- Cu Reduction taking place in this cell

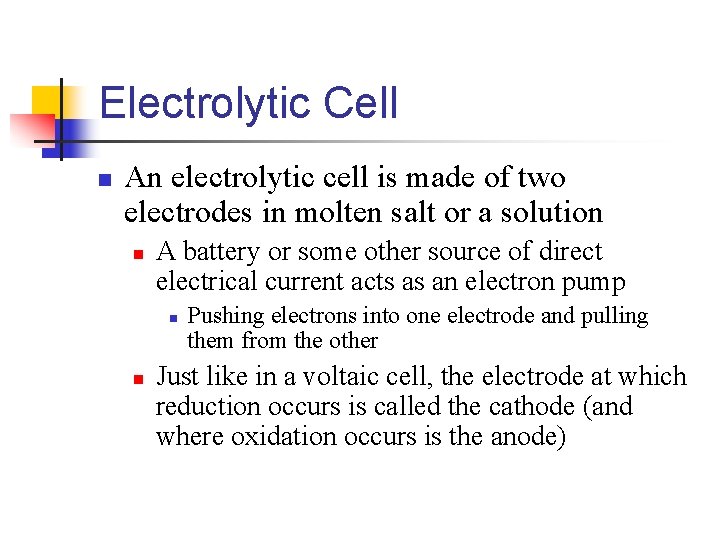
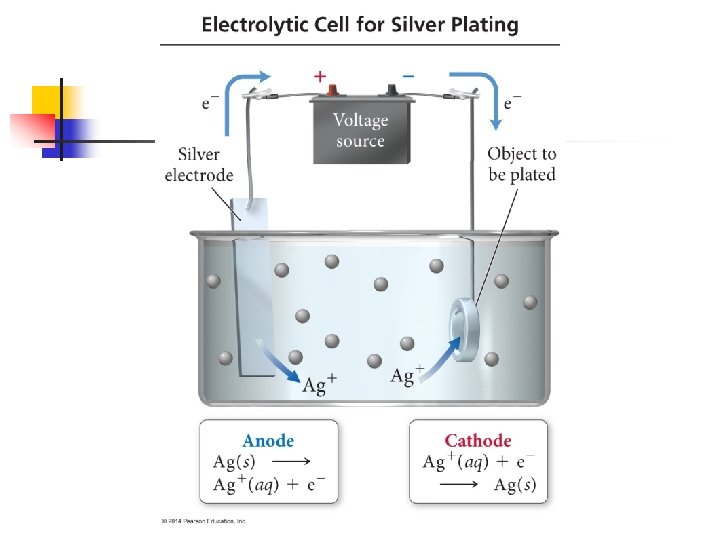
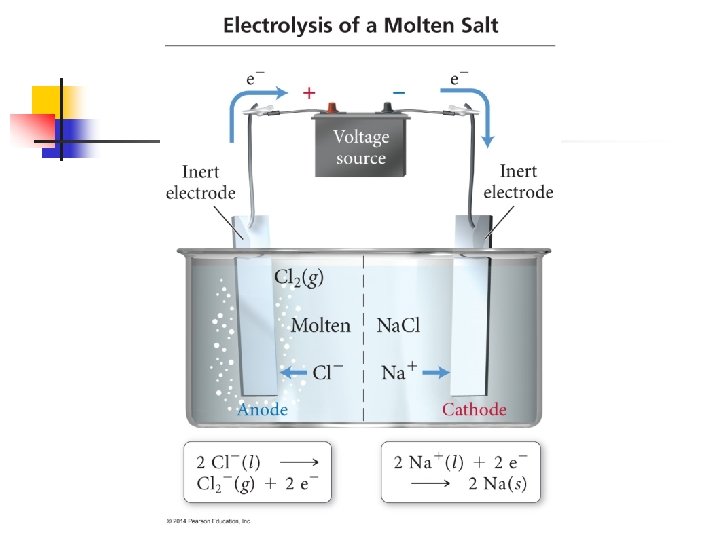
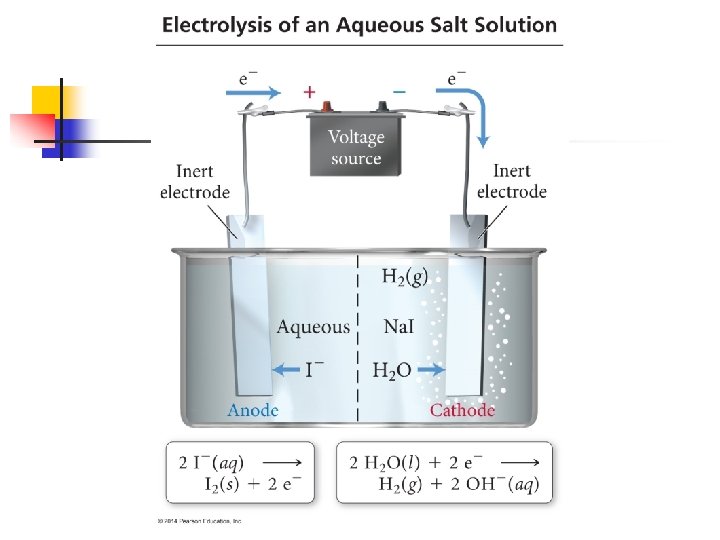
18. 8 - Electrolysis** n Voltaic cells are based on spontaneous redox reactions n It is possible to use electrical energy to cause nonspontaneous redox reactions to occur n n For example, we can use electricity to decompose molten sodium chloride into its component elements 2 Na. Cl(l) 2 Na(l) + Cl 2(g) A nonspontaneous process driven by outside electrical energy is called an electrolysis reaction These reactions take place is electrolytic cells

Electrolytic Cell n An electrolytic cell is made of two electrodes in molten salt or a solution n A battery or some other source of direct electrical current acts as an electron pump n n Pushing electrons into one electrode and pulling them from the other Just like in a voltaic cell, the electrode at which reduction occurs is called the cathode (and where oxidation occurs is the anode)


Things to notice n In a voltaic cell (or any other source of direct current) n n So the electrode of the electrolytic cell that is connected to the negative terminal of the voltage source is the cathode n n electrons move from the negative terminal It receives the electrons that are used to reduce the substance The electrons that are removed during the oxidation process at the anode travel to the positive terminal of the voltage source

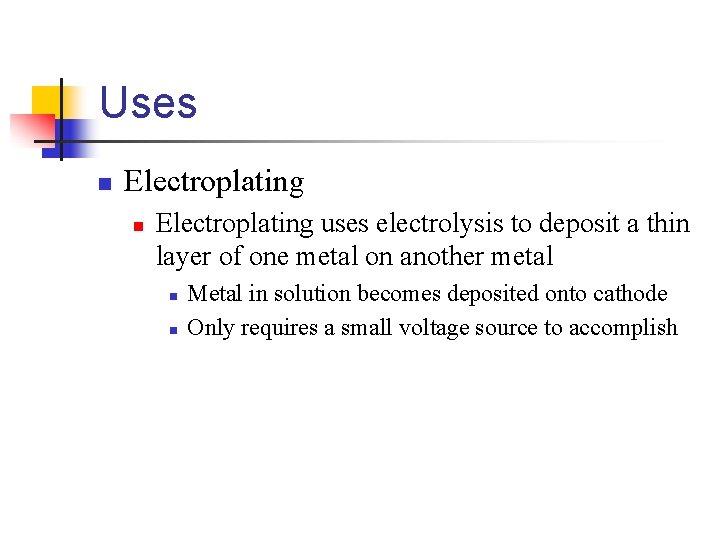
Uses n Electroplating uses electrolysis to deposit a thin layer of one metal on another metal n n Metal in solution becomes deposited onto cathode Only requires a small voltage source to accomplish


Electrical Work n Remember n A positive value of E is associated with a negative value of free-energy change n n And thus spontaneous processes We also know that for any spontaneous process ΔG measures the maximum useful work, wmax for the process n ΔG = wmax

n Since ΔG = -n. FE n n wmax = -n. FE The cell emf for a voltaic cell is positive, so wmax will be negative n Negative work means work is being done by the system on the surroundings

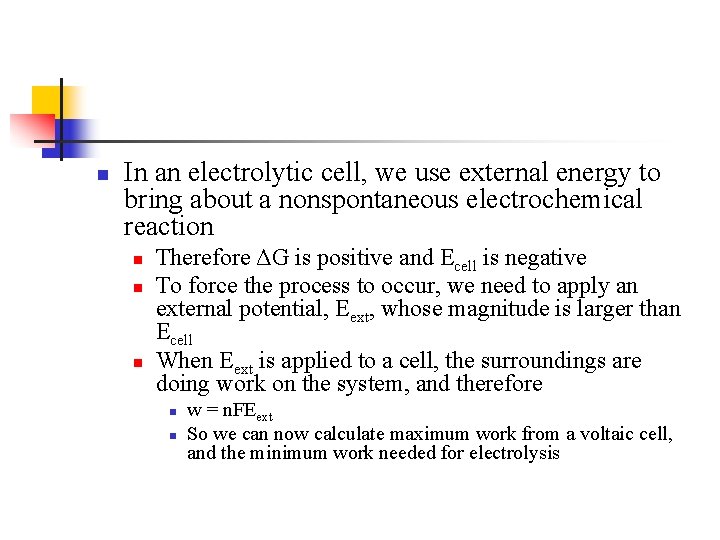
n In an electrolytic cell, we use external energy to bring about a nonspontaneous electrochemical reaction n Therefore ΔG is positive and Ecell is negative To force the process to occur, we need to apply an external potential, Eext, whose magnitude is larger than Ecell When Eext is applied to a cell, the surroundings are doing work on the system, and therefore n n w = n. FEext So we can now calculate maximum work from a voltaic cell, and the minimum work needed for electrolysis



A few final notes. . . n Electrical work is expressed in terms of watts time n n n 1 W = 1 J/s Therefore, a watt-second = 1 J The unit power companies use is the kilowatthour (k. Wh), which is equal to 3. 6 x 106 J

Electricity Misc. n Current n n The flow of the electrons is called current Measured in the unit of the ampere (A) n n Is really Coulomb/second Faraday’s constant n F = 96500 coulombs per mole of electrons n Which means that 1 mole of electrons has a charge of 96500 C

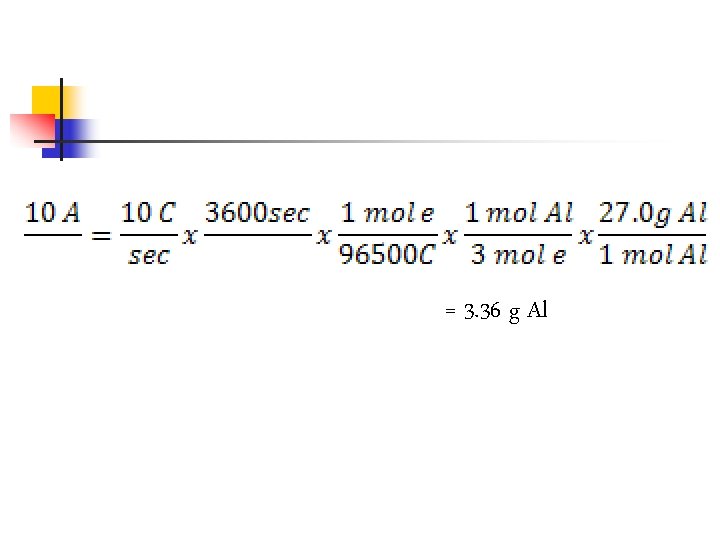
Electrolysis Example n Calculate the number of grams of aluminum produced in 1. 00 h by the electrolysis of molten Al. Cl 3 if the electrical current is 10. 0 A. n Note: Al 3+ + 3 e- Al

Note: n n 10 A = 10 C/sec 1. 00 h = 3600 sec There are 3 moles of electrons per mole Al Faradays constant brings this together (96000 C/mol e)

= 3. 36 g Al