Chapter 17 Understanding Heat Transfer Conduction Convection and



























- Slides: 52

Chapter 17: Understanding Heat Transfer, Conduction, Convection and Radiation

The Evolution of the Discovery of Heat • 1798: Benjamin Thompson (Count Rumford) performed experiments to test how heat is produced. – He concluded that action or work produces heat. – He concluded that heat is a form of energy • 1838: James Prescott Joule further investigated the relationship between heat and motion. – He concluded that heat and motion is relative to each other

What is Heat! • Heat: is a form of energy caused by the internal motion of molecules of matter. What are molecules? • Atoms combined in a covalent bond. Module 1: Basic Concepts - Kinetics #1 (Low Position) | Basic Concepts in Environmental Sciences | APTI | USEPA

How and what is heat transfer? • Heat can be transferred 3 ways: 1. Conduction 2. Convection 3. Radiation • Heat transfer is when matter passes heat on to another object. – Important! Heat is transferred from a warmer place to a cooler place.

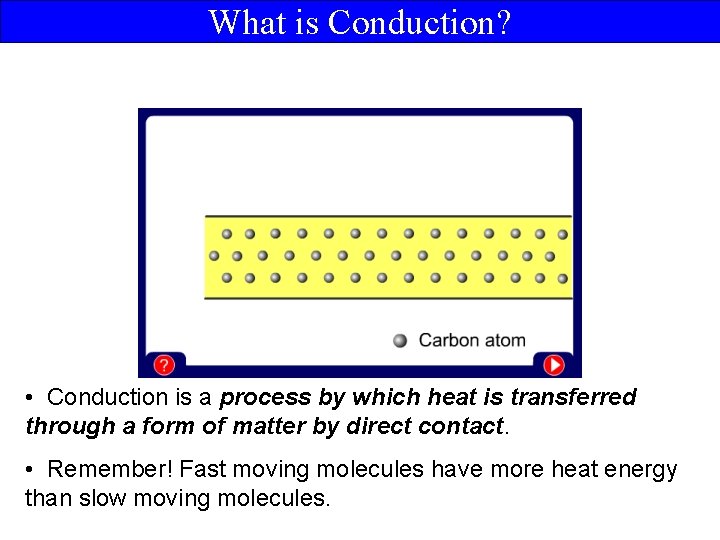
What is Conduction? • Conduction is a process by which heat is transferred through a form of matter by direct contact. • Remember! Fast moving molecules have more heat energy than slow moving molecules.

How can you cook or maintain heat instead of losing heat when cooking or heating a house? • You need an insulator! What is an insulator? • An insulator is a type of material that is used to trap heat and prohibit heat from being transferred.

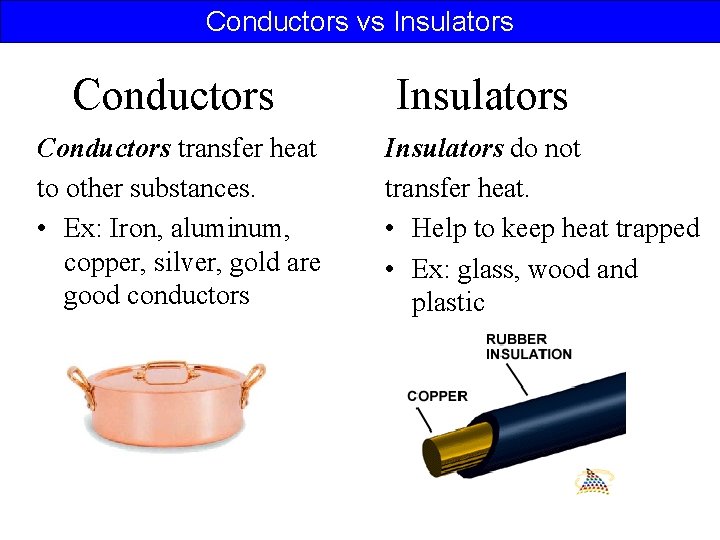
Conductors vs Insulators Conductors transfer heat to other substances. • Ex: Iron, aluminum, copper, silver, gold are good conductors Insulators do not transfer heat. • Help to keep heat trapped • Ex: glass, wood and plastic

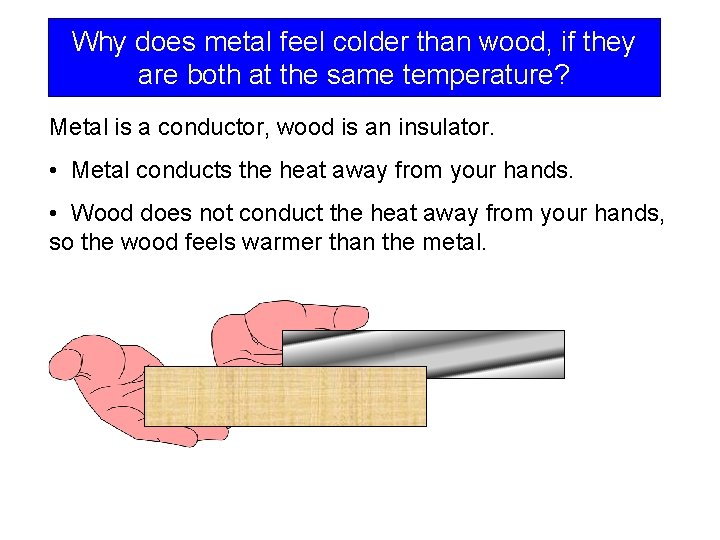
Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. • Metal conducts the heat away from your hands. • Wood does not conduct the heat away from your hands, so the wood feels warmer than the metal.

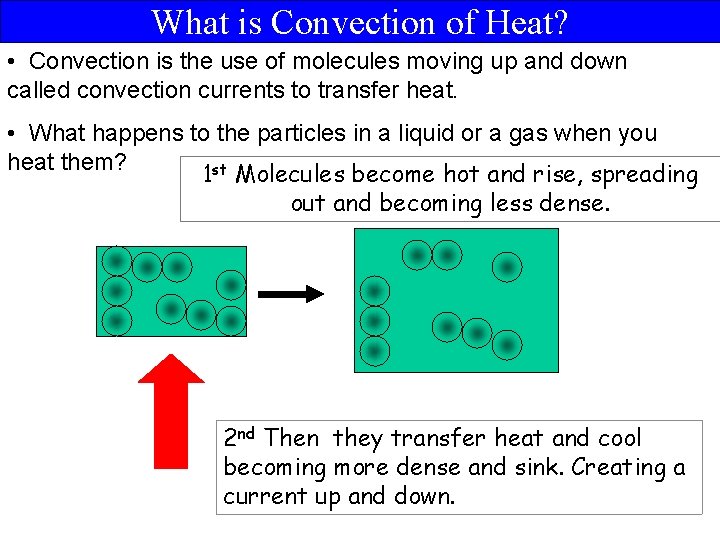
What is Convection of Heat? • Convection is the use of molecules moving up and down called convection currents to transfer heat. • What happens to the particles in a liquid or a gas when you heat them? 1 st Molecules become hot and rise, spreading out and becoming less dense. 2 nd Then they transfer heat and cool becoming more dense and sink. Creating a current up and down.

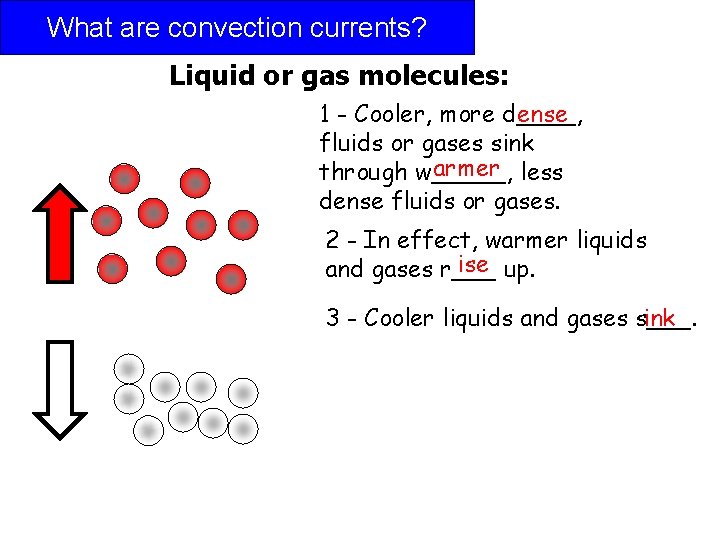
What are convection currents? Liquid or gas molecules: 1 - Cooler, more d____, ense fluids or gases sink armer less through w_____, dense fluids or gases. 2 - In effect, warmer liquids ise up. and gases r___ 3 - Cooler liquids and gases s___. ink

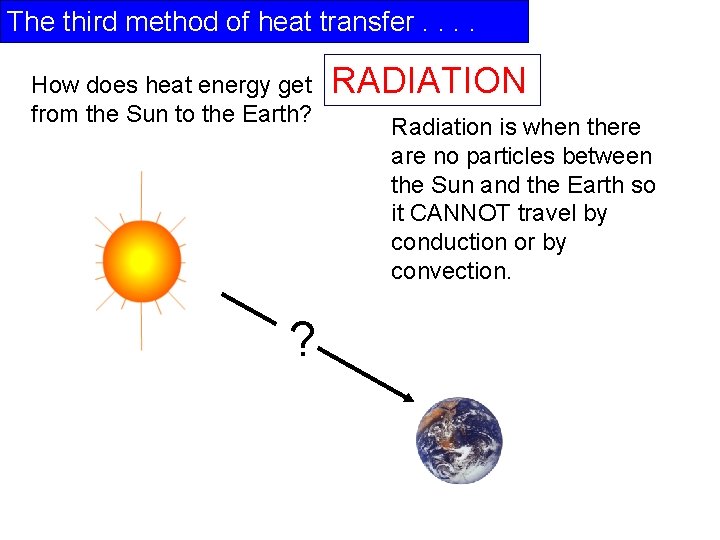
The third method of heat transfer. . How does heat energy get from the Sun to the Earth? ? RADIATION Radiation is when there are no particles between the Sun and the Earth so it CANNOT travel by conduction or by convection.

Measuring Temperature What is temperature? Temperature is the measurement of the average kinetic energy within molecules. What is kinetic energy? Kinetic energy is the amount of energy an object possesses because it is in motion. What is potential energy? Potential energy is the amount of energy stored in matter. Ex: gas or fuel when burned there is a release of stored heat energy There are 3 temperature scales: 1. Celsius ( Metric System) (ºC) 2. Kelvin (Metric System) (K) 3. Fahrenheit

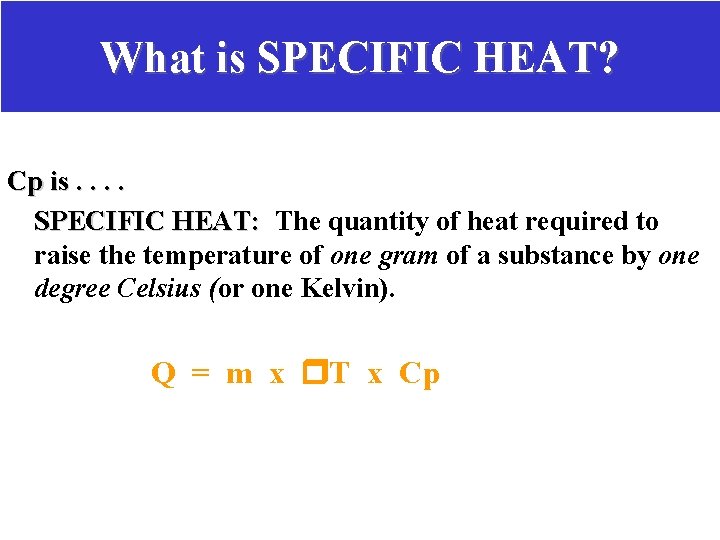
WHAT IS SPECIFIC HEAT? Cp is. . – SPECIFIC HEAT: The amount of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin). – Specific Heat is a physical property of matter. – Each sample of matter has its own specific heat number just like each type of matter has its own density value. Q = m x T x Cp

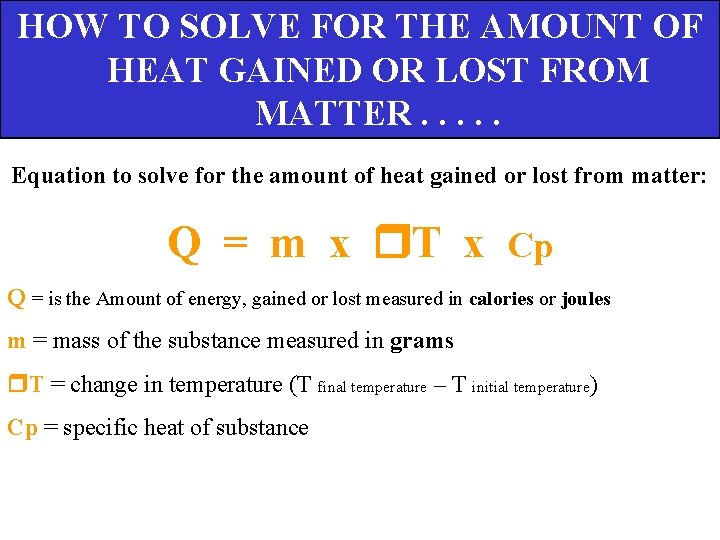
HOW TO SOLVE FOR THE AMOUNT OF HEAT GAINED OR LOST FROM MATTER. . . Equation to solve for the amount of heat gained or lost from matter: Q = m x T x Cp Q = is the Amount of energy, gained or lost measured in calories or joules m = mass of the substance measured in grams T = change in temperature (T final temperature – T initial temperature) Cp = specific heat of substance

Specific Heat of Water CONSTANT: The Specific Heat of water is always: 4. 184 J/g x C = 1 calorie Remember: The specific heat of any substance is a constant number!


UNITS for HEAT ENERGY Q = (Quantity of heat energy) Heat energy is measured in either Joules(J), kilojoules (k. J), calories (cal) or kilocalories (kcal).

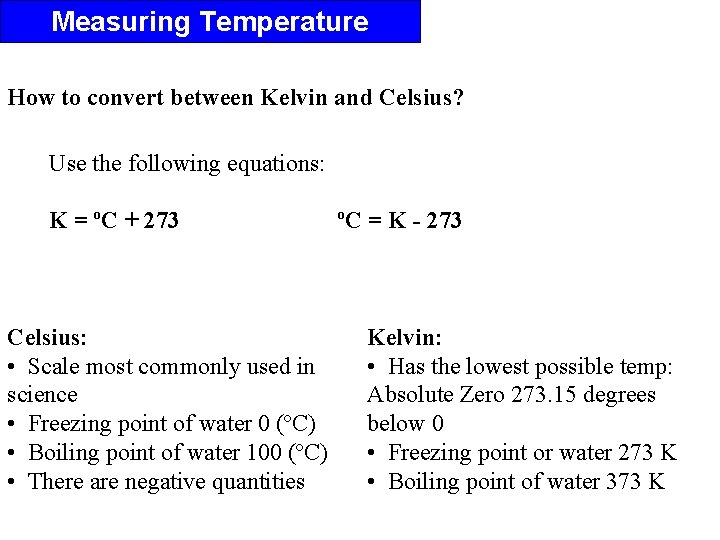
Measuring Temperature How to convert between Kelvin and Celsius? Use the following equations: K = ºC + 273 Celsius: • Scale most commonly used in science • Freezing point of water 0 (ºC) • Boiling point of water 100 (ºC) • There are negative quantities ºC = K - 273 Kelvin: • Has the lowest possible temp: Absolute Zero 273. 15 degrees below 0 • Freezing point or water 273 K • Boiling point of water 373 K

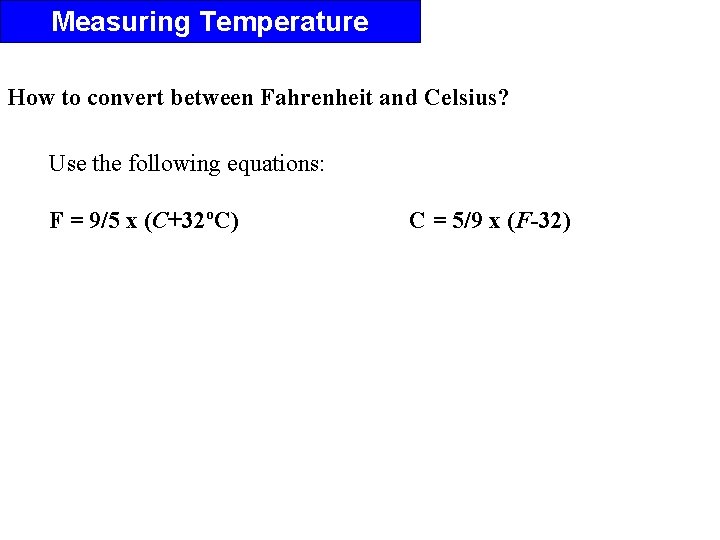
Measuring Temperature How to convert between Fahrenheit and Celsius? Use the following equations: F = 9/5 x (C+32ºC) C = 5/9 x (F-32)

QUICK WRITE QUESTIONS 1. The faster molecules of an object move, the more ______ the object possesses. 2. _______ is the measurement of how much kinetic energy is present in a substance at a molecular level. 3. Convert -273 degrees C = ______ Fahrenheit. 4. How do convection currents take place? Include the words density, molecules, temperature, drop and rise in your answer. 5. What type of heat transfer occurred in your lab at station 3 with the gold ring? Explain what, how and why it occurred. 6. What is heat of fusion and heat of vaporization?

Example: If you place a metal spoon in a hot pot. What happens to the metal spoon? The heat from the hot pot is transferred to the metal spoon. This is called? Conduction

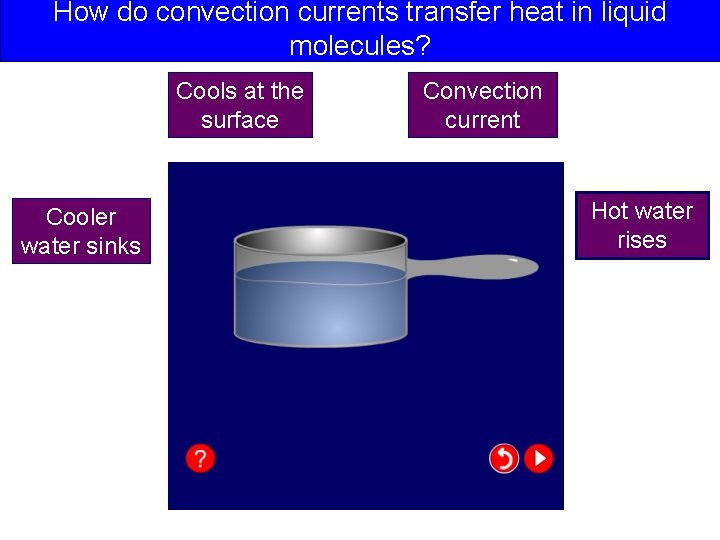
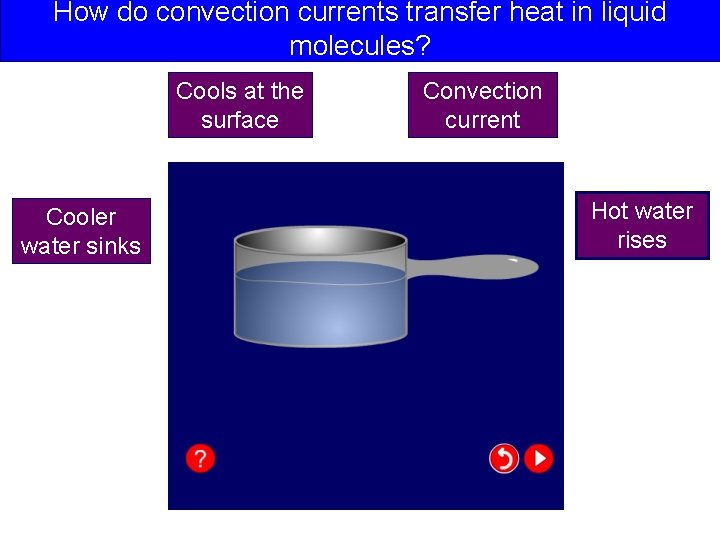
How do convection currents transfer heat in liquid molecules?

How do convection currents transfer heat in liquid molecules? Cools at the surface Cooler water sinks Convection current Hot water rises

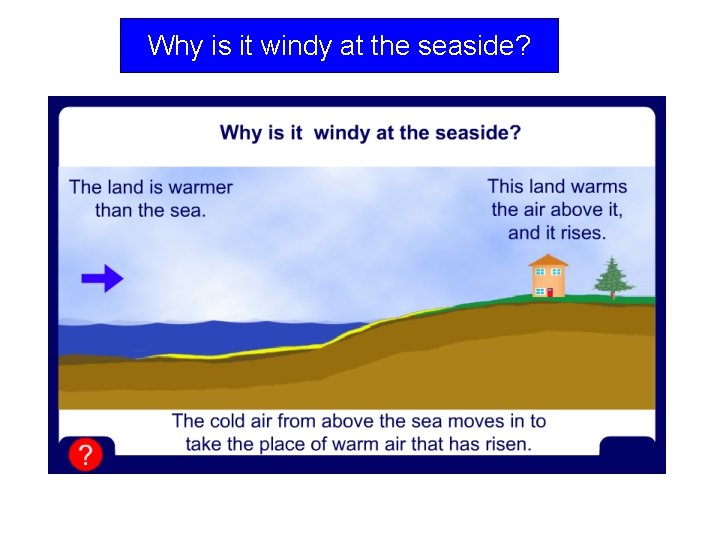
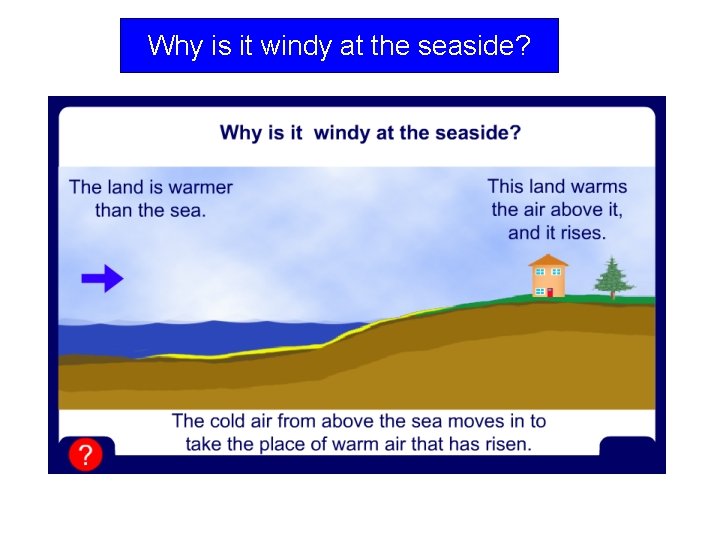
Why is it windy at the seaside?

Why is it windy at the seaside?

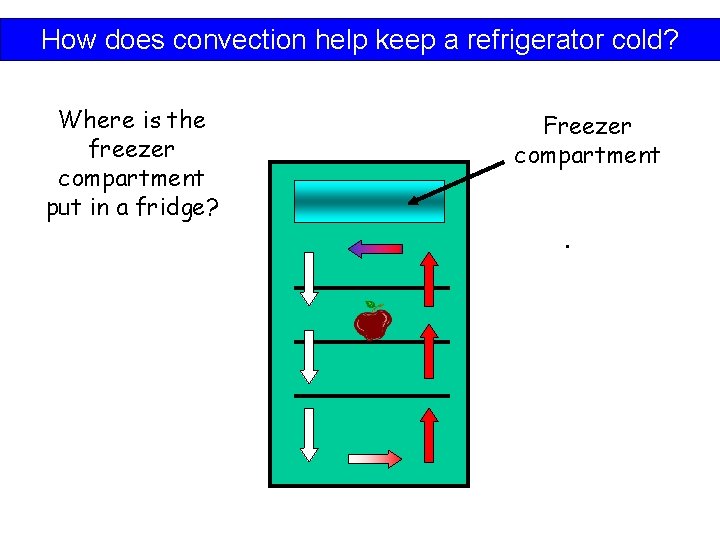
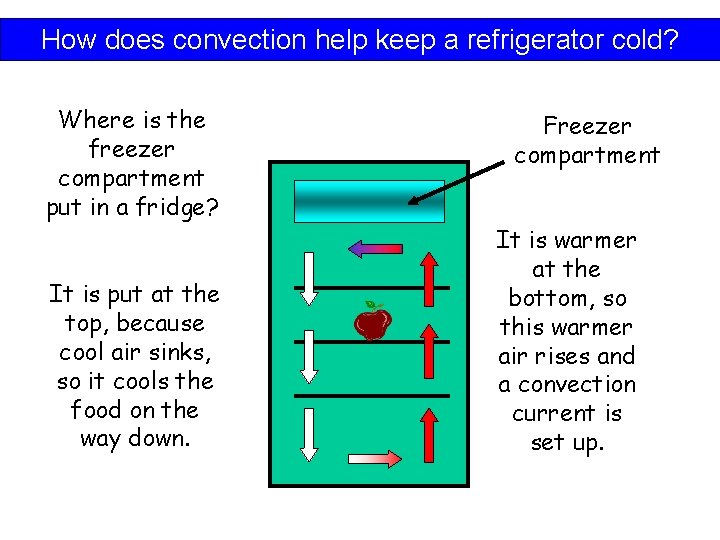
How does convection help keep a refrigerator cold?

How does convection help keep a refrigerator cold? Where is the freezer compartment put in a fridge? Freezer compartment.

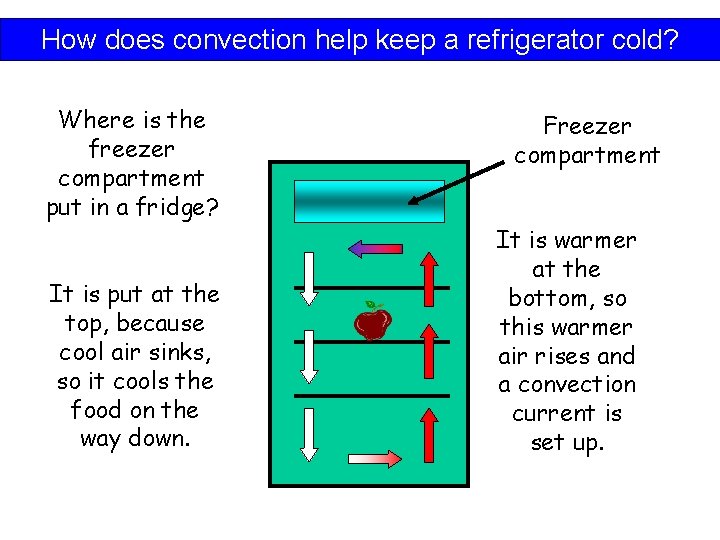
How does convection help keep a refrigerator cold? Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up.

Convection Q and A Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air.

Quick Write: Determine the specific heat of an unknown metal that required 2, 560. 0 cal of heat to raise the temperature of 150. 00 g from 15. 0 o. C to 200. 0 o. C? Steps to Solve for Q 1. 2. 3. 4. 5. Label each unit in the word problem according to which value it is in the Specific Heat equation. Identify what you are solving for? Plug in each value in the equation with correct labels. Solve. Label your answer with correct units! Cp = 0. 0923 cal /g -o. C

Thursday, March 8 th Quick Write : ) 1. Iron metal has a specific heat of 0. 449 J/go. C. How much heat is transferred to a 5. 00 g piece of iron, initially at 20. 0 o. C, when it is placed in a beaker of boiling water? Homework: 1. 2. Objectives: . See Mrs. Fugarino during Activity 1. Critically analyze and use the appropriate math skills to Period to make corrections to your quiz Calculate specific heat Today! 2. Solve specific heat word Test Friday on Specific problems. Heat Word Problems, Temperature 3. Label units appropriately Conversions, Forms of Heat transfer and correct labelling units and decimal points during calculations.

PRACTICE PROBLEM #7 1. Iron metal has a specific heat of 0. 449 J/go. C. How much heat is transferred to a 5. 00 g piece of iron, initially at 20. 0 o. C, when it is placed in a beaker of boiling water? 180. J 2. How many calories of energy are given off to lower the temperature of 100. 0 g of iron from 150. 0 o. C to 35. 0 o. C? 1. 23 x 103 cal 3. If 3. 47 k. J were absorbed by 75. 0 g H 2 O at 20. 0 o. C, what would be the final temperature of the water? 31. 1 o. C 4. A 100. g sample of water at 25. 3 o. C was placed in a calorimeter. 45. 0 g of lead shots (at 100 o. C) was added to the calorimeter and the final temperature of the mixture was 34. 4 o. C. What is the specific heat of lead? 1. 28 J/g o. C 5. A 17. 9 g sample of unknown metal was heated to 48. 31 o. C. It was then added to 28. 05 g of water in an insulted cup. The water temperature rose from 21. 04 o. C to 23. 98 o. C. What is the specific heat of the metal in J/go. C? 0. 792 J/go. C

Radiation Q and A Radiation travels in straight lines True/False Radiation can travel through a vacuum True/False Radiation requires particles to travel True/False Radiation travels at the speed of light True/False

Calculate how to solve for. . . • Specific Heat Cp • m • Change in T From Q=m. C T

Radiation Q and A Why are houses painted white in hot countries? White reflects heat radiation and keeps the house cooler. Why are shiny foil blankets wrapped around marathon runners at the end of a race? The shiny metal reflects the heat radiation from the runner back in, this stops the runner getting cold.

Heat of Fusion 1. Is the amount of energy absorbed to bring a solid to liquid form during melting. 2. Is the phase change called fusion or melting on a phase change diagram. 3. During this phase: Potential Energy increases and Kinetic Energy is constant. 4. The equation to solve for the heat of fusion is: Q = m x Hf(heat of fusion) A constant value for water = 334 j/g

Heat of Vaporization 1. Is the amount of energy absorbed to bring a liquid to gas form during vaporization. 2. Is the phase change called vaporization on a phase change diagram. 3. During this phase: Potential Energy increases and Kinetic Energy is constant. 4. The equation to solve for the heat of fusion is: Q = m x Hv(heat of vaporization) A constant value for water = 2, 260 j/g

Emission experiment Four containers were filled with warm water. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black shiny metal container would be the warmest after ten The _____ radiation back minutes because its shiny surface reflects heat _______ dull black container into the container so less is lost. The ____ emitting heat would be the coolest because it is the best at _______ radiation.

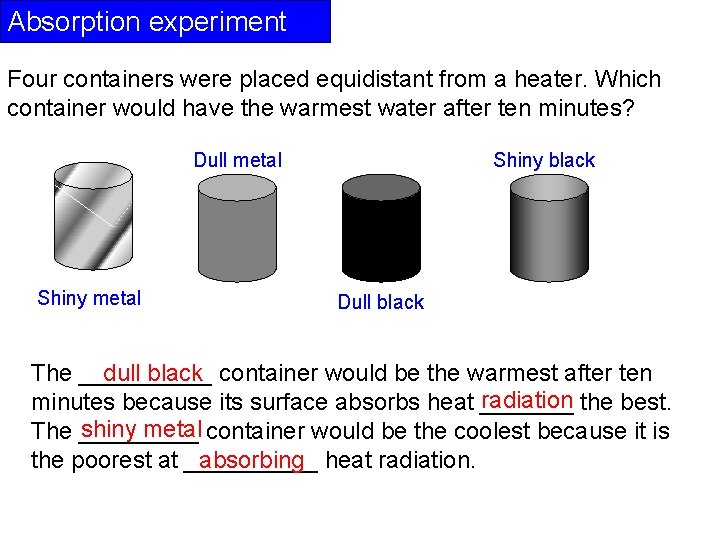
Absorption experiment Four containers were placed equidistant from a heater. Which container would have the warmest water after ten minutes? Dull metal Shiny black Dull black dull black container would be the warmest after ten The _____ radiation the best. minutes because its surface absorbs heat _______ shiny metal container would be the coolest because it is The _____ the poorest at _____ absorbing heat radiation.

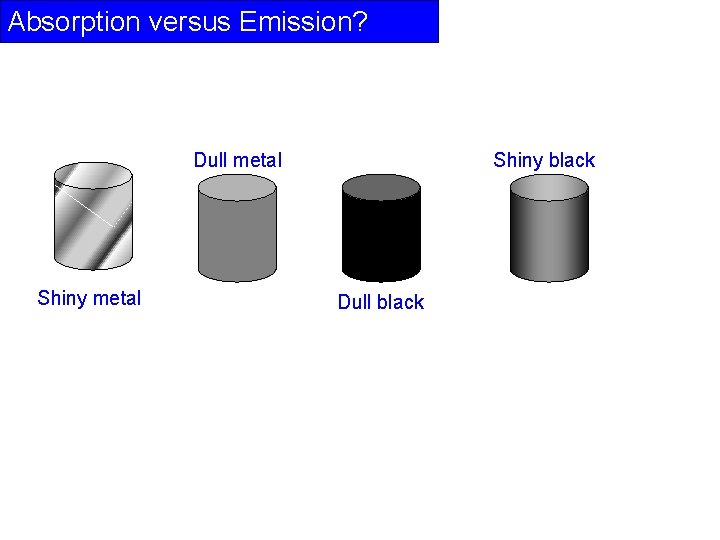
Absorption versus Emission? Dull metal Shiny black Dull black

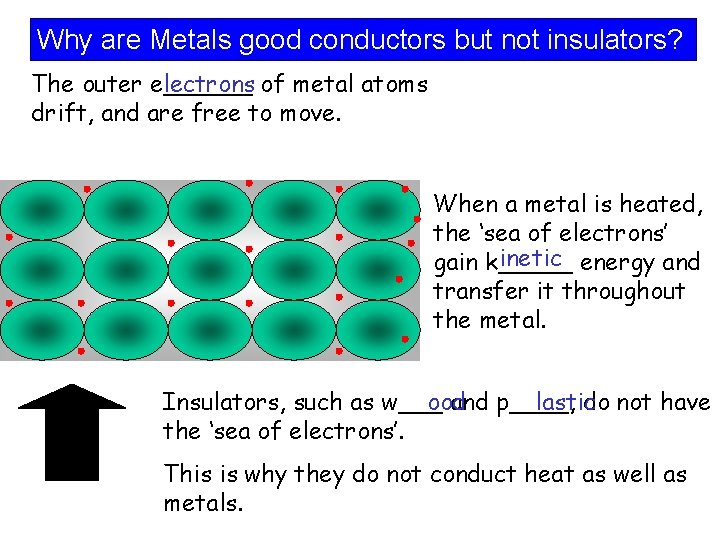
Why are Metals good conductors but not insulators? The outer e______ lectrons of metal atoms drift, and are free to move. When a metal is heated, the ‘sea of electrons’ inetic energy and gain k_____ transfer it throughout the metal. Insulators, such as w___ ood and p____, lastic do not have the ‘sea of electrons’. This is why they do not conduct heat as well as metals.

A calorimeter is used to help measure the specific heat of a substance. Knowing its Q value, its mass, and its T, its Cp can be calculated T is measured for water to First, mass and temperature help getare its heat gain of water measured This gives the heat lost by the substance Then heated sample is put inside and heat flows into water

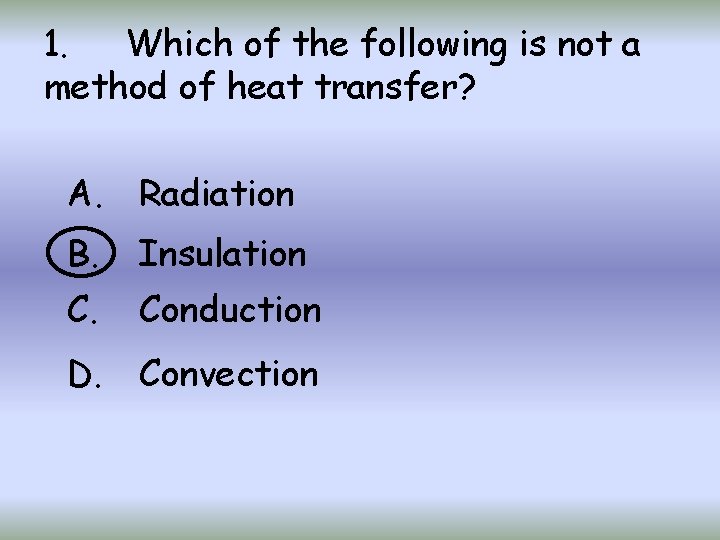
1. Which of the following is not a method of heat transfer? A. Radiation B. Insulation C. Conduction D. Convection

2. In which of the following are the particles closest together? A. Solid B. Liquid C. Gas D. Fluid

3. How does heat energy reach the Earth from the Sun? A. Radiation B. Conduction C. Convection D. Insulation

4. Which is the best surface for reflecting heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black

5. Which is the best surface for absorbing heat radiation? A. Shiny white B. Dull white C. Shiny black D. Dull black

What is SPECIFIC HEAT? Cp is. . SPECIFIC HEAT: The quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one Kelvin). Q = m x T x Cp

How do convection currents transfer heat in liquid molecules? Cools at the surface Cooler water sinks Convection current Hot water rises

Why is it windy at the seaside?

How does convection help keep a refrigerator cold? Where is the freezer compartment put in a fridge? It is put at the top, because cool air sinks, so it cools the food on the way down. Freezer compartment It is warmer at the bottom, so this warmer air rises and a convection current is set up.

Convection Q and A Why does hot air rise and cold air sink? Cool air is more dense than warm air, so the cool air ‘falls through’ the warm air.