Chapter 17 Spontaneity entropy and free energy Spontaneous



















- Slides: 26

Chapter 17 Spontaneity, entropy and free energy

Spontaneous l. A reaction that will occur without outside intervention. l We can’t determine how fast. l We need both thermodynamics and kinetics to describe a reaction completely. l Thermodynamics compares initial and final states. l Kinetics describes pathway between.

Thermodynamics l Entropy (S) – Number of ways things can be arranged – Looks like disorder or randomness l 2 nd Law: The entropy of the universe increases in any change

Entropy l Defined in terms of probability. l Substances take the arrangement that is most likely. l The most likely is the most random. l Calculate the number of arrangements for a system.

Why does that happen? Why does a gas tend to fill an empty container? It can be answered by using the idea of entropy.

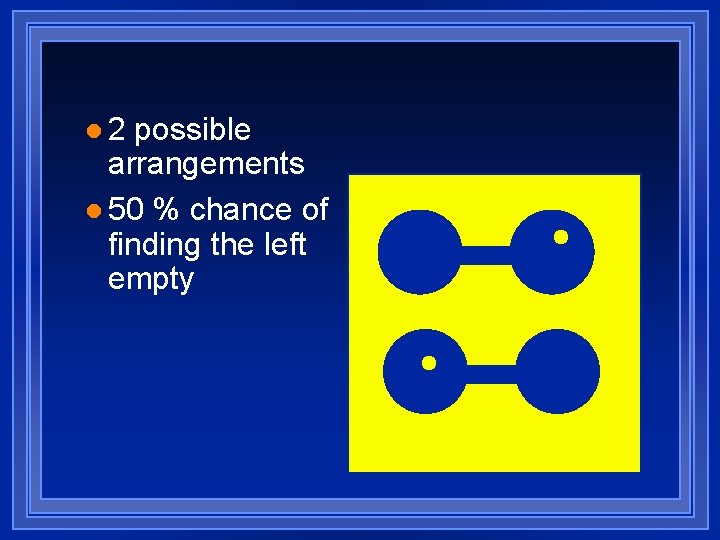
l 2 possible arrangements l 50 % chance of finding the left empty

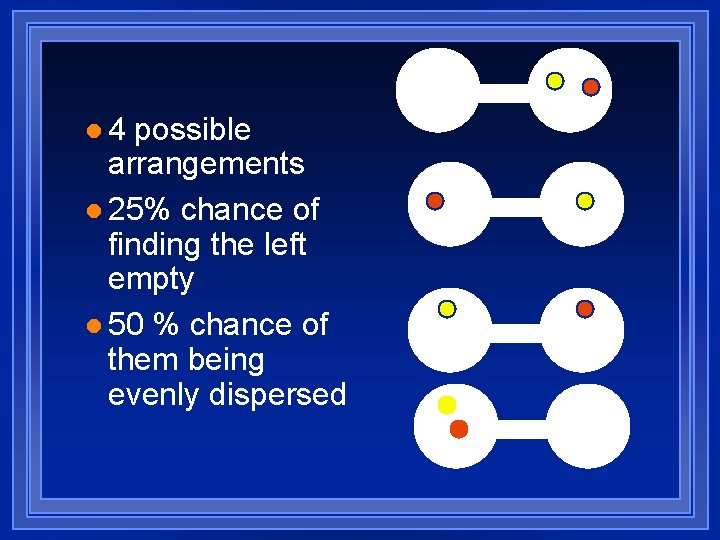
l 4 possible arrangements l 25% chance of finding the left empty l 50 % chance of them being evenly dispersed

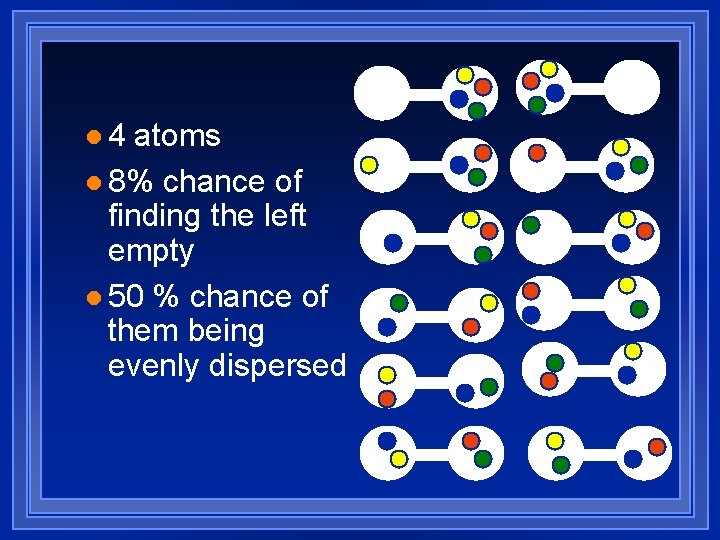
l 4 atoms l 8% chance of finding the left empty l 50 % chance of them being evenly dispersed

Gases l Gases completely fill their chamber because there are many more ways to do that than to leave half empty. l. Ssolid <Sliquid <<Sgas l there are many more ways for the molecules to be arranged as a liquid than a solid. l Gases have a huge number of positions possible.

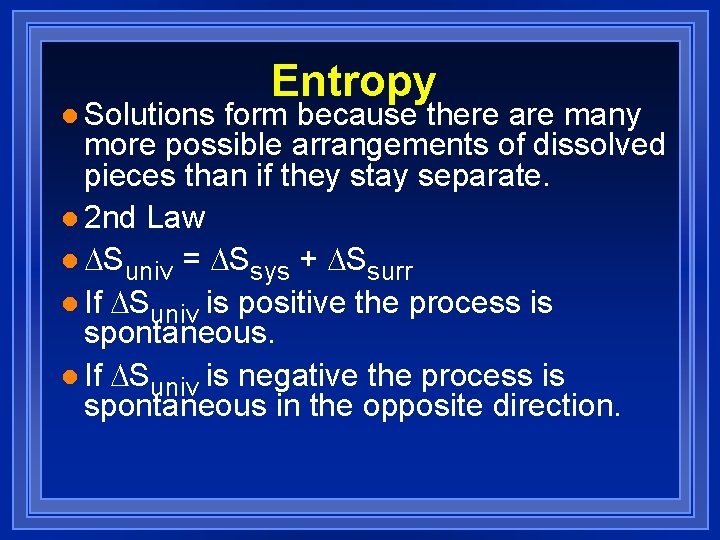
l Solutions Entropy form because there are many more possible arrangements of dissolved pieces than if they stay separate. l 2 nd Law l Suniv = Ssys + Ssurr l If Suniv is positive the process is spontaneous. l If Suniv is negative the process is spontaneous in the opposite direction.

l Consider this process H 2 O(l)® H 2 O(g) – Ssys is positive

Temperature and Spontaneity l Entropy changes in the surroundings are determined by the heat flow. l An exothermic process is favored because by giving up heat the entropy of the surroundings increases. l The size of Ssurr depends on temperature l Ssurr = - H/T

Gibb's Free Energy l The energy associated with a chemical reaction that can be used to do work. G=H-TS l Never used this way. l G= H-T S at constant temperature G is negative at constant T and P, the Process is spontaneous. l If

Let’s Check the reaction H 2 O(s) ® H 2 O(l) l Sº = 22. 1 J/K mol Hº =6030 J/mol l Calculate G at 10ºC and -10ºC l When does it become spontaneous? l Look at the equation G= H-T S l Spontaneity can be predicted from the sign of H and S. l For

Let’s Check the reaction H 2 O(s) ® H 2 O(l) l Sº = 22. 1 J/Kmol Hº =6030 J/mol l Calculate G at 10ºC and -10ºC l For – G = 6030 J/mol-283 K(22. 1 J/Kmol) – G = -224. 3 J/mol spontaneous

Let’s Check the reaction H 2 O(s) ® H 2 O(l) l Sº = 22. 1 J/Kmol Hº =6030 J/mol l Calculate G at 10ºC and -10ºC l For – G = 6030 J/mol-263 K(22. 1 J/Kmol) – G = 217. 7 J/mol not spontaneous

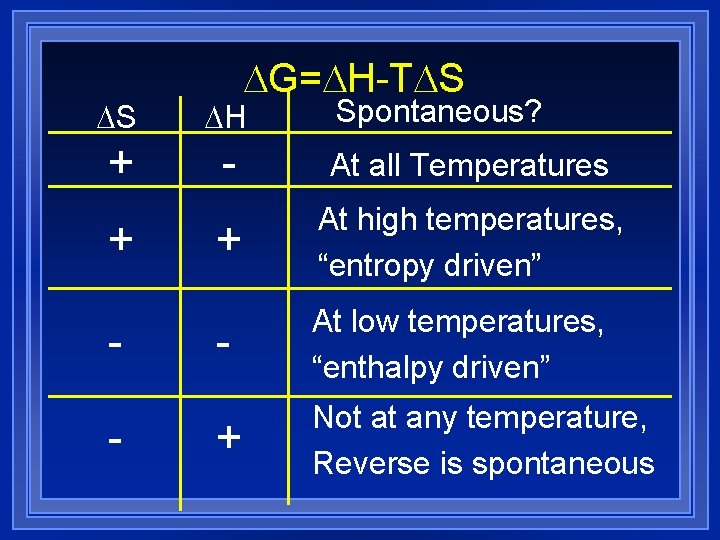
G= H-T S Spontaneous? S H + - At all Temperatures + At high temperatures, “entropy driven” - At low temperatures, “enthalpy driven” + Not at any temperature, Reverse is spontaneous + -

Third Law of Thermo l The entropy of a pure crystal at 0 K is 0. l Gives us a starting point. l All others must be>0. l More complex molecules higher Sº.

Third Law of Thermo l Srxn =∑n Sproducts - ∑n Sreactants – We can use this because entropy is a state function l Standard Entropies Sº ( at 298 K and 1 atm) of substances are listed.

Free Energy in Reactions l Grxn – We =∑n Gproducts - ∑n Greactants can use this because free energy is a state function l Can’t be measured directly, can be calculated from other measurements. l Gº= Hº-T Sº l Use Hess’s Law with known reactions.

Free Energy in Reactions are tables of Gºf. l The standard free energy of formation for any element in its standard state is 0. l Remember- Spontaneity tells us nothing about rate. l There

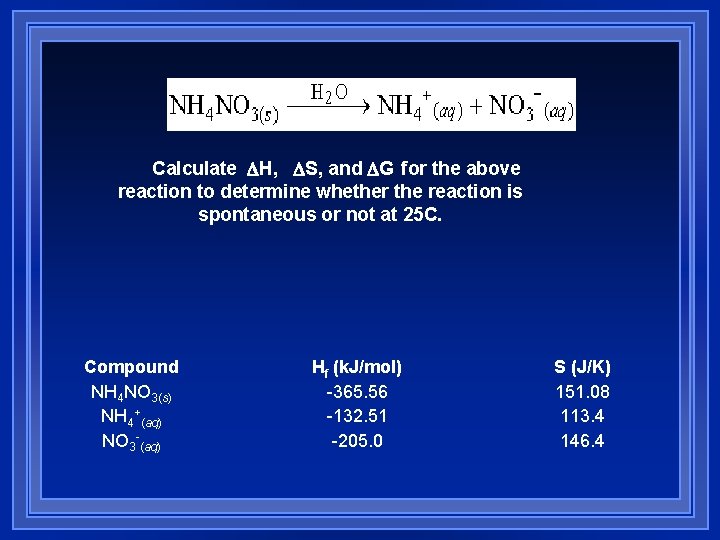
Calculate DH, DS, and DG for the above reaction to determine whether the reaction is spontaneous or not at 25 C. Compound NH 4 NO 3(s) NH 4+(aq) NO 3 -(aq) Hf (k. J/mol) -365. 56 -132. 51 -205. 0 S (J/K) 151. 08 113. 4 146. 4

H = 28. 05 k. J S = 108. 7 J/K G = 28. 05 k. J – 298 K(. 1087 k. J/K) = 28. 05 k. J – 32. 39 k. J = -4. 34 k. J (Spontaneous)

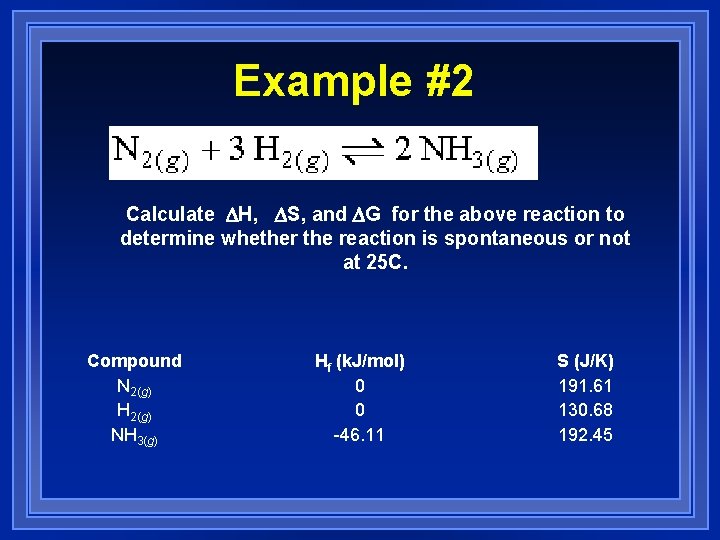
Example #2 Calculate DH, DS, and DG for the above reaction to determine whether the reaction is spontaneous or not at 25 C. Compound N 2(g) H 2(g) NH 3(g) Hf (k. J/mol) 0 0 -46. 11 S (J/K) 191. 61 130. 68 192. 45

H = -92. 22 k. J S = -198. 75 J/K G = -92. 22 k. J – 298 K(-. 199 k. J/K) = -92. 22 k. J + 59. 30 k. J = -32. 91 k. J (Spontaneous)

Free energy And Work l Free energy is that energy free to do work. l The maximum amount of work possible at a given temperature and pressure. l E = q + w l Never really achieved because some of the free energy is changed to heat during a change, so it can’t be used to do work. l Can’t be 100% efficient