Chapter 17 Section 1 Electric Charge and Force










- Slides: 16

Chapter 17 Section 1 Electric Charge and Force • What is an Electric Charge? • How do Electric Charges affect each other?

Do Now • What is an Electric Charge? • Electric Charge – An electrical property of matter • An object can have a negative charge, a positive charge or no charge at all. • How do Electric Charges affect each other? • Like charges repel and unlike charges attract each other

Chapter 1 Section 1 • When you speak into a telephone, the microphone in the handset changes sound waves into electrical signals. • Tip: Electrons are thought to be negative made by a guess by Benjamin Franklin • Positive and Negative Electric charges are said to be opposite, because an object with an equal amount of positive and negative charge has no net charge. • Electric Charge – An electrical property of matter that creates electric and magnetic forces and interactions • An object can have a negative charge, a positive charge or no charge at all.

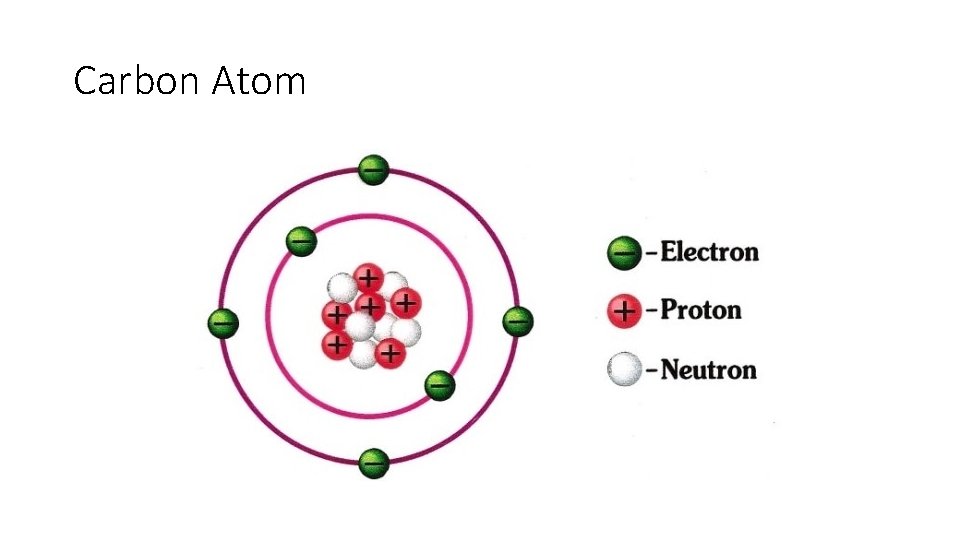
Carbon Atom

Electric Charge • Electric charge depends on an imbalance of protons and electrons • All matter (including you) is made up of atoms • Atoms are made from Electrons, Neutrons and Protons • Electrons=negatively charged • Protons = Positively Charged • Neutrons = Neutral No Charge No gain or Loss • Whenever an imbalance in the number of protons and electrons in an atom, molecule or other object • The object would have Net Electric Charge

Electric Charge • Coulomb – SI Unit of Electric Charge • Electron and Proton = 1. 6 x 10 ^-19 C • Proton = + 1. 6 x 10 ^-19 C • Electron = - 1. 6 x 10 ^-19 C • Object with charge -1. 0 C has 6. 25 x 10 ^18 have excess electrons • Because the amount of electric charge on an object depends on the number of protons and electrons the net charge is always a multiple of 1. 6 x 10^-19 C



Section 1 Review Answers • 1. Like charges repel each other • 2. a. conductor B. Conductor c. Insulator 3. A. Force will be 1/9 as large as the original force B. doubled 4. Proton = positive Electron = Negative Neutron=neutral 5. On board 6. Negative

Do Now – Put Phones in the caddy • How can electrons move between objects? • What is a Coulomb?

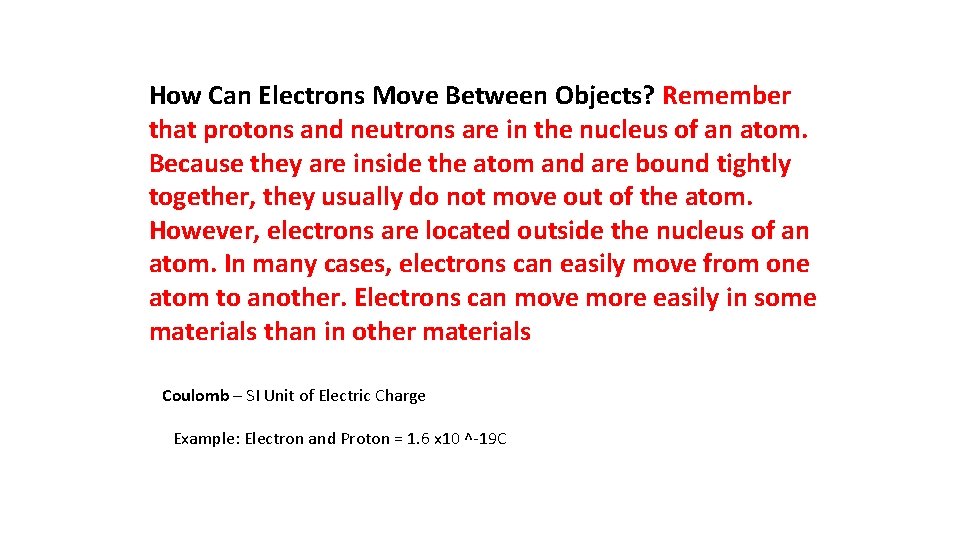
How Can Electrons Move Between Objects? Remember that protons and neutrons are in the nucleus of an atom. Because they are inside the atom and are bound tightly together, they usually do not move out of the atom. However, electrons are located outside the nucleus of an atom. In many cases, electrons can easily move from one atom to another. Electrons can move more easily in some materials than in other materials Coulomb – SI Unit of Electric Charge Example: Electron and Proton = 1. 6 x 10 ^-19 C

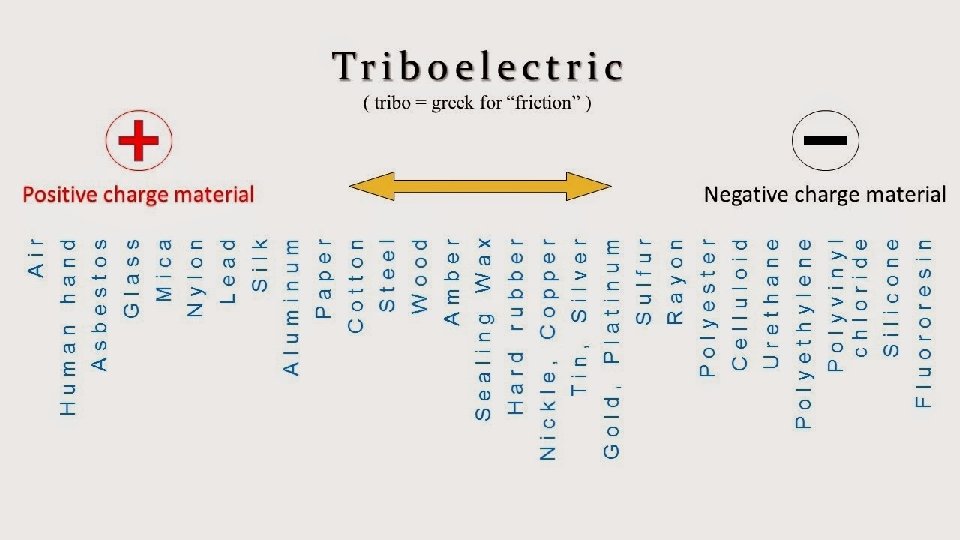
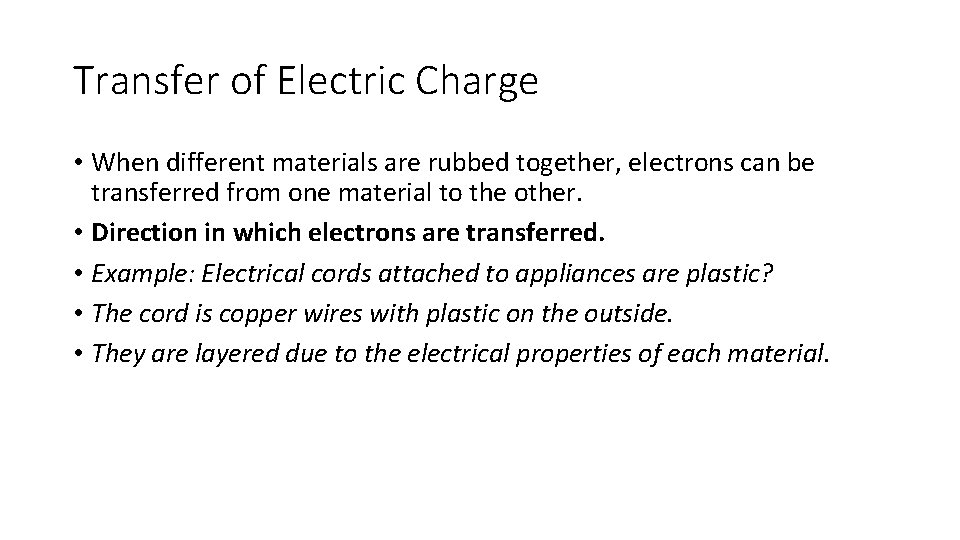
Transfer of Electric Charge • When different materials are rubbed together, electrons can be transferred from one material to the other. • Direction in which electrons are transferred. • Example: Electrical cords attached to appliances are plastic? • The cord is copper wires with plastic on the outside. • They are layered due to the electrical properties of each material.

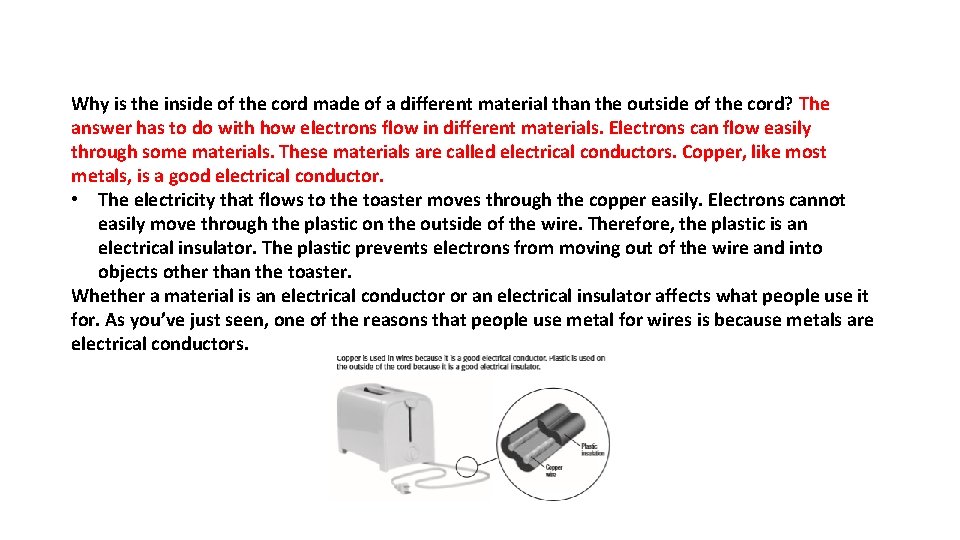
Why is the inside of the cord made of a different material than the outside of the cord? The answer has to do with how electrons flow in different materials. Electrons can flow easily through some materials. These materials are called electrical conductors. Copper, like most metals, is a good electrical conductor. • The electricity that flows to the toaster moves through the copper easily. Electrons cannot easily move through the plastic on the outside of the wire. Therefore, the plastic is an electrical insulator. The plastic prevents electrons from moving out of the wire and into objects other than the toaster. Whether a material is an electrical conductor or an electrical insulator affects what people use it for. As you’ve just seen, one of the reasons that people use metal for wires is because metals are electrical conductors.

1. Explain Why can electrons move between atoms more easily than protons? (6) 2. Predict Would the toaster work if the entire cord was made of plastic? Explain your answer. (7)

Group Activity- For Do Now • Brainstorm Make a list of five substances that you think are electrical conductors and five substances that you think are electrical insulators. Share your list with a small group. Explain why you think each substance is an electrical conductor or insulator.

Induced Charges in Conductors • INDUCED CHARGES IN CONDUCTORS If an object gains or loses electrons, it will have an electric charge. • However, sometimes part of an object can have an electric charge, even if the whole object does not. • For example, the end of the rod in the figure below has a negative electric charge. The negative electric charge on the rod repels electrons in the doorknob. • The electrons in the doorknob move away from the rod. Therefore, part of the doorknob has a slight negative charge. The other part has a slight positive charge. However, the whole doorknob does not have an electric charge, because it has not gained or lost electrons.