Chapter 17 Reaction Kinetics 17 1 The Reaction




![Rate Orders 1 st order ln [reactants] 0 order 1/[reactants] • 0, 1 st Rate Orders 1 st order ln [reactants] 0 order 1/[reactants] • 0, 1 st](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-23.jpg)

![Calculating for k A + 2 B C Rate = k[A][B]2 Experiment Initial [A] Calculating for k A + 2 B C Rate = k[A][B]2 Experiment Initial [A]](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-26.jpg)
![Calculating for k Experiment Initial [A] Initial [B] Rate of Formation of C 1 Calculating for k Experiment Initial [A] Initial [B] Rate of Formation of C 1](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-27.jpg)

- Slides: 30

Chapter 17 Reaction Kinetics 17 -1 The Reaction Process

Can you remember the first time you ever made a friend? What had to happen before the friendship could begin? Mutual Friend Accidentally Bumped into each other How did you meet? Eye Contact

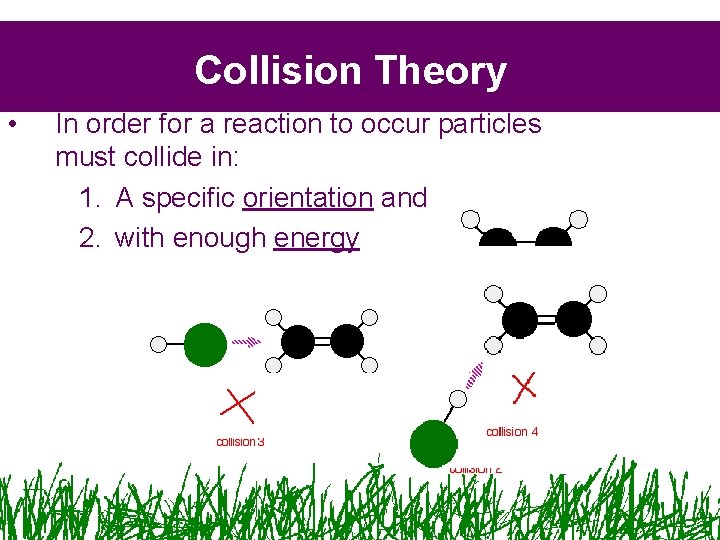
Collision Theory • In order for a reaction to occur particles must collide in: 1. A specific orientation and 2. with enough energy

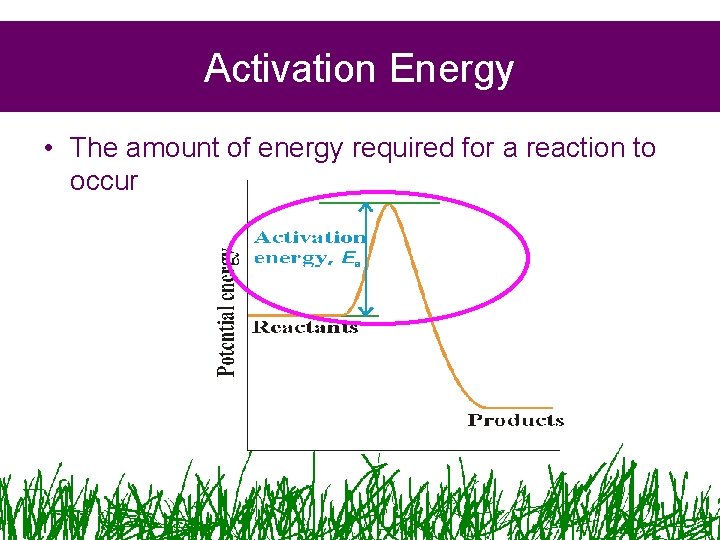
Activation Energy • The amount of energy required for a reaction to occur

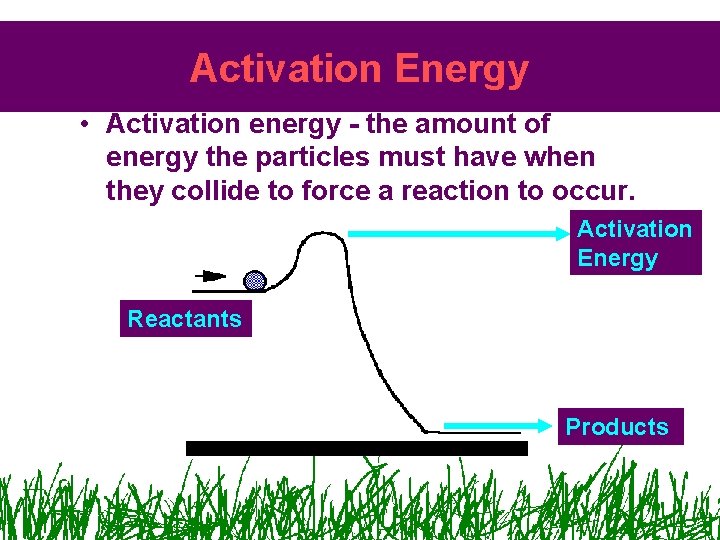
Activation Energy • Activation energy - the amount of energy the particles must have when they collide to force a reaction to occur. Activation Energy Reactants Products

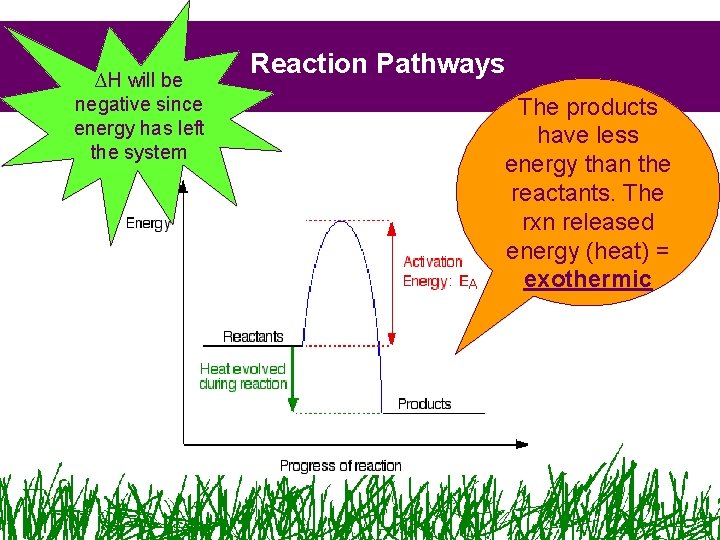
∆H will be negative since energy has left the system Reaction Pathways The products have less energy than the reactants. The rxn released energy (heat) = exothermic

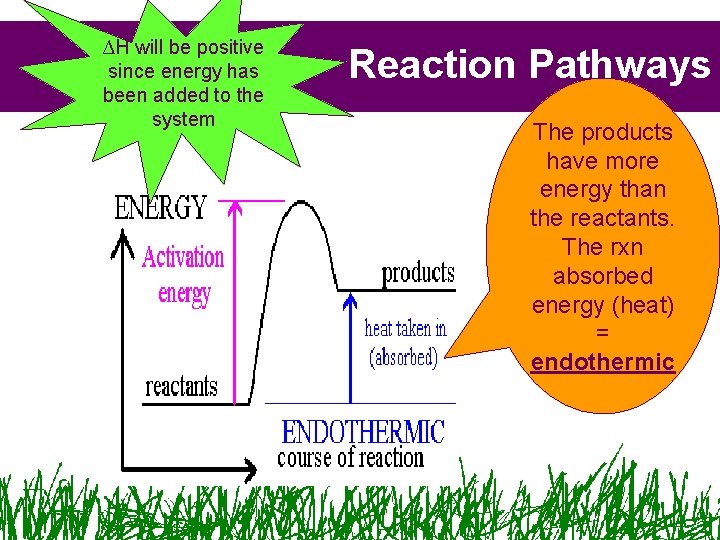
∆H will be positive since energy has been added to the system Reaction Pathways The products have more energy than the reactants. The rxn absorbed energy (heat) = endothermic

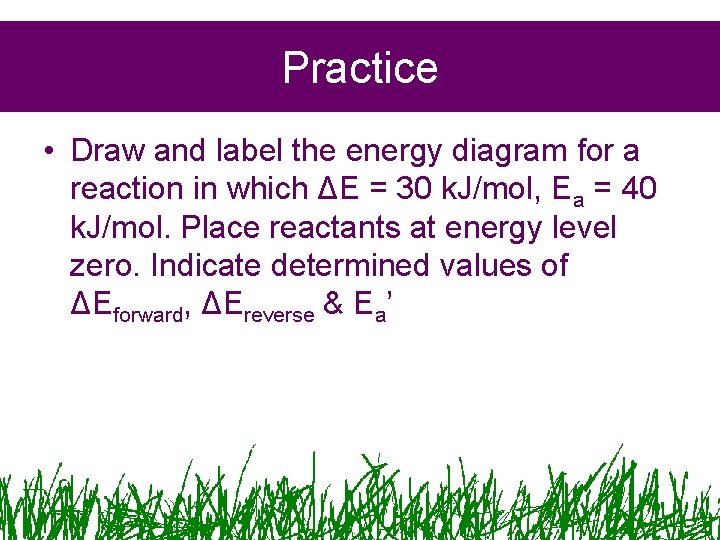
Practice • Draw and label the energy diagram for a reaction in which ΔE = 30 k. J/mol, Ea = 40 k. J/mol. Place reactants at energy level zero. Indicate determined values of ΔEforward, ΔEreverse & Ea’

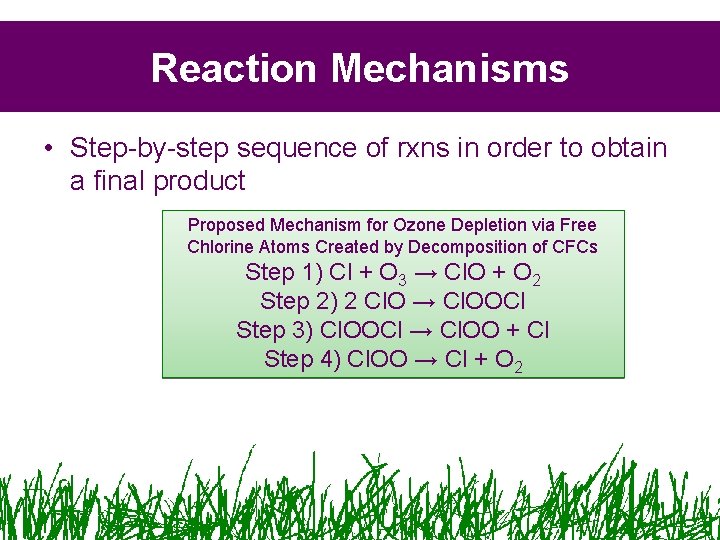
Reaction Mechanisms • Step-by-step sequence of rxns in order to obtain a final product Proposed Mechanism for Ozone Depletion via Free Chlorine Atoms Created by Decomposition of CFCs Step 1) Cl + O 3 → Cl. O + O 2 Step 2) 2 Cl. O → Cl. OOCl Step 3) Cl. OOCl → Cl. OO + Cl Step 4) Cl. OO → Cl + O 2

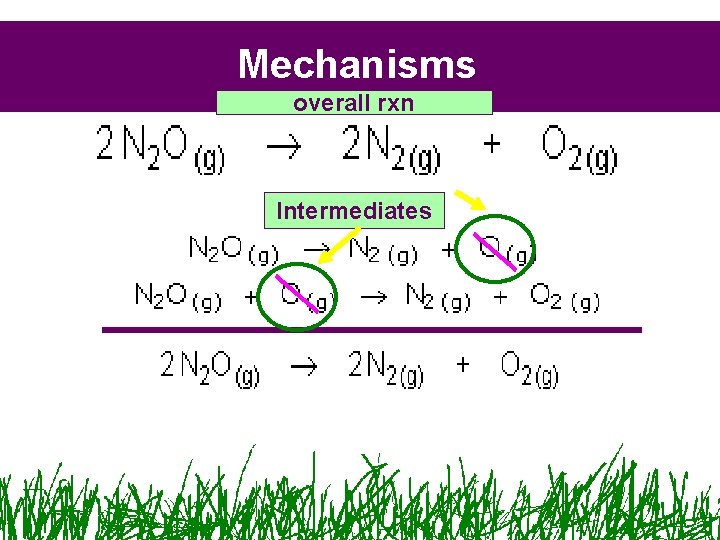
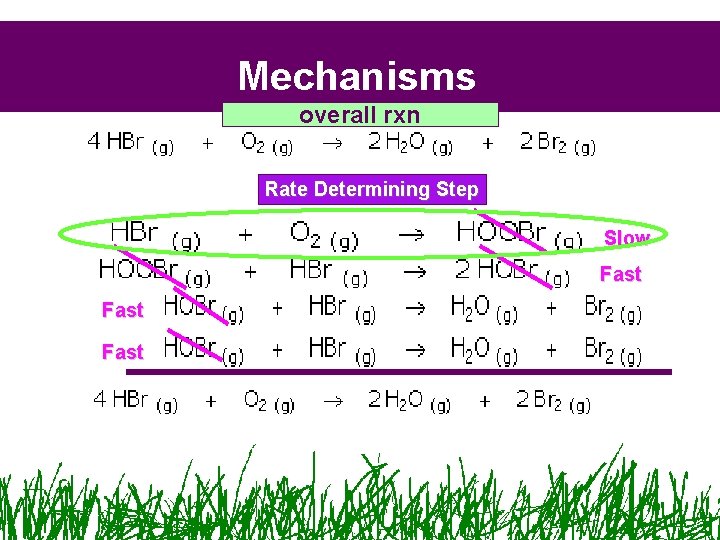
Mechanisms overall rxn Intermediates

Mechanisms overall rxn Rate Determining Step Slow Fast

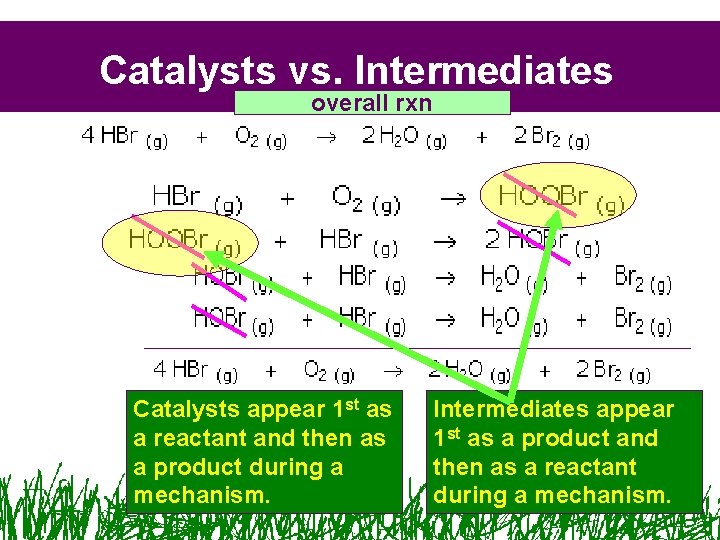
Catalysts vs. Intermediates overall rxn Catalysts appear 1 st as a reactant and then as a product during a mechanism. Intermediates appear 1 st as a product and then as a reactant during a mechanism.

Chapter 17 Reaction Kinetics 17 -2 Reaction Rate

How can we increase the rate of a reaction? 1. 2. 3. 4. 5. Increase Surface Area Increase Temperature Increase Concentration Increase in Pressure Add a Catalyst

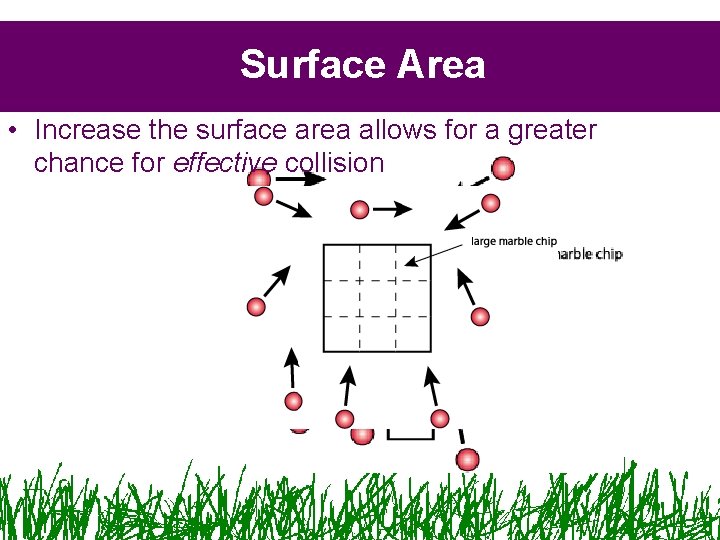
Surface Area • Increase the surface area allows for a greater chance for effective collision

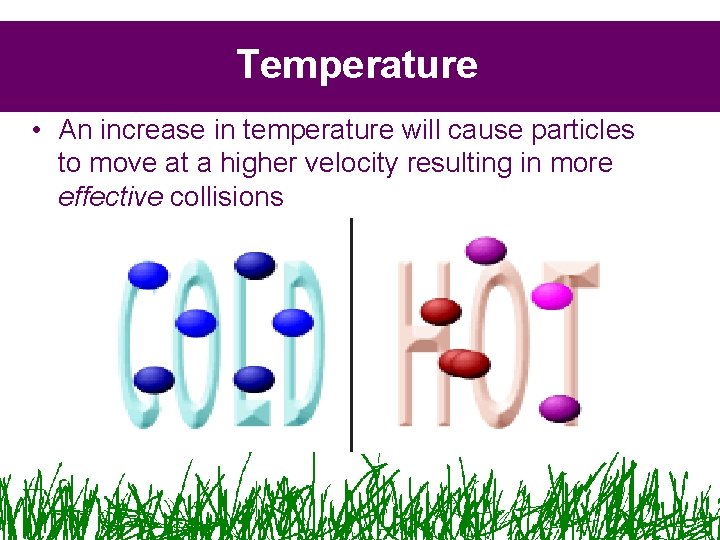
Temperature • An increase in temperature will cause particles to move at a higher velocity resulting in more effective collisions

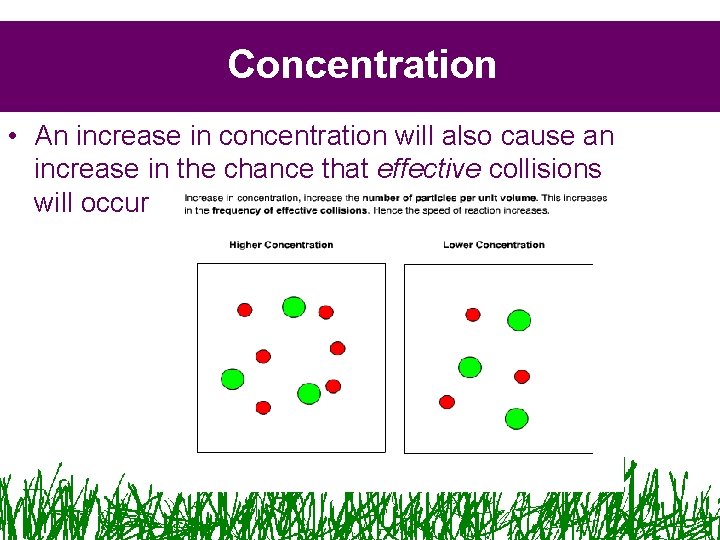
Concentration • An increase in concentration will also cause an increase in the chance that effective collisions will occur

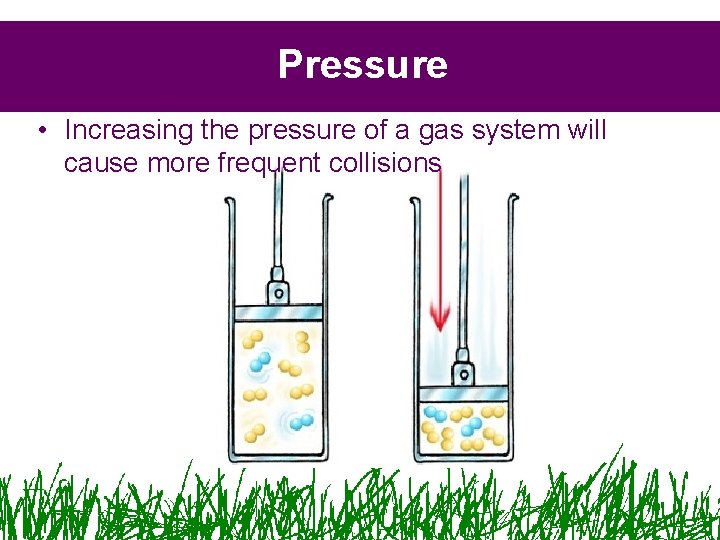
Pressure • Increasing the pressure of a gas system will cause more frequent collisions

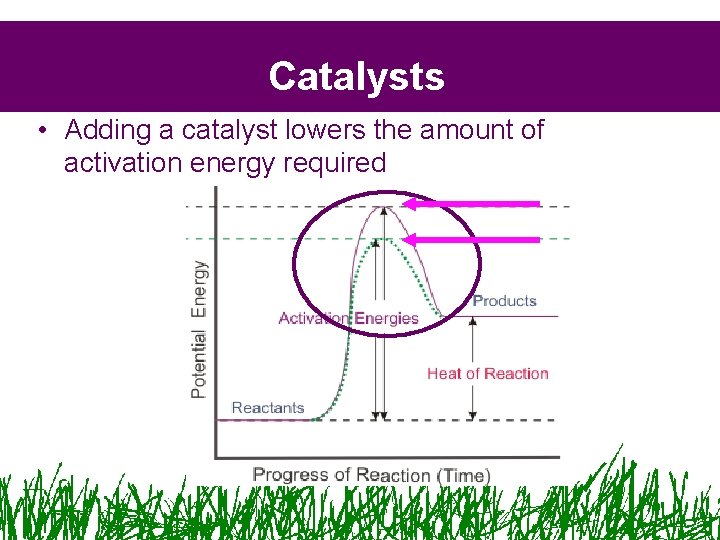
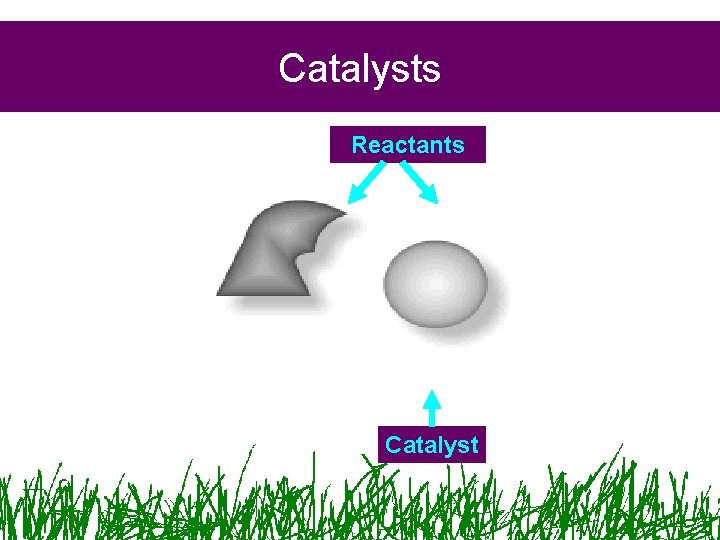
Catalysts • Adding a catalyst lowers the amount of activation energy required

Catalysts Reactants Catalyst

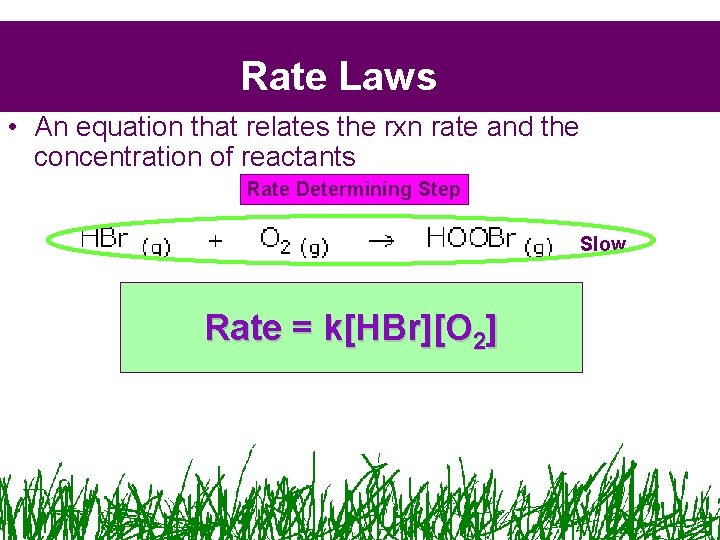
Rate Laws • An equation that relates the rxn rate and the concentration of reactants Rate Determining Step Slow Rate = k[HBr][O 2]

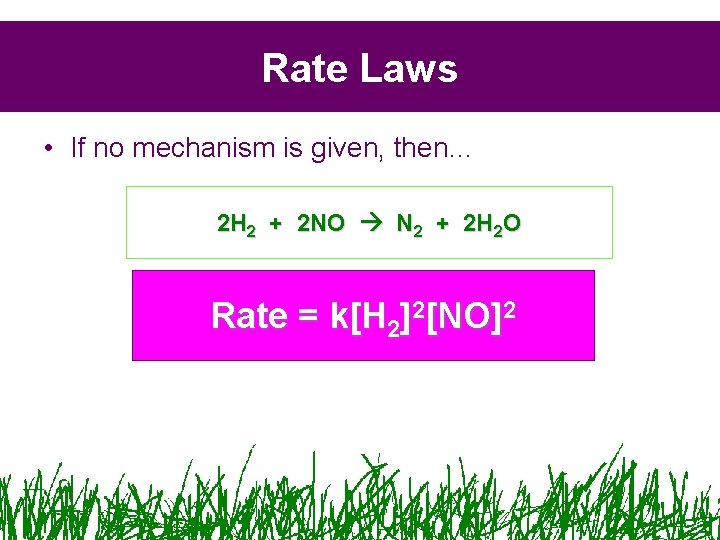
Rate Laws • If no mechanism is given, then… 2 H 2 + 2 NO N 2 + 2 H 2 O Rate = k[H 2]2[NO]2
![Rate Orders 1 st order ln reactants 0 order 1reactants 0 1 st Rate Orders 1 st order ln [reactants] 0 order 1/[reactants] • 0, 1 st](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-23.jpg)
Rate Orders 1 st order ln [reactants] 0 order 1/[reactants] • 0, 1 st and 2 nd order rates • Order is dependent upon what will yield a straight line 2 nd order

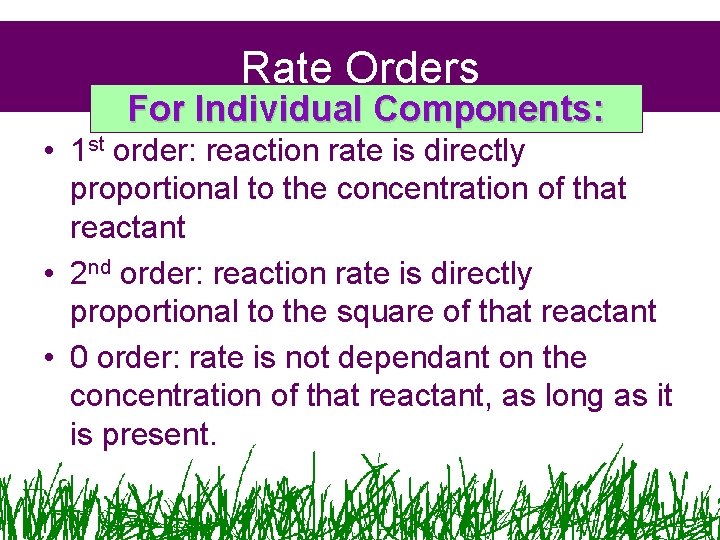
Rate Orders For Individual Components: • 1 st order: reaction rate is directly proportional to the concentration of that reactant • 2 nd order: reaction rate is directly proportional to the square of that reactant • 0 order: rate is not dependant on the concentration of that reactant, as long as it is present.

Rate Orders For Overall Order: • Overall reaction orders is equal to the sum of the reactant orders. • Always determined experimentally!
![Calculating for k A 2 B C Rate kAB2 Experiment Initial A Calculating for k A + 2 B C Rate = k[A][B]2 Experiment Initial [A]](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-26.jpg)
Calculating for k A + 2 B C Rate = k[A][B]2 Experiment Initial [A] Initial [B] Rate of Formation of C 1 0. 20 M 2. 0 x 10 -4 M/min 2 0. 20 M 0. 40 M 8. 0 x 10 -4 M/min 3 0. 40 M 1. 6 x 10 -3 M/min What is the value of k, the rate constant?
![Calculating for k Experiment Initial A Initial B Rate of Formation of C 1 Calculating for k Experiment Initial [A] Initial [B] Rate of Formation of C 1](https://slidetodoc.com/presentation_image/2f40751551795fd6801f6860aff8587f/image-27.jpg)
Calculating for k Experiment Initial [A] Initial [B] Rate of Formation of C 1 0. 20 M 2. 0 x 10 -4 M/min 2 0. 20 M 0. 40 M 8. 0 x 10 -4 M/min 3 0. 40 M 1. 6 x 10 -3 M/min Rate = k[A][B]2 2. 0 x 10 -4 = k[0. 20]2 2. 0 x 10 -4 = k(0. 008) k = 2. 50 x 10 -2 min-1 M-2

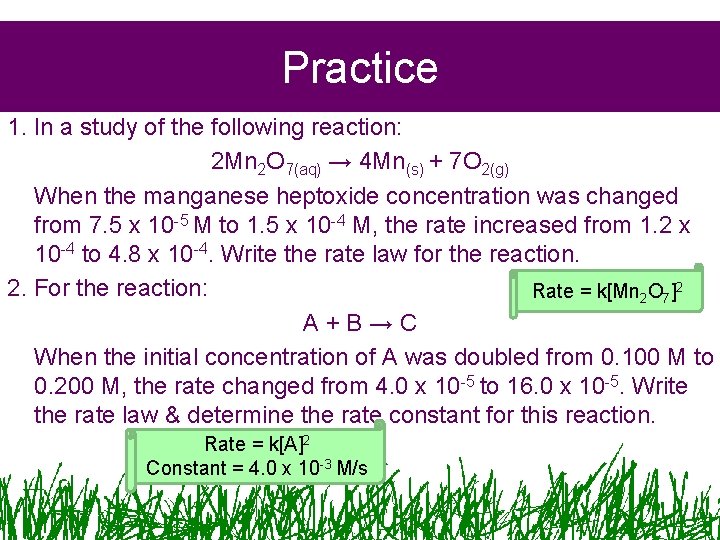
Practice 1. In a study of the following reaction: 2 Mn 2 O 7(aq) → 4 Mn(s) + 7 O 2(g) When the manganese heptoxide concentration was changed from 7. 5 x 10 -5 M to 1. 5 x 10 -4 M, the rate increased from 1. 2 x 10 -4 to 4. 8 x 10 -4. Write the rate law for the reaction. 2. For the reaction: Rate = k[Mn 2 O 7]2 A+B→C When the initial concentration of A was doubled from 0. 100 M to 0. 200 M, the rate changed from 4. 0 x 10 -5 to 16. 0 x 10 -5. Write the rate law & determine the rate constant for this reaction. Rate = k[A]2 Constant = 4. 0 x 10 -3 M/s

More Practice 3. The following reaction is first order: CH 3 NC(g) → CH 3 CN(g) The rate of this reaction is 1. 3 x 10 -4 M/s when the reactant concentration is 0. 040 M. Predict the rate when [CH 3 NC] = 0. 025. New Rate = 8. 1 x 10 -5 M/s 4. The following reaction is first order: (CH 2)3(g) → CH 2 CHCH 3 (g) What change in reaction rate would you expect if the pressure of the reactant is doubled? An increase by a factor of 2

Even More Practice 5. The rate law for a single step reaction that forms one product, C is R = k[A][B]2. Write the balanced reaction of A & B to form C. A + 2 B → C 6. The rate law of a reaction is found to be R = k[X]3. By what factor does the rate increase if the concentration of X is tripled? The rate will increase by a factor of 27 7. The rate of reaction, involving 2 reactants, X & Z, is found to double when the concentration of X is doubled, and to quadruple when the concentration of Z is doubled. Write the rate law for this reaction. R = k[X][Z]2