Chapter 17 Rates of Reaction EQUILIBRIUM Collision Theory

Chapter 17 Rates of Reaction EQUILIBRIUM

Collision Theory • Reactions can occur: • Very fast – such as a firecracker • Very slow – such as the time it took for dead plants to make coal • A “rate” is a measure of the speed of any change that occurs within an interval of time • In chemistry, reaction rate is expressed as the amount of reactant changing per unit time. https: //www. youtube. com/watch? v=Sbap. BWj. DA 74
Collision Model • Key Idea: Molecules must collide to react. • However, only a small fraction of collisions produces a reaction. Why? • Particles lacking the necessary kinetic energy to react bounce apart unchanged when they collide https: //www. youtube. com/watch? v=m. BTSw. Jn. Z 6 Sk

Collision Model • Collisions must have enough energy to produce the reaction (must equal or exceed the activation energy – the minimum energy needed to react). • Pushing a stalled car: it takes a lot of force to overcome the inertia. Once it is rolling, it becomes easy to push. https: //www. youtube. com/watch? v=e. SIn. I 1 x. Hv h 4
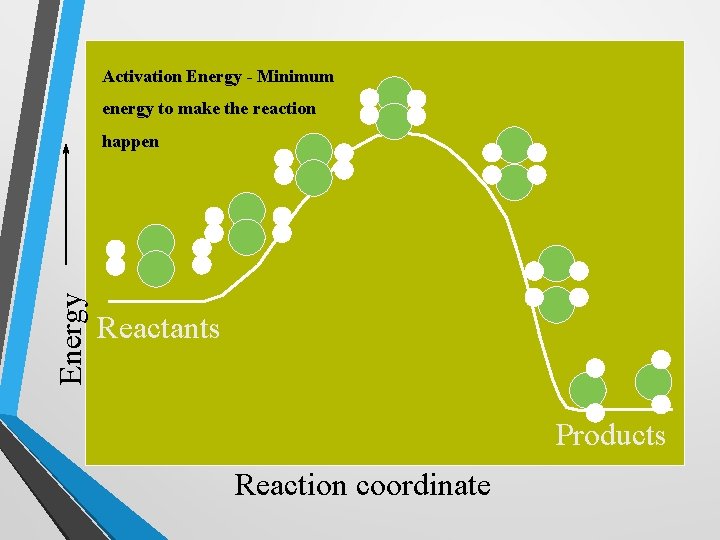
Activation Energy - Minimum energy to make the reaction Energy happen Reactants Products Reaction coordinate
Collision Model • An “activated complex” is an unstable arrangement of atoms that forms momentarily (typically about 1013 seconds (femptasecond) at the peak of the activation-energy barrier. • This is sometimes called the transition state • Results in either a) forming products, or b) reformation of reactants • Both outcomes are equally likely • https: //www. youtube. com/watch? v=7 q. OFt. L 3 VEBc
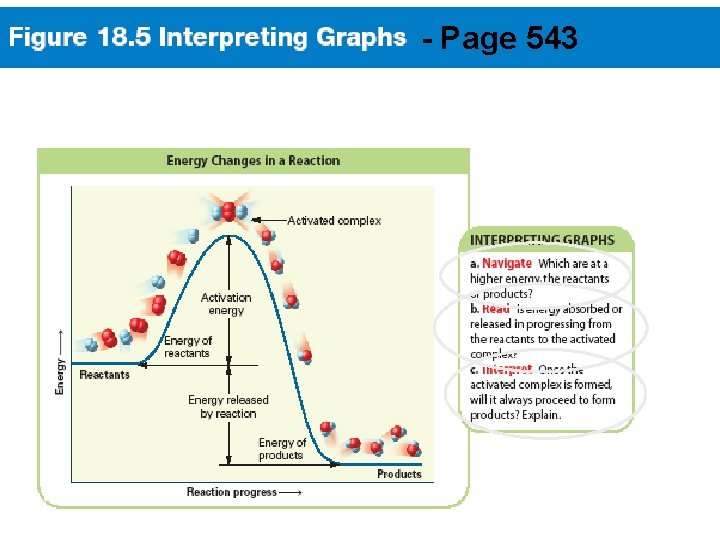
- Page 543
Collision Model The collision theory explains why some naturally occurring reactions are very slow Carbon and oxygen react when charcoal burns, but this has a very high activation energy At room temperature, the collisions between carbon and oxygen are not enough to cause a reaction
Factors Affecting Rate Temperature Increasing temperature always increases the rate of a reaction. 2) Surface Area Increasing surface area increases the rate of a reaction 3) Concentration Increasing concentration USUALLY increases the rate of a reaction 4) Presence of Catalysts 1)

Catalysts • Catalyst: A substance that speeds up a reaction, without being consumed itself in the reaction • Enzyme: A large molecule (usually a protein) that catalyzes biological reactions. • Human body temperature = 37 o. C, much too low for digestion reactions without catalysts. • Inhibitors – interfere with the action of a catalyst; reactions slow or even stop
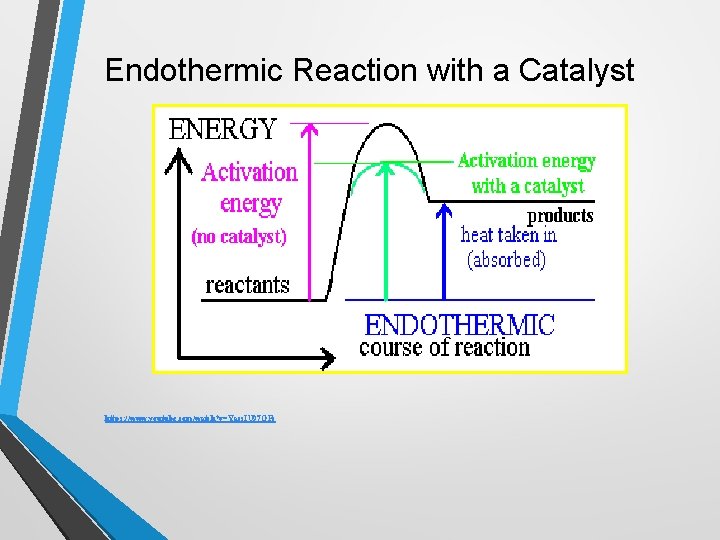
Endothermic Reaction with a Catalyst https: //www. youtube. com/watch? v=Yacs. IU 97 OFc
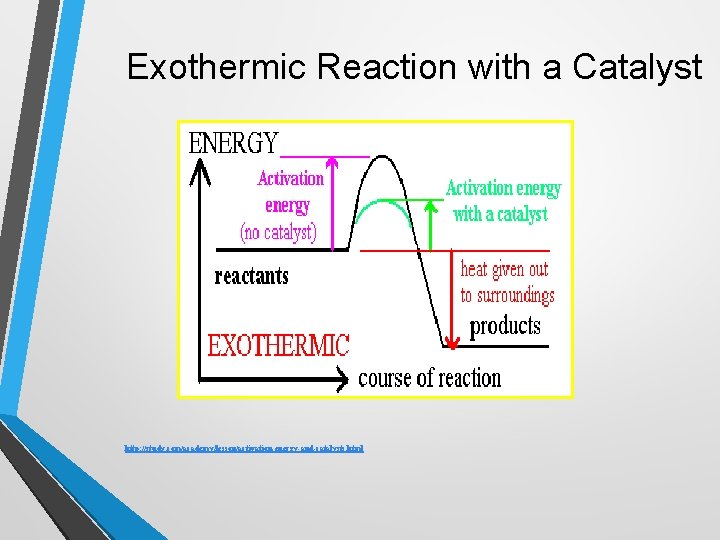
Exothermic Reaction with a Catalyst http: //study. com/academy/lesson/activation-energy-and-catalysts. html
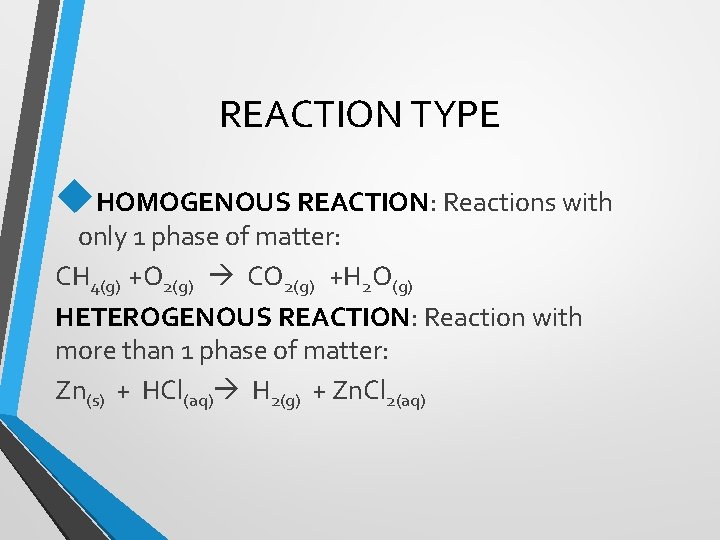
REACTION TYPE HOMOGENOUS REACTION: Reactions with only 1 phase of matter: CH 4(g) +O 2(g) CO 2(g) +H 2 O(g) HETEROGENOUS REACTION: Reaction with more than 1 phase of matter: Zn(s) + HCl(aq) H 2(g) + Zn. Cl 2(aq)

FORWARD REVERSE • Up to now, we have assumed reactions go to completion. Meaning the reaction goes to product until 1 of the reactants runs out. • Not true for some reactions. • CHEMICAL EQUILIBRIUM IS REACHED WHEN THE EXACT BALANCE BETWEEN FORWARD AND REVERSE REACTIONS IS REACHED.

CHEMICAL EQUILIBRIUM • N 2 O 4(g) NO 2(g) +NO 2(g) • Forward is always towards the product side. • Reverse is always towards the reactant side. • Dynamic Equilibrium: concentration of reactants and products remains constant as long as conditions remain unchanged. https: //www. youtube. com/watch? v=g 5 w. Ng_d. Ks. YY

Reversible Reactions • Some reactions do not go to completion as we have assumed • They may be reversible – a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously • Forward: 2 SO 2(g) + O 2(g) → 2 SO 3(g) • Reverse: 2 SO 2(g) + O 2(g) ← 2 SO 3(g) http: //study. com/academy/lesson/dynamic-equilibrium-physical-and-chemical. html 3

Reversible Reactions • The two equations can be combined into one, by using a double arrow, which tells us that it is a reversible reaction: 2 SO 2(g) + O 2(g) ↔ 2 SO 3(g) üA chemical equilibrium occurs, and no net change occurs in the actual amounts of the components of the system.
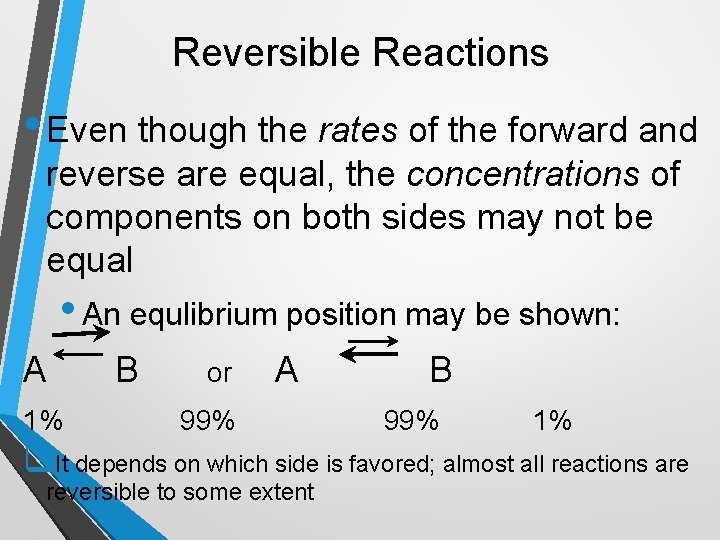
Reversible Reactions • Even though the rates of the forward and reverse are equal, the concentrations of components on both sides may not be equal • An equlibrium position may be shown: A 1% B or A 99% B 99% 1% q. It depends on which side is favored; almost all reactions are reversible to some extent

Le Chatelier’s Principle The French chemist Henri Le Chatelier (1850 -1936) studied how the equilibrium position shifts as a result of changing conditions Le Chatelier’s principle: If stress is applied to a system in equilibrium, the system changes in a way that relieves the stress https: //www. youtube. com/watch? v=7 zu. UV 455 z. Fs Fuse 4. 0

Le Chatelier’s Principle • • What items did he consider to be stress on the equilibrium: 1)Concentration 2)Temperature 3)Pressure Each of these will now be discussed in detail Concentration – adding more reactant produces more product, and removing the product as it forms will produce more product
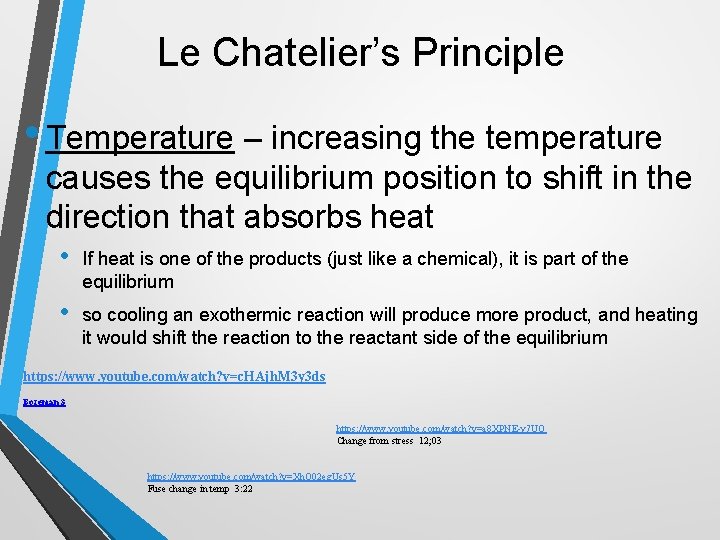
Le Chatelier’s Principle • Temperature – increasing the temperature causes the equilibrium position to shift in the direction that absorbs heat • If heat is one of the products (just like a chemical), it is part of the equilibrium • so cooling an exothermic reaction will produce more product, and heating it would shift the reaction to the reactant side of the equilibrium https: //www. youtube. com/watch? v=c. HAjh. M 3 y 3 ds Bozeman 3 https: //www. youtube. com/watch? v=a 8 XPNE-y 7 UQ Change from stress 12; 03 https: //www. youtube. com/watch? v=Xh. Q 02 eg. Us 5 Y Fuse change in temp 3: 22
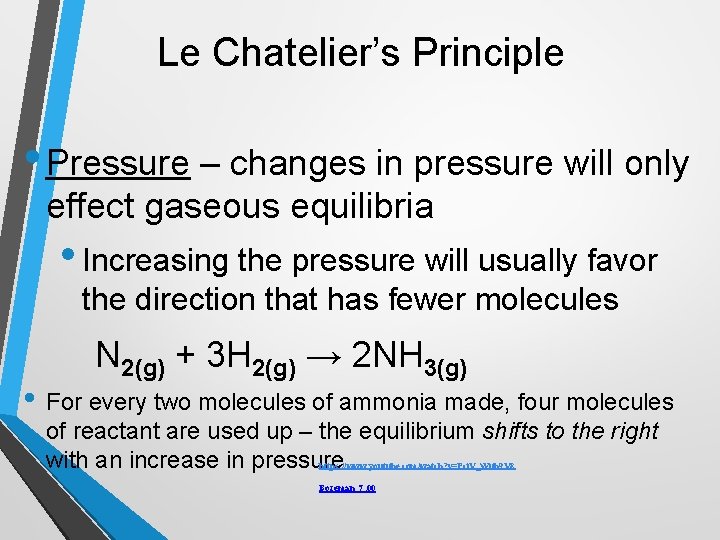
Le Chatelier’s Principle • Pressure – changes in pressure will only effect gaseous equilibria • Increasing the pressure will usually favor the direction that has fewer molecules N 2(g) + 3 H 2(g) → 2 NH 3(g) • For every two molecules of ammonia made, four molecules of reactant are used up – the equilibrium shifts to the right with an increase in pressure. https: //www. youtube. com/watch? v=Pci. V_Wuh 9 V 8 Bozeman 7; 00

PREDICT THE SHIFT IN EQUILBRIUM WHEN PRESSURE IS INCREASED/VOLUME IS REDUCED N 2 (g) + 3 H 2(g) 2 NH 3 (g) right P 4 (s) + 6 Cl 2 (g) 4 PCl 3 (l) PCl 3 (g) + Cl 2 (g) PCl 5 (g) right No change PCl 3(g) + 3 NH 3 (g) P(NH ) + 3 HCl (g) right 2 3(g) WHEN NH 3 IS REMOVED left N 2 (g) + 3 H 2(g) 2 NH 3 (g) PCl 3(g) + 3 NH 3 (g) P(NH 2)3(g) + 3 HCl (g)

EQUILBRIUM ANALOGY Before the movie starts, people are filling up all the seats until they are full. The movie theater is at equilibrium when the movie starts. The same number of people are coming in as going out. The “people” remain constant. Equilbrium will Design an analogy which demonstrates before equilibrium and during equilibrium. Explain
Equilibrium Constants • Law of chemical equilibrium: states that the equilibrium • condition is demonstrated by the equilibrium expression. Chemists generally express the position of equilibrium in terms of numerical values • These values relate to the amounts of reactants and products at equilibrium • This is called the equilibrium constant, and abbreviated Keq https: //www. youtube. com/watch? v=DP-v. WN 1 y. Xr. Y Crash course 9. 26
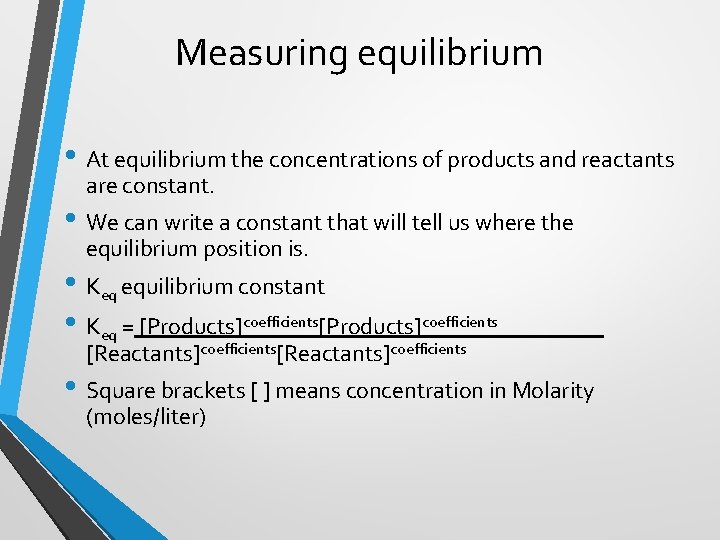
Measuring equilibrium • At equilibrium the concentrations of products and reactants are constant. • We can write a constant that will tell us where the equilibrium position is. • Keq equilibrium constant • Keq = [Products]coefficients [Reactants]coefficients • Square brackets [ ] means concentration in Molarity (moles/liter)
![Writing Equilibrium Expressions • General equation • Keq = [C]c [D]d • a. A Writing Equilibrium Expressions • General equation • Keq = [C]c [D]d • a. A](http://slidetodoc.com/presentation_image/30c3b81dfe472216641813445eb167f0/image-27.jpg)
Writing Equilibrium Expressions • General equation • Keq = [C]c [D]d • a. A + b. B c. C + d. D [A]a [B]b • Write the equilibrium expressions for the following reactions. • 2 H 2 O(g) 2 H 2(g) + O 2(g) • [H 2]2 [O 2 ] • [H 2 O]2
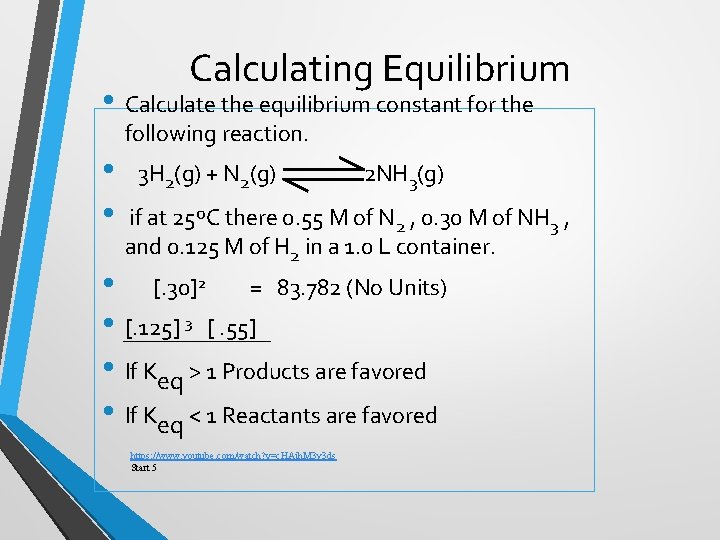
Calculating Equilibrium • Calculate the equilibrium constant for the following reaction. • • 3 H 2(g) + N 2(g) 2 NH 3(g) if at 25ºC there 0. 55 M of N 2 , 0. 30 M of NH 3 , and 0. 125 M of H 2 in a 1. 0 L container. • [. 30]2 = 83. 782 (No Units) • [. 125] 3 [. 55] • If Keq > 1 Products are favored • If Keq < 1 Reactants are favored https: //www. youtube. com/watch? v=c. HAjh. M 3 y 3 ds Start 5

Heterogenous Equilibria The concentrations of pure solids or liquids cannot be included in an equilibrium expression because their concentrations do not change. Water is a pure liquid and it concentration is constant. H 2 O(l) O 2(g) +H 2(g) Keq = [H 2]2[O 2]

TOexpression. KNOW FOR TEST How. WHAT to write a Keq How to solve for Keq Pure solids and liquids are not included in Keq expressions. Vocab words: equilibrium, forward reaction, reverse reaction Le. Chatlier’s principle, activation energy catalyst, collision theory, transition state, entropy, enthalpy, spontaneous reaction, endothermic and exothermic. Which direction the reaction will go if pressure, temperature, or concentration is changed. Keq>1 favors products and Keq<1 favors reactants. What is dynamic equilibrium How to interpret graph.

Entropy • Entropy is a measure of disorder, and is measured. in units of J/mol K; there are no negative values of entropy • The law of disorder states the natural tendency is for systems to move to the direction of maximum disorder, not vice-versa • Your room NEVER cleans itself (disorder to order? ) • An increase in entropy favors the spontaneous chemical reaction • A decrease in entropy favors the nonspontaneous reaction http: //www. youtube. com/watch? feature=player_embedded&v=Cgpp. Gozb. Fd 4
Factors that affect Entropy • Temperature – increase, Entropy increase • Crushing or dividing a substance increases Entropy • When a molecule changes state from solid to liquid to gas, entropy increases • Entropy increases when the chemical reaction yields more product molecules than reactant. • Ex. 2 H 2 O 2 H 2 + O 2 http: //www. sciencechannel. com/tv-shows/wonders-with-brian-cox/videos/wonders-of -the-universe-entropy/
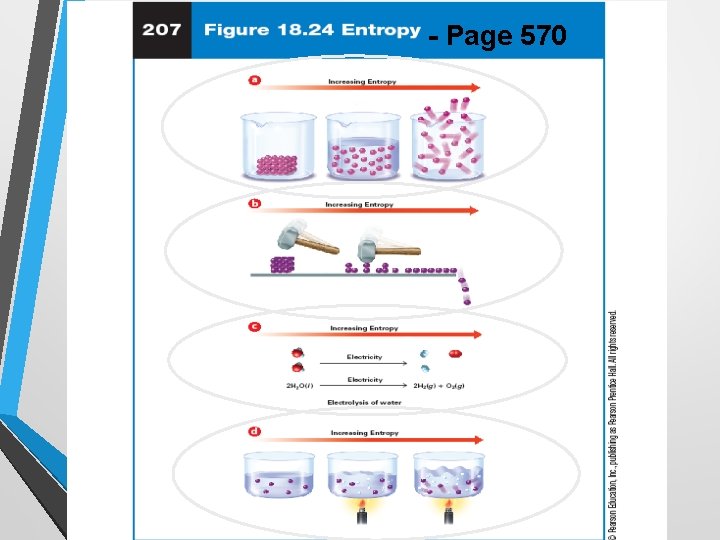
- Page 570

Your Mission • Create an analogy for the Collision model. • Product: 2 pictures showing the collision has enough energy to make it happen and a picture showing too little energy to make it happen. Use same analogy in both pics. Write a short description for each pic. https: //www. youtube. com/watch? v=Jty. Byef. Ovg. Q

COLLISION MODEL: My car ran out of gas and I have to push it to the gas station. I am pushing as hard as I can and the car will not move. My friends came by to help me push my car and now it is rolling. More energy was needed to make the car move.
- Slides: 35