CHAPTER 17 Organic Chemistry 17 3 Organic Reactions


- Slides: 23

CHAPTER 17 Organic Chemistry 17. 3 Organic Reactions

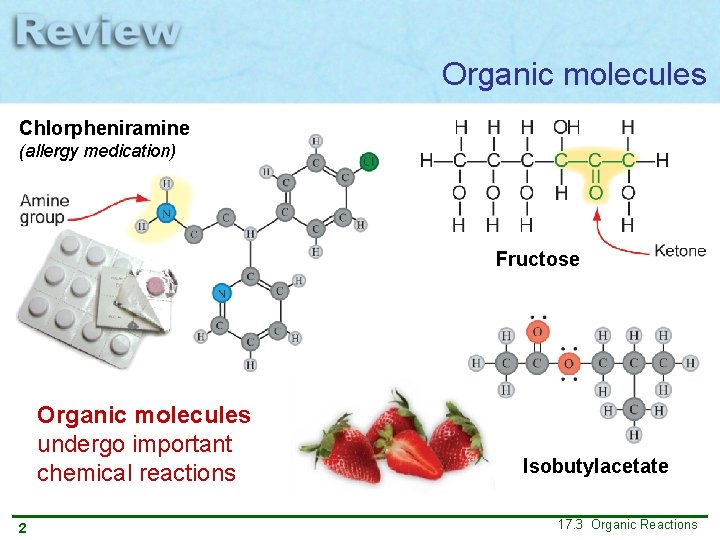
Organic molecules Chlorpheniramine (allergy medication) Fructose Organic molecules undergo important chemical reactions 2 Isobutylacetate 17. 3 Organic Reactions

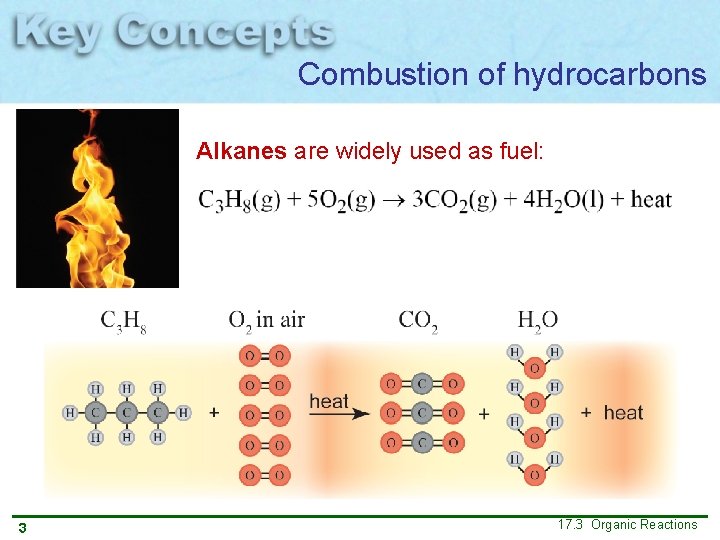
Combustion of hydrocarbons Alkanes are widely used as fuel: 3 17. 3 Organic Reactions

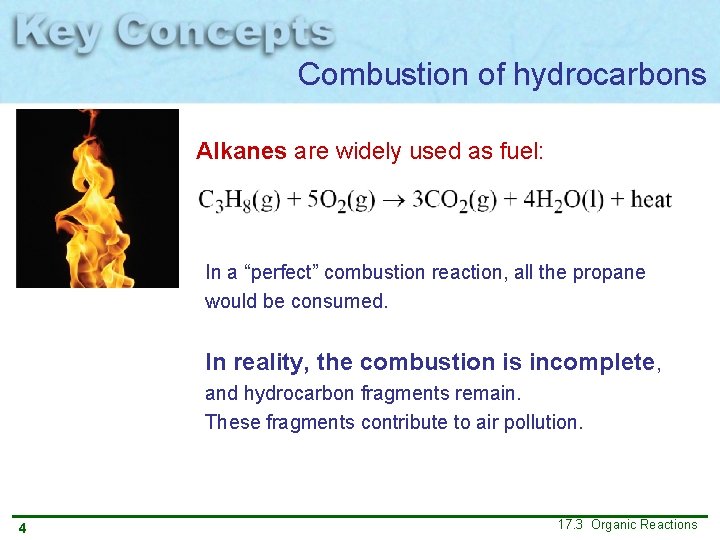
Combustion of hydrocarbons Alkanes are widely used as fuel: In a “perfect” combustion reaction, all the propane would be consumed. In reality, the combustion is incomplete, and hydrocarbon fragments remain. These fragments contribute to air pollution. 4 17. 3 Organic Reactions

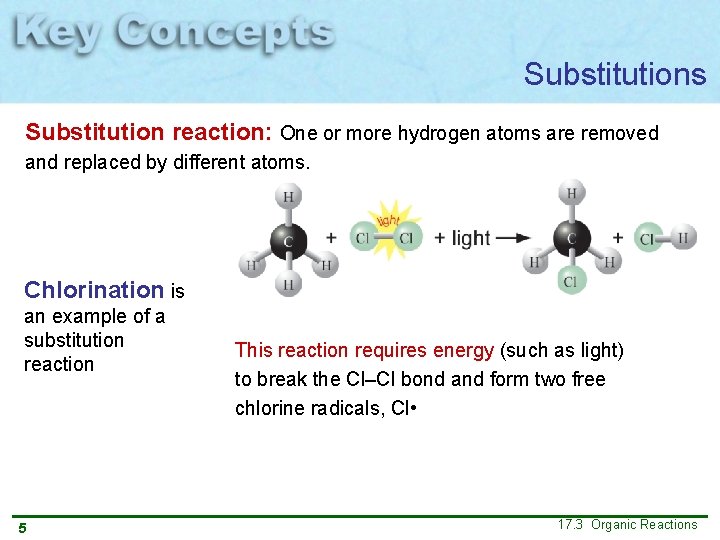
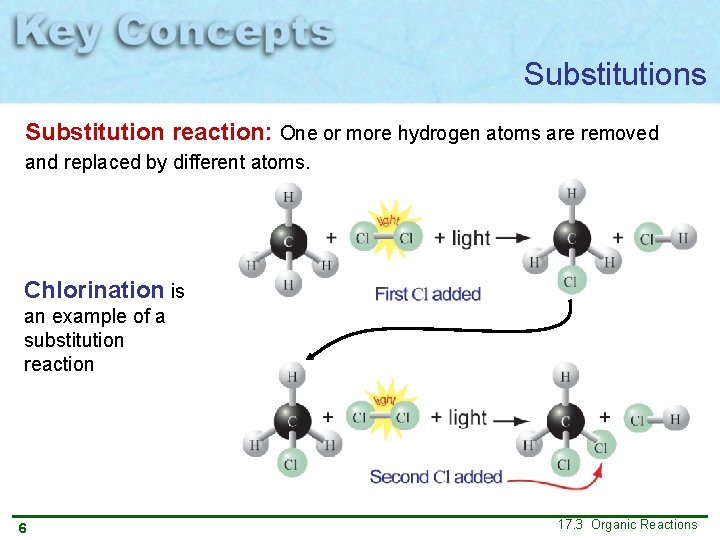
Substitutions Substitution reaction: One or more hydrogen atoms are removed and replaced by different atoms. Chlorination is an example of a substitution reaction 5 This reaction requires energy (such as light) to break the Cl–Cl bond and form two free chlorine radicals, Cl • 17. 3 Organic Reactions

Substitutions Substitution reaction: One or more hydrogen atoms are removed and replaced by different atoms. Chlorination is an example of a substitution reaction 6 17. 3 Organic Reactions

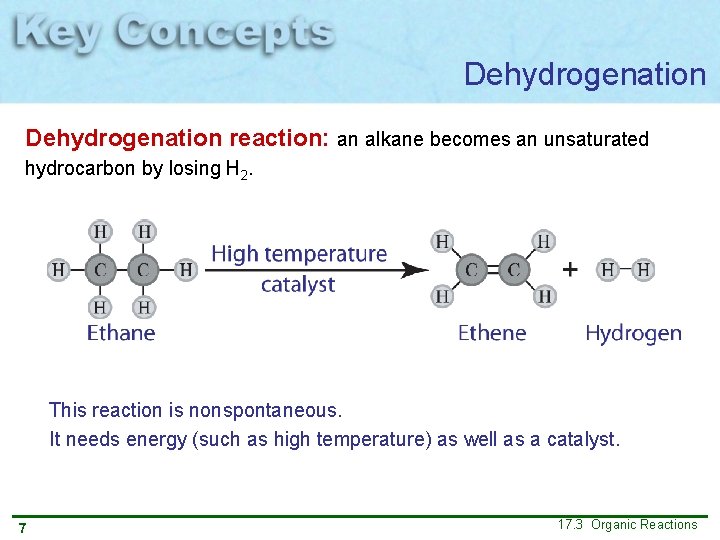
Dehydrogenation reaction: an alkane becomes an unsaturated hydrocarbon by losing H 2. This reaction is nonspontaneous. It needs energy (such as high temperature) as well as a catalyst. 7 17. 3 Organic Reactions

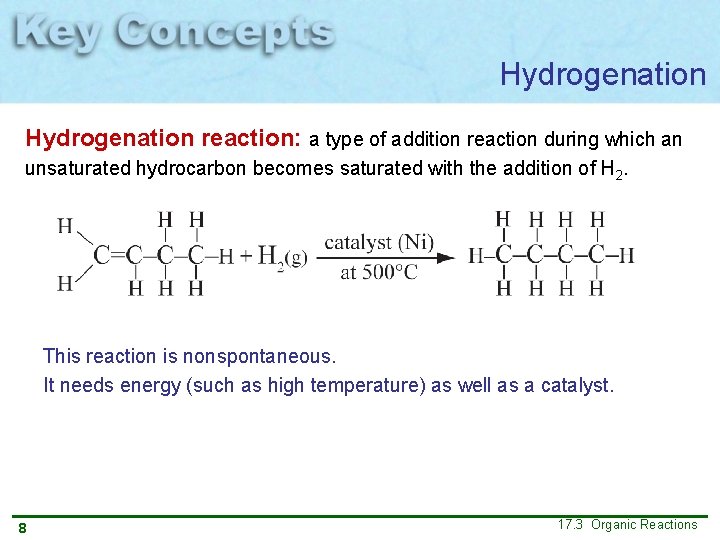
Hydrogenation reaction: a type of addition reaction during which an unsaturated hydrocarbon becomes saturated with the addition of H 2. This reaction is nonspontaneous. It needs energy (such as high temperature) as well as a catalyst. 8 17. 3 Organic Reactions

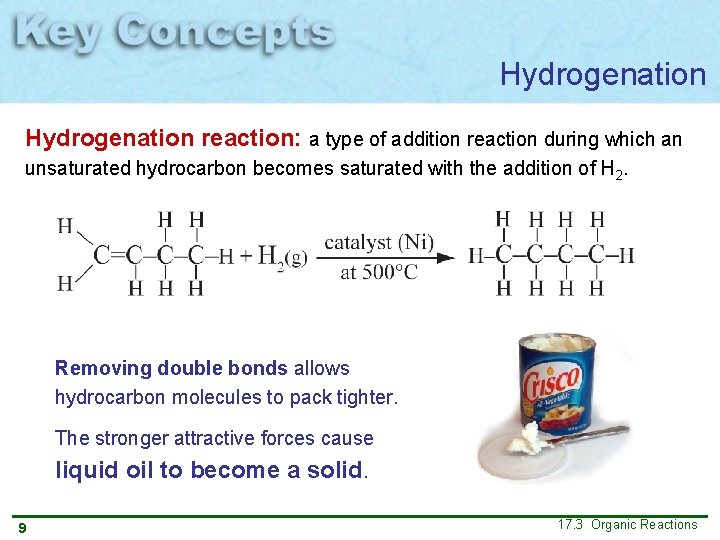
Hydrogenation reaction: a type of addition reaction during which an unsaturated hydrocarbon becomes saturated with the addition of H 2. Removing double bonds allows hydrocarbon molecules to pack tighter. The stronger attractive forces cause liquid oil to become a solid. 9 17. 3 Organic Reactions

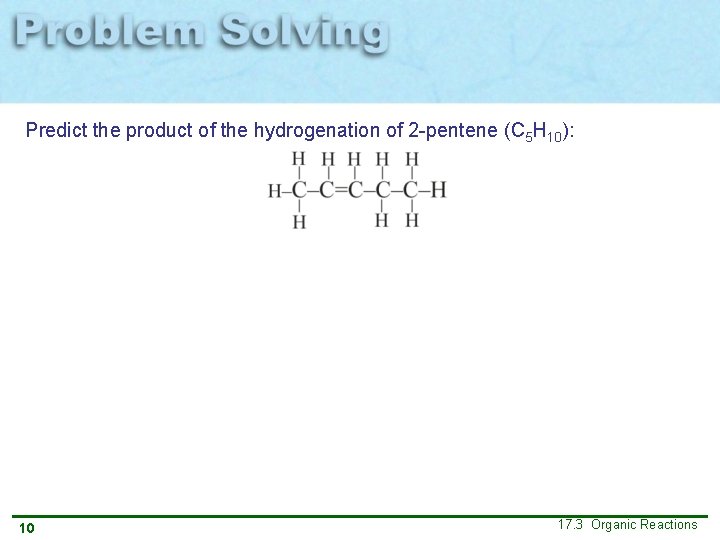
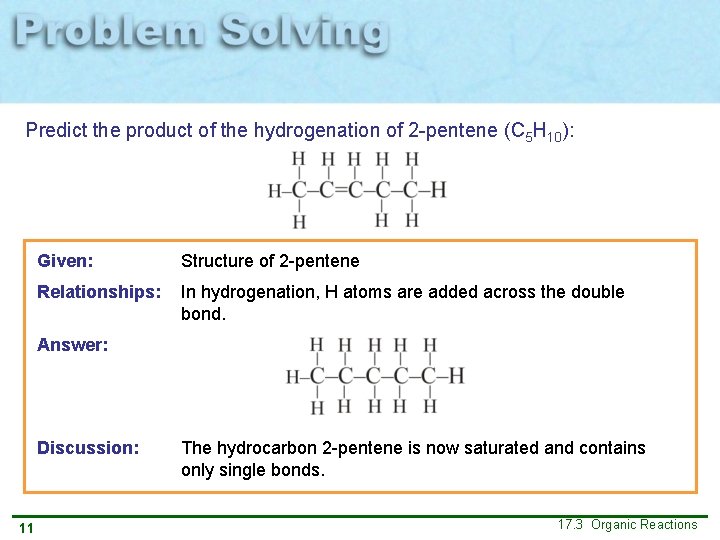
Predict the product of the hydrogenation of 2 -pentene (C 5 H 10): 10 17. 3 Organic Reactions

Predict the product of the hydrogenation of 2 -pentene (C 5 H 10): Given: Structure of 2 -pentene Relationships: In hydrogenation, H atoms are added across the double bond. Answer: Discussion: 11 The hydrocarbon 2 -pentene is now saturated and contains only single bonds. 17. 3 Organic Reactions

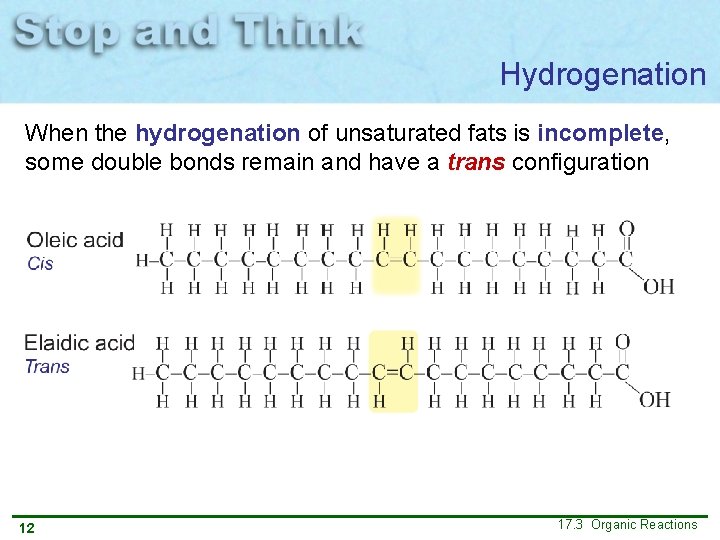
Hydrogenation When the hydrogenation of unsaturated fats is incomplete, some double bonds remain and have a trans configuration 12 17. 3 Organic Reactions

Hydrogenation Partial hydrogenation causes food that contains oil to have a longer shelf life. However, research now shows that trans fats are harmful to our health. 13 17. 3 Organic Reactions

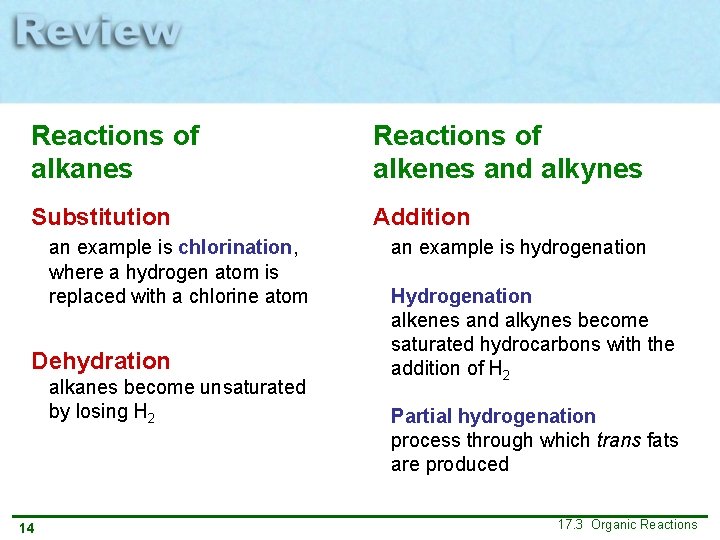
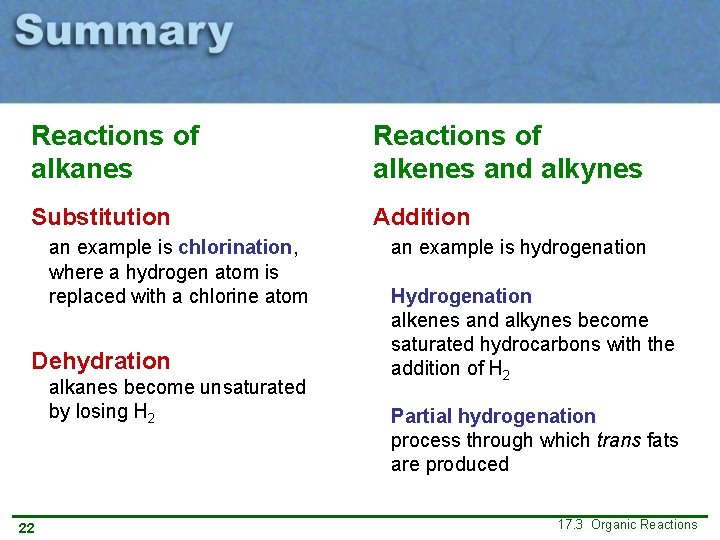
Reactions of alkanes Reactions of alkenes and alkynes Substitution Addition an example is chlorination, where a hydrogen atom is replaced with a chlorine atom Dehydration alkanes become unsaturated by losing H 2 14 an example is hydrogenation Hydrogenation alkenes and alkynes become saturated hydrocarbons with the addition of H 2 Partial hydrogenation process through which trans fats are produced 17. 3 Organic Reactions

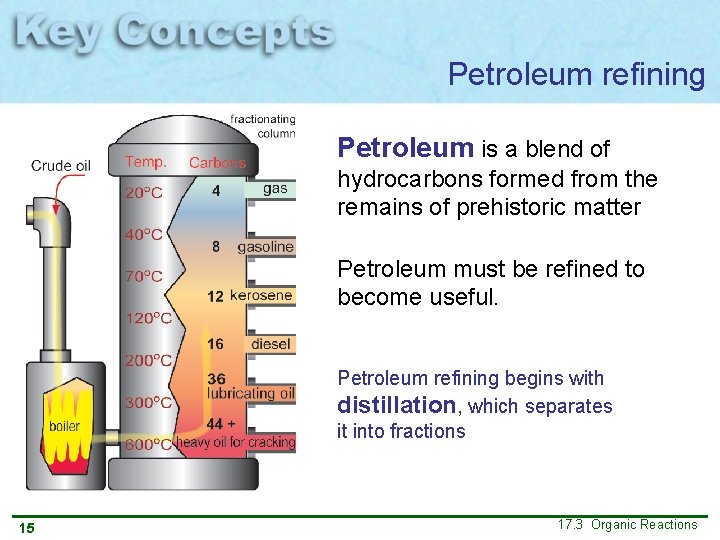
Petroleum refining Petroleum is a blend of hydrocarbons formed from the remains of prehistoric matter Petroleum must be refined to become useful. Petroleum refining begins with distillation, which separates it into fractions 15 17. 3 Organic Reactions

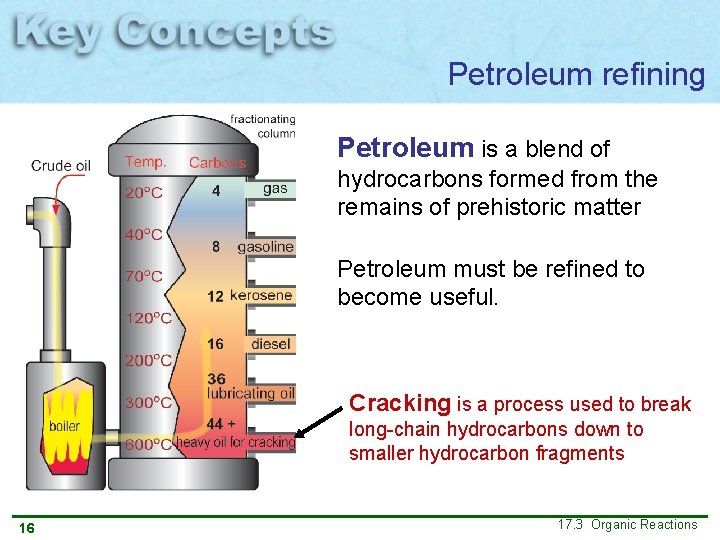
Petroleum refining Petroleum is a blend of hydrocarbons formed from the remains of prehistoric matter Petroleum must be refined to become useful. Cracking is a process used to break long-chain hydrocarbons down to smaller hydrocarbon fragments 16 17. 3 Organic Reactions

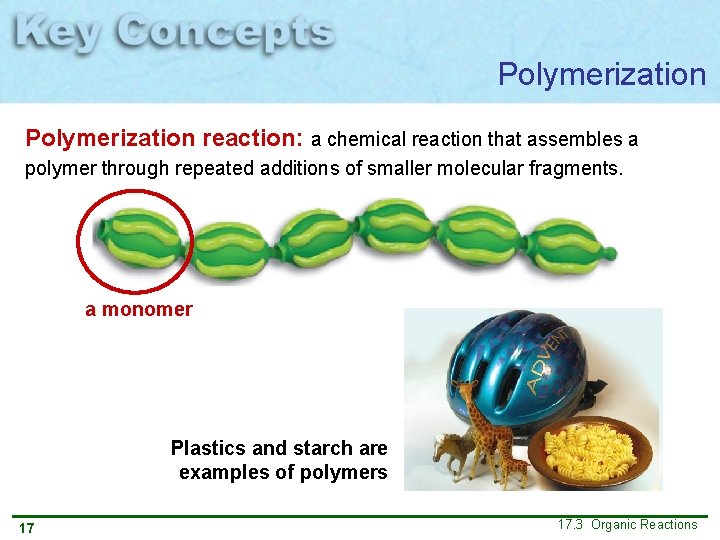
Polymerization reaction: a chemical reaction that assembles a polymer through repeated additions of smaller molecular fragments. a monomer Plastics and starch are examples of polymers 17 17. 3 Organic Reactions

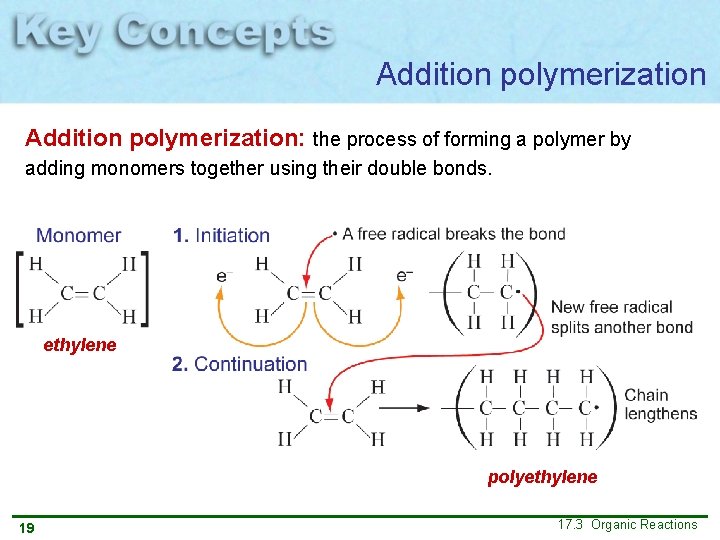
Addition polymerization: the process of forming a polymer by adding monomers together using their double bonds. Polyethylene is the one of the simplest and most widely used polymers in the world. It is used to make plastic bags and shampoo bottles 18 17. 3 Organic Reactions

Addition polymerization: the process of forming a polymer by adding monomers together using their double bonds. ethylene polyethylene 19 17. 3 Organic Reactions

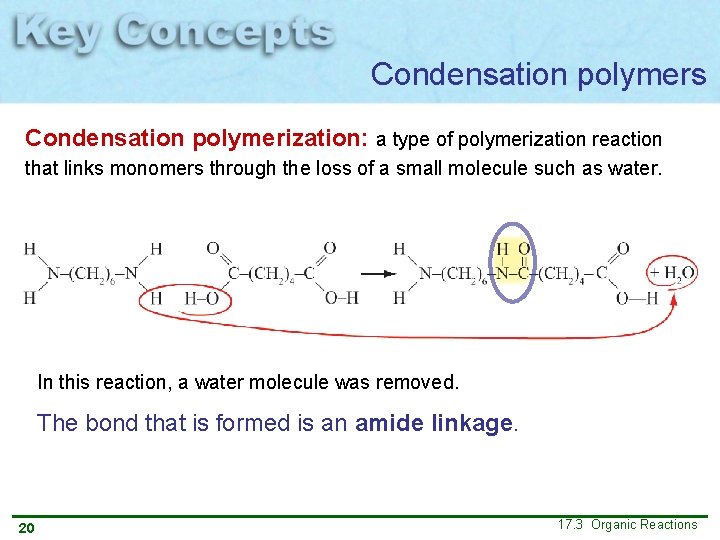
Condensation polymers Condensation polymerization: a type of polymerization reaction that links monomers through the loss of a small molecule such as water. In this reaction, a water molecule was removed. The bond that is formed is an amide linkage. 20 17. 3 Organic Reactions

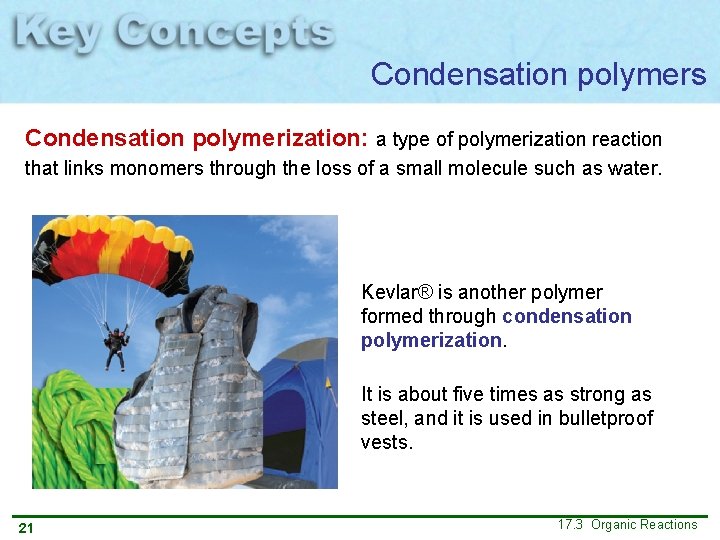
Condensation polymers Condensation polymerization: a type of polymerization reaction that links monomers through the loss of a small molecule such as water. Kevlar® is another polymer formed through condensation polymerization. It is about five times as strong as steel, and it is used in bulletproof vests. 21 17. 3 Organic Reactions

Reactions of alkanes Reactions of alkenes and alkynes Substitution Addition an example is chlorination, where a hydrogen atom is replaced with a chlorine atom Dehydration alkanes become unsaturated by losing H 2 22 an example is hydrogenation Hydrogenation alkenes and alkynes become saturated hydrocarbons with the addition of H 2 Partial hydrogenation process through which trans fats are produced 17. 3 Organic Reactions

Reactions of alkanes Substitution Dehydration Polymerization Addition polymerization monomers are linked using their double bonds Condensation polymerization Reactions of alkenes and alkynes a small molecule such as water is lost during linkage formation Addition (ex: hydrogenation) 23 17. 3 Organic Reactions